Polydextrose is a polysaccharide of randomly cross-linked glucose molecules, created by the vacuum polymerisation of glucose using a sorbitol catalyst. It is resistant to digestion by human alimentary enzymes, and therefore it reaches the colon largely undigested. Approximately 50 % of ingested polydextrose is fermented by the colonic microflora to yield CO2 and volatile SCFA such as propionate and butyrate(Reference Jie, Bang-Yao and Ming-Jie1), while the remaining 50 % is excreted intact in the faeces(Reference Auerbach, Craig and Howlett2). The textural similarity to sucrose, low energy content and sweet taste allows polydextrose to be commonly used as a bulking agent, as well as a sugar and partial fat replacer in low-energy and diet products(Reference Craig, Holden, Troup and Cho3).
A previous study reported that when 25 g polydextrose were consumed in a yogurt preload, subjective appetite ratings following consumption of the preload, and ad libitum energy intake at a buffet-style test meal served 90 min after the preload, were significantly reduced compared with when a control yogurt, matched for weight and volume, but containing no polydextrose was consumed(Reference King, Craig and Pepper4). A further study by Schwab et al. (Reference Schwab, Louheranta and Torronen5) reported that consuming a drink enriched with 16 g polydextrose, daily for 12 weeks, reduced subjective hunger ratings in response to a standard test meal compared with when a control drink, which contained no polydextrose, was consumed over the same time period. Consequently, polydextrose may be a useful ingredient to include in food products designed to limit energy intake at meals served shortly after it has been consumed. However, excess consumption of polydextrose is associated with symptoms of laxation, which include but are not limited to bloating, flatulence and diarrhoea(6). It is currently unknown what the optimal dose of polydextrose would be in order to achieve the desired effects on satiety, without causing the adverse side effects associated with excess consumption, which may represent a hazard to health.
The aim of the present study was to investigate the effects of different doses of polydextrose, consumed in a mixed macronutrient isoenergetic liquid preload as a mid-morning between-meal ‘snack’, on subjective appetite and subsequent energy intake, in healthy-weight men and women.
Subjects and methods
Participants
Participants were recruited from the staff and student populations of the University of Nottingham and Queen's Medical Centre via poster advertisement. Inclusion criteria for volunteers were weight stable ( < 3 kg change in body weight in previous 6 months), healthy weight (BMI 19–25 kg/m2), aged 18–45 years, non-smokers, with no current diseases and not currently taking any medications. Females were not pregnant or lactating. Volunteers were excluded if they scored >7 for restraint on the Three-Factor Eating Questionnaire(Reference Stunkard and Messick7), >10 for symptoms of clinical depression on the Beck Depression Inventory(Reference Beck, Ward and Mendelson8), reported previous gastrointestinal diseases or any undiagnosed gastrointestinal problems lasting longer than 3 weeks, or had an allergy, intolerance or particular dislike of any of the foods provided during the study. Written informed consent was obtained from all participants before their participation in the study, and the study was approved by the University of Nottingham Medical School Ethics Committee. All participants were recruited and studied between March and October of 2007.
Experimental design
Data were collected using the Sussex Ingestion Pattern Monitor (University of Sussex), a computer-based system modified from the Universal Eating Monitor(Reference Kissileff, Klingsberg and Van Itallie9) for measuring food intake and recording subjective appetite ratings(Reference Yeomans10).
A total of four liquid preloads were tested in a single-blind, randomised, cross-over design. Participants attended the Ingestion Laboratory at the School of Biomedical Sciences, University of Nottingham on four occasions, with at least 7 d between each visit. Female participants were always studied during days 6–12 of their menstrual cycle to avoid differences in hormonal fluctuations which may influence appetite and satiety(Reference Brennan, Feltrin and Nair11, Reference Dye and Blundell12). Participants were advised to refrain from consuming alcohol and undertaking extensive exercise for 24 h before arrival in the laboratory. Participants were provided with a menu of foods and instructed to consume this meal at approximately 20.00 hours the evening before each study day. They were instructed not to consume any other foods or drinks (apart from water) after this meal until the next morning. A standardised breakfast was provided to participants to consume at home, at approximately 08.00 hours on the morning of each study visit. Following this, participants were asked to refrain from eating or drinking (apart from water) until they arrived at the laboratory at approximately 10.45 hours. On arrival at the laboratory, the investigator confirmed that participants were compliant with the pre-study standardisation procedures before participants completed baseline ratings of appetite sensations using computerised visual analogue scales. Participants were then provided with a liquid preload (used to represent a mid-morning snack) which they were asked to consume within 15 min. After the preload had been consumed, participants rated the taste properties using visual analogue scales. Further appetite ratings were collected immediately and 30, 60 and 90 min later. After 90 min, participants were provided a homogeneous pasta-based test meal and instructed to consume as much as they wished until they felt comfortably full. Following the voluntary termination of the test meal, participants remained resting in the laboratory for a further 60 min. Participants were provided with a food diary and were then free to leave the laboratory. They recorded all foods and drinks consumed during the remainder of the day.
Procedures
Screening visit
Before commencing the trials, all volunteers attended a screening visit to gain informed consent and to establish that they met the inclusion criteria for the study as described previously(Reference Astbury, Stevenson and Morris13, Reference Astbury, Taylor and Macdonald14). Eligible volunteers were provided with a food and activity diary, and asked to record food intake and physical activities for 3 d (2 weekdays, 1 weekend day) so that habitual energy intake and total energy expenditure could be estimated as described previously(Reference Astbury, Stevenson and Morris13, Reference Astbury, Taylor and Macdonald14).
Test foods
Evening meal
Participants were supplied with a list of food items to consume as their evening meal, at home, at approximately 20.00 hours the day before each trial. This meal comprised foods that participants reported to consume in the screening food diary and was designed to provide 30 % of participants' total energy expenditure and to consist of 48, 35 and 13 % energy from carbohydrate, fat and protein, respectively; these macronutrient values were based on average UK intakes from the 2003 National Diet and Nutrition Survey(Reference Henderson, Gregor and Irving15).
Breakfast
A standardised breakfast of Rice Krispies (Kelloggs) and semi-skimmed milk (1·7 % fat) was supplied to the participants, to consume, at home, at approximately 08.00 hours on the morning of the trial. This meal was equivalent to 10 % of participants' total energy expenditure. Each 30 g of cereal was matched with 125 ml of semi-skimmed milk.
Preloads
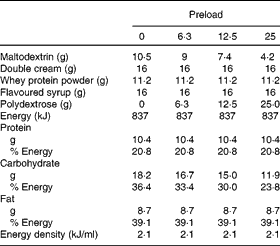
One of four chocolate-flavoured liquid preloads was provided to the participants in a random order determined by a computer-generated randomisation plan. Preloads were prepared using maltodextrin (Cerestar), whey protein powder (Davisco), double cream, polydextrose (Litesse Ultra®; Danisco) and were all flavoured with the same amount of chocolate milkshake syrup (Crusha; The Silver Spoon Company). All preloads were 837 kJ and were made up to 400 ml using cold water (Table 1). The amount of polydextrose added to each preload was offset by reducing the amount of maltodextrin in the preload (energy conversion for polydextrose = 4 kJ/g(Reference Auerbach, Craig and Howlett2)) while keeping all other ingredients constant. All preloads were served in opaque, covered containers which allowed the participants to consume the contents through a straw to minimise any olfactory or texture differences that may influence appetite. Litesse Ultra® is a commercially available polydextrose that is processed so that it contains no reducing sugars, making it suitable for application in products where a heat-related colour change would be considered undesirable.
Table 1 Composition of the preloads
Lunchtime test meal
The lunchtime test meal was a pasta-based meal providing 657 kJ/100 g with 13, 38 and 49 % energy provided by protein, fat and carbohydrate, respectively. The homogeneous nature of this meal meant that energy and macronutrient intake could be easily determined by the weight of food consumed (see Astbury et al. (Reference Astbury, Stevenson and Morris13, Reference Astbury, Taylor and Macdonald14) for details). Participants were provided with an initial bowl, containing approximately 500 g of the test meal. They were instructed to eat as much of the food as they wished, and that they should stop eating only when they felt comfortably full. After the participants had consumed approximately 300 g, the researcher was prompted by the Sussex Ingestion Pattern Monitor (University of Sussex) to add a new portion of food (approximately 250 g) into the bowl. This process was repeated, as many times as necessary, until the participants indicated that they wished to terminate the meal by using the mouse to click on a button marked ‘Finished’ which was visible throughout on the computer screen in front of them. This process has previously been shown to be a sensitive method to detect differences in ad libitum energy intake(Reference Astbury, Stevenson and Morris13, Reference Bertenshaw, Lluch and Yeomans16, Reference Lara, Taylor and Macdonald17) and allows participants to terminate the test meal as a result of internal satiation cues, opposed to cues relating to learnt behaviours such as the presence of an empty bowl.
Subjective appetite sensations
Subjective ratings were presented to participants using computerised visual analogue scales (Sussex Ingestion Pattern Monitor, University of Sussex). The questions appeared on a monitor for participants to score at each visual analogue scale collection point. The questions appeared on the monitor, with a horizontal line displayed below each question. The questions were in the form ‘How (rating) do you feel?’ and the ratings were ‘much of a desire to eat’, ‘full’, ‘hungry’, ‘nauseous’ and ‘thirsty’. The taste ratings were in the same format where the question appeared ‘How (rating) is the food?’, where the rating was ‘creamy’, ‘pleasant’, ‘strong’, ‘sweet’, ‘fruity’ and ‘salty’. The terms ‘Extremely’ or ‘Not at all’ were anchored at either end of the line with the polarity randomised throughout the experiment. A vertical bar appeared in the centre of the line and participants were instructed to move the bar, using the mouse or cursor keys, to the position which best described their rating for the current question. Confirmation of the response was made by clicking a button placed in the corner of the screen labelled ‘Done’. All ratings were automatically scored on a scale from 0 (Not at all) to 500 (Extremely).
Gastrointestinal symptoms questionnaire
Participants were provided with a short questionnaire to take away and complete. The questionnaire asked participants, ‘Did you experience any abnormal side effects, including any abnormal gastrointestinal symptoms or discomfort (e.g. bloating, nausea, loose bowel movement or flatulence) for a period of 24 h after you left the laboratory?’.
Statistical analysis
Statistical analysis was conducted using SPSS for Windows (version 14, SPSS, Inc.). Data are presented as means with their standard errors, unless otherwise stated.
Two-way repeated-measures ANOVA with polydextrose dose (with four levels) as a within-participant factor and sex as a between-participant factor was conducted on ad libitum energy intake at the lunchtime test meal. One-way repeated-measures ANOVA with polydextrose dose (with four levels) as a within-subject factor was conducted on the ad libitum intakes of male and female participants separately. Subjective taste properties of the preload were analysed using a one-way repeated-measures ANOVA. Changes from baseline subjective appetite responses to the preload (from baseline until lunch was served) were analysed using two-way repeated-measures ANOVA with polydextrose (four levels) and time as within-subject factors. Where significant main effects were obtained, paired t tests with Holm–Bonferroni corrections for multiple comparisons were used to determine the location of the difference. Differences were considered significant at P< 0·05.
Results
A total of twenty-four participants were enrolled onto the study (n 12 men, n 12 women) and were randomly assigned a preload sequence using a computer-generated random number. Of these, one female participant withdrew from the study before undergoing any of the main experimental visits. A further two female participants withdrew during the study before they had completed the study. Complete outcome data for twenty-one participants (n 12 men, n 9 women) were analysed for outcome measures (Table 2 ).
Table 2 Participant characteristics (Mean values and standard deviations)
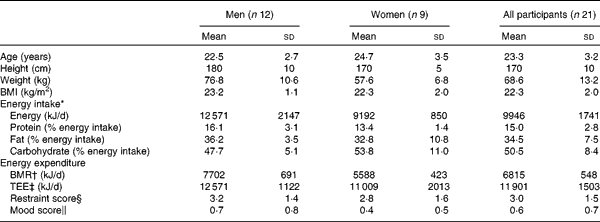
TEE, total energy expenditure.
* Energy intake measured using the 3-d food diary completed during screening.
† BMR calculated using the Schofield equation(Reference Schofield33).
‡ TEE calculated by multiplying BMR with physical activity level determined using the 3-d activity diary.
§ Restraint score as determined by the Three-Factor Eating Questionnaire(Reference Stunkard and Messick7).
∥ Mood score assessed using the Beck Depression Inventory(Reference Beck, Ward and Mendelson8).
Taste ratings of preload
There was a significant main effect of polydextrose for ratings of ‘creamy’ (P< 0·05; Fig. 1). ‘Creamy’ ratings demonstrated a significant within-subject linear contrast (P< 0·01): as the amount of polydextrose in the preload increased, so did the ‘creamy’ rating. The control preload (0 g polydextrose) was rated significantly less ‘creamy’ than the preload containing 25 g polydextrose (P< 0·01). There was a significant main effect of polydextrose for ratings of ‘pleasantness’ (P< 0·05). ‘Pleasantness’ ratings demonstrated a significant within-subject linear contrast (P< 0·01): as the amount of polydextrose in the preload increased, participants' rating of ‘pleasant’ increased; however, despite the main effect of polydextrose for ‘pleasant’ ratings, post hoc tests did not reveal any differences between the preloads. Ratings of ‘sweetness’ tended to increase as the amount of polydextrose in the preload increased (P= 0·07); however, there were no significant differences in ‘sweetness’ ratings between the preloads. There were no differences in the ratings of ‘fruity’, ‘salty’ and ‘strong’ between the preloads.

Fig. 1 Hedonic evaluation ((a) ‘creamy’, (b) ‘pleasant’, (c) ‘salty’, (d) ‘strong’, (e) ‘sweet’ and (f) ‘fruity’) of fixed energy liquid preloads containing 25, 12·5, 6·3 g polydextrose or an energy-matched control which contained no polydextrose (0 g). Values are means (n 21; twelve men, nine women), with their standard errors represented by vertical bars. There was a significant effect of polydextrose for ‘creamy’ and ‘pleasant’ ratings (P< 0·05; repeated-measures ANOVA). ** Mean value was significantly different from that of the control preload (P< 0·01).
Subjective appetite responses
There were no differences between the preloads at baseline for any of the subjective appetite ratings collected. Change from baseline ratings of fullness, hunger, thirst, desire to eat and nausea all displayed a significant main effect of time (P< 0·01) in response to the preload (Fig. 2). Ratings of fullness and nausea increased, and ratings of hunger, thirst and desire to eat decreased following consumption of the preload, before gradually returning to baseline values. However, the post hoc analyses revealed that there were no differences in subjective appetite responses between the preload conditions following corrections for multiple comparisons.

Fig. 2 Change from baseline (BL) subjective ratings of (a) fullness, (b) hunger, (c) desire to eat, (d) thirst and (e) nausea in response to a fixed energy liquid preload containing 25 g (![]() ), 12·5 g (
), 12·5 g (![]() ), 6·3 g (
), 6·3 g (![]() ) polydextrose, and a control preload containing no polydextrose (0 g,
) polydextrose, and a control preload containing no polydextrose (0 g, ![]() ). Values are means (n 21; twelve men, nine women), with their standard errors represented by vertical bars. There was a significant main effect of time for ratings of fullness (P< 0·05), hunger (P< 0·05), thirst (P< 0·05) and desire to eat (P< 0·05) in response to the preloads. Post hoc tests revealed that there were no significant differences at any of the time points for any of the ratings between the preload conditions.
). Values are means (n 21; twelve men, nine women), with their standard errors represented by vertical bars. There was a significant main effect of time for ratings of fullness (P< 0·05), hunger (P< 0·05), thirst (P< 0·05) and desire to eat (P< 0·05) in response to the preloads. Post hoc tests revealed that there were no significant differences at any of the time points for any of the ratings between the preload conditions.
Energy intake at the test meal
There was a significant effect of sex (P< 0·05) and polydextrose (P< 0·05) on energy intake at the ad libitum test meal (Fig. 3). Over all the four conditions, men consumed more energy at the test meal than women (mean difference 2428 (sem 276) kJ). Both men and women demonstrated a significant main effect of polydextrose (P< 0·05), and there was a significant within-subject linear contrast (P< 0·05) in both sexes. Increasing the amount of polydextrose in the preload was accompanied by a stepwise reduction in energy intake at the test meal. Mean energy intake (combined men and women) following the control preload (5756 (sem 423) kJ) was significantly higher than following the preloads containing 6·3 g (5048 (sem 384) kJ), 12·5 g (4722 (sem 384) kJ) or 25 g (4362 (sem 316) kJ) polydextrose (P< 0·01), and intake following the 6·3 g polydextrose preload was significantly greater than following the 25 g polydextrose preload (P< 0·01) (Fig. 3).

Fig. 3 Energy intake at the ad libitum lunchtime test meal following fixed energy liquid preloads containing different amounts of polydextrose. Values are means (n 21; twelve men (■), nine women (![]() )), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA) and polydextrose (P< 0·05; repeated-measures ANOVA). * Mean value was significantly different from that of the control preload (P< 0·05). † Mean value was significantly different from that of the 6·3 g polydextrose preload (P< 0·05). ‡ Mean value was significantly different from that of the 12·5 g polydextrose preload (P< 0·05).
)), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA) and polydextrose (P< 0·05; repeated-measures ANOVA). * Mean value was significantly different from that of the control preload (P< 0·05). † Mean value was significantly different from that of the 6·3 g polydextrose preload (P< 0·05). ‡ Mean value was significantly different from that of the 12·5 g polydextrose preload (P< 0·05).
Test-day food diary analysis
Self-reported intake during the remainder of the experimental day was significantly greater in men than in women (mean difference 1256 kJ, P< 0·01). However, there was no significant difference for the self-reported energy intake during the remainder of the day between the preload conditions (combined intake of men and women; 4391 (sem 507), 4600 (sem 494), 4203 (sem 347) and 4500 (sem 347) kJ in the control preload and the preloads containing 6·3, 12·5 and 25 g polydextrose, respectively) (Fig. 4).

Fig. 4 Self-reported energy intake for the remainder of the day, after participants had left the laboratory following fixed energy liquid preloads containing different amounts of polydextrose. Values are means (n 21; twelve men (■), nine women (![]() )), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA), although there was no significant main effect of polydextrose.
)), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA), although there was no significant main effect of polydextrose.
Total energy intake
Total daily energy intake was calculated as the sum of all components of energy intake (breakfast+preload+test meal+food diary). There was a significant main effect of sex (P< 0·05) and polydextrose (P< 0·05) on total daily energy intake (Fig. 5). The total daily energy intake of men was significantly greater than that of women (mean difference 3655 kJ, P< 0·01). Both men and women displayed a significant main effect of polydextrose (P< 0·05), and a significant within-subject linear contrast (P< 0·05). Increasing the amount of polydextrose in the preload was accompanied by a stepwise reduction in total daily energy intake. However, total daily energy intake (combined men and women) was only significantly higher in the control preload condition (12 051 (sem 805) kJ) compared with the 12·5 and 25 g polydextrose preload conditions (10 854 (sem 589) and 10 656 (sem 506) kJ, respectively). There was no significant difference in total energy intake following the control preload condition compared with the 6·3 g polydextrose preload condition (11 555 (sem 705) kJ; Fig. 4).

Fig. 5 Total daily energy intake on the days 837 kJ liquid preloads containing different amounts of polydextrose were consumed. Values are means (n 21; twelve men (■), nine women (![]() )), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA) and polydextrose (P< 0·05; repeated-measures ANOVA). * Mean value was significantly different from that of the control preload (P< 0·05).
)), with their standard errors represented by vertical bars. There was a significant main effect of sex (P< 0·05; repeated-measures ANOVA) and polydextrose (P< 0·05; repeated-measures ANOVA). * Mean value was significantly different from that of the control preload (P< 0·05).
Gastrointestinal distress
There were no reports of gastrointestinal distress from any of the participants during the study.
Discussion
The findings of the present study show that including polydextrose in a mixed macronutrient, liquid preload served as a between-meal, mid-morning snack reduces energy intake at a subsequent lunchtime test meal in a dose-dependent manner. Furthermore, consuming preloads containing 12·5 and 25 g polydextrose was associated with a lower total daily energy intake than the energy-matched preload containing no polydextrose.
Sustaining a reduction in overall daily energy intake, even by a relatively small amount, over a prolonged period of time may help individuals achieve a reduction in body weight, or a prevention of body-weight gain or regain(Reference Astrup18, Reference Webster and Garrow19). The present findings indicate that polydextrose may be a useful ingredient to include in foods that aim to limit total daily energy intake.
The present findings are consistent with those of a previous study(Reference King, Craig and Pepper4), which demonstrated that the addition of 25 g polydextrose to a preload was able to reduce energy intake at a test meal served 90 min later compared with a control. Furthermore, in the present study, we demonstrate the dose-dependent effect of polydextrose on subsequent voluntary food intake, and we have shown that total daily energy intake also shows a dose-dependent response too; however, the threshold dose at which there is a significant reduction in total energy intake relative to the control preload is 12·5 g, with only marginally greater effects when the 25 g preload is consumed.
It is important to note that both these studies were conducted in a laboratory setting, where participants' behaviours could be closely monitored and controlled. Furthermore, the remainder-of-the-day component of intake was based on self-reported semi-quantitative measures, which are subject to the well-documented phenomena of misreporting and under-reporting of energy intake(Reference Black, Prentice and Goldberg20–Reference Prentice, Black and Coward22). We believe that the failure to detect differences in self-reported energy intake for the remainder of the day between the preload conditions demonstrates that participants are not compensating for the differences in energy intake at the lunchtime test meal by eating more during the remainder of the day. However, we cannot rule out the possibility that the lack of differences between the conditions may have occurred as a result of observational or reporting effects associated with misreporting of dietary intake. Interestingly, the effects on energy intake were not coupled with concomitant changes in subjective satiety, which suggests that the study may be underpowered to detect differences in subjective appetite ratings.
Previous reports have suggested that gastrointestinal distress might limit the application of polydextrose in food products(6). However, consistent with other studies(Reference Jie, Bang-Yao and Ming-Jie1), study participants did not report any symptoms relating to gastrointestinal distress (bloating, nausea, loose bowel movement or flatulence) during the 24 h period following each test day. These findings indicate that polydextrose is well tolerated in amounts up to 25 g.
In the present study, we used 4 kJ/g as the energy conversion factor for polydextrose, which is based on several studies that specifically investigated the net metabolisable energy provided by polydextrose(Reference Auerbach, Craig and Howlett2, Reference Figdor and Rennhard23). However, for labelling purposes, polydextrose will fall under the definition of a dietary fibre, and it is suggested that an energy conversion factor of 8 kJ/g should be used for all fibres(24).
Although by recalculating the energy content of the preloads using these revised values there are small differences in the energy contents of the preloads (0 g, 837 kJ; 6·3 g, 864 kJ; 12·5 g, 890 kJ; 25 g, 942 kJ), the differences in energy intake at the lunchtime test meal cannot be explained simply by the difference in the energy content of the preloads using the alternative energy value. It should also be noted that although we did not measure the weight of the preloads in the present study, due to the exchange of polydextrose (4 kJ/g) with maltodextrin (17 kJ/g), it is likely that the preload ingredients increased in weight as the polydextrose dose increased, and we cannot rule out the possibility that these small differences may have influenced subsequent energy intakes.
Taste may play an important role in controlling appetite, and the hedonic properties of foods may influence satiety via a learned mechanism(Reference Yeomans, Bertenshaw, Harris and Mattes25). Despite no significant differences in subjective pleasantness ratings between the preloads, the significant within-subject linear contrast demonstrates that as the amount of polydextrose in the preload increased, this was associated with greater subjective pleasantness ratings.
We believe that this may be linked to the higher perceived sweetness of preloads containing increasing amounts of polydextrose, since humans have an innate preference for sweet foods(Reference Lawless26).
Humans sense ‘sweetness’ when sweet tastants (including sugars and sweeteners) bind to a broadly tuned sweet taste receptor, which is a heterodimer of two G-protein coupled receptors (T1R2+T1R3)(Reference Nelson, Hoon and Chandrashekar27, Reference Vigues, Dotson and Munger28). Recent findings have suggested that the T1R2+T1R3 sweet taste receptor is not only present on the lingual taste buds, but is also expressed on the enteroendocrine cells located throughout the small intestine(Reference Dyer, Salmon and Zibrik29, Reference Kokrashvili, Mosinger and Margolskee30). When nutrients are present in the intestinal lumen, these cells that secrete a variety of peptides associated with gastrointestinal function, the regulation of appetite and the control of energy intake such as glucagon-like peptide 1, polypeptide YY and cholecystokinin(Reference Chaudhri, Small and Bloom31).
Similarly, specific taste receptors for SCFA taste (GPR41 and GPR43) have been identified on the enteroendocrine cells of the colonic mucosa(Reference Tazoe, Otomo and Karaki32), thus, giving rise to the possibility that this is the mechanism by which the colon can sense the presence of SCFA, either as a result of their presence in certain food products (i.e. vinegar, sourdough bread), or due to their production during the fermentation of non-digestible carbohydrates such as polydextrose. However, due to the short duration of the present study, it is unlikely that any ingested polydextrose would have had sufficient time to reach the colon, where it would be available for fermentation by the colonic microflora. In order to assess the possible effects of the products of fermentation on appetite and energy intake, future studies should observe participants for several hours after consumption, and investigate the effects of long-term daily consumption of polydextrose as the gut microflora and SCFA production may be modified in response to longer-term exposure.
In order to determine whether the reduction in daily energy intake observed in the present study may have implications for the management of body weight, future studies should investigate whether the effects observed in these acute laboratory-based studies can be replicated under free-living conditions, and whether these findings can be sustained when polydextrose is habitually consumed as part of the diet.
In summary, polydextrose can influence short-term energy intake in a dose-dependent manner, and thus may be a beneficial ingredient to include in foods designed to limit subsequent energy intake. Further investigations are required to elucidate possible mechanisms associated with the effects of polydextrose on energy intake, as well as the effects of habitual consumption of foods containing polydextrose on changes in body weight and body composition.
Acknowledgements
The present study was supported by a BBSRC CASE PhD Studentship for N. M. A., with Mars UK as industrial partner (BBSSD200513300). M. A. T. and I. A. M. gained funding for the project from Mars UK. I. A. M. is a consultant for the Mars Europe Nutrition Advisory Board. The authors' contributions are as follows: N. M. A. designed the experimental protocol, conducted the research, performed the statistical analysis, interpreted the results, wrote the manuscript and had primary responsibility for the final content. M. A. T. and I. A. M. refined the experimental design, had input into the interpretation of the results and helped produce a final draft of the manuscript. All authors read and approved the final manuscript.









