Normal wound healing is a complex process involving a series of sequential and overlapping phases, including inflammation, granulation tissue formation and remodelling, which result in scar formation. This process requires interaction among a variety of cells, multiple cytokines and growth factors and extracellular matrix molecules. For normal wound healing, the temporal sequence of these events should be kept up, as it is necessary that the early events of coagulation and inflammation occur in a short time lapse and then cease to allow the subsequent reparative processes to occur (Singer & Clark, Reference Singer and Clark1999). Adipose tissue produces and secretes inflammatory cytokines (Mohamed-Ali et al. Reference Mohamed-Ali, Pinkney and Coppack1998; Sethi & Hotamisligil, Reference Sethi and Hotamisligil1999) that, when in excess, can perpetuate the inflammation and delay the wound repair.
Over-consumption of a high-energy diet may contribute to positive energy balance and lead to overweight and obesity development (Swinburn & Egger, Reference Swinburn and Egger2002). Administration of a high-fat diet is an animal model that reproduces many features of human obesity (Lauterio et al. Reference Lauterio, Bond and Ulman1994). Rats fed a high-fat diet exhibit a bimodal pattern in body weight gain similar to that observed in human subjects (Lauterio et al. Reference Lauterio, Bond and Ulman1994) and the increase in body weight reflects an increase in adipose mass (Dobrian et al. Reference Dobrian, Davies, Prewitt and Lauterio2000). Therefore, the main feature of obesity is the increase in adipose mass, and pathophysiology is the increased secretion of factors associated with enlarged fat cells (Bray, Reference Bray2003).
White adipose tissue was traditionally considered as an energy storage cell. In recent years, we have become more aware that fat cells are metabolically active, producing adipokines, soluble hormone-like substances (including adiponectin and leptin) that have a wide range of metabolic effects (Rajala & Scherer, Reference Rajala and Scherer2003). Targets of these mediators seem to include immune and inflammatory systems (Fantuzzi & Faggioni, Reference Fantuzzi and Faggioni2000; Rajala & Scherer, Reference Rajala and Scherer2003). White adipose tissue increase is associated with an increase in pro-inflammatory mediators such as TNF-α and various IL, including IL-1β, IL-6 and IL-8 (Coppack, Reference Coppack2001; Grimble, Reference Grimble2002; Black, Reference Black2003; Dandona et al. Reference Dandona, Aljada and Bandyopadhyay2004).
The effects of overweight on morbidity and mortality have been known for more than 2000 years (Bray, Reference Bray2004). Overweight individuals often undergo surgery, experience trauma and develop chronic wounds (Wilson & Clark, Reference Wilson and Clark2004). Providing wound care for an overweight patient requires an understanding of the intrinsic changes in body systems induced by an increase of adipose tissue mass and in particular the modifications induced in the wound-healing process. The aim of the present study was to investigate the effects of overweight induced by a high-fat diet on rat excisional cutaneous wound healing, evaluating wound contraction and re-epithelialization, inflammatory infiltrate, granulation tissue development and angiogenesis.
Material and methods
Animals and diets
Male Wistar rats (120–150 g) were randomly separated in control and diet groups. The control group (n 5) was fed with commercial pellets (Nuvital; Nuvilab®, Colombo, PR, Brazil) and the diet group (n 15) was fed with a high-fat diet (containing 30 % fat) (Aoyama et al. Reference Aoyama, Fukui, Takamatsu, Hashimoto and Yamamoto2000). The diet group contained more rats to accommodate the variability displayed by this group in weight gain. Animals were housed individually with free access to food and water throughout the experimental period. Table 1 shows the composition of the diets. All animal procedures were approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro.
Table 1 Experimental high-fat diet composition*

* For details of diets and procedures, see p. 1070.
† Standard rat chow (Nuvital, Nuvilab®, Colombo, PR, Brazil).
‡ Nestlé®, São Paulo, SP, Brazil.
§ Primor®, Florianópolis, SC, Brazil. Shortening is a semi-solid fat composed of hydrogenated soyabean oil, containing 30 % trans fatty acids.
Body weight was measured weekly from the beginning of experiment and naso–anal length was measured monthly. The systolic blood pressure was verified using the non-invasive method of the tail-cuff plethysmography in conscious rats (Letica LE 5100; Panlab, Barcelona, Spain) and the glucose was assessed with Accu-Chek (Roche Diagnostics, Mannheim, Germany) at the beginning and at 15 and 18 weeks of the experiment. Obesity Index of Lee was calculated monthly by dividing the cubic root of the body weight (g) by the naso–anal length (mm) × 104 (Cattaneo et al. Reference Cattaneo, De Gennaro Colonna, Zoli, Muller and Cocchi1997). All data are presented as means and standard deviations.
Wounding procedure
Since the pattern in body weight gain is bimodal in rats fed with a high-fat diet (Lauterio et al. Reference Lauterio, Bond and Ulman1994), at the 15th week of the diet the ten animals with greatest weight gain in the diet group were selected to continue the experiment. After 15 weeks of diet, on day 0, the animals were anaesthetized with ketamine (5 mg/kg, intraperitoneally) and xylazine (2 mg/kg, intraperitoneally), the dorsal surface was shaved and a full-thickness excisional wound (4 cm2) was made on the back of each rat by removing the skin (epidermis and dermis) and exposing paniculus carnosus. The wound was neither sutured nor covered and healed by second intension, as already described (Souza et al. Reference Souza, Cardoso, Amadeu, Desmouliere and Costa2005; Amadeu & Costa, Reference Amadeu and Costa2006).
Wound area
To evaluate the wound contraction and re-epithelialization, its margins were traced on a transparent plastic sheet placed over it. Wound area was measured soon after injury and 7, 14 and 21 d later; re-epithelialized wound area was measured 14 and 21 d after wounding. Lesion tracings were digitalized and the wound area was evaluated using Image-Pro® Plus 5.0.2 image-processing program (Media Cybernetics, Silver Spring, MD, USA). Wound contraction was estimated by total lesion area and wound re-epithelialization was estimated by the difference between the total lesion area and the wound area still uncovered with epidermis, as shown in Fig. 1. Wound contraction data are expressed as a percentage of the initial wound area and re-epithelialization data are expressed as a percentage of re-epithelialized wound area at each measurement (means and standard deviations).
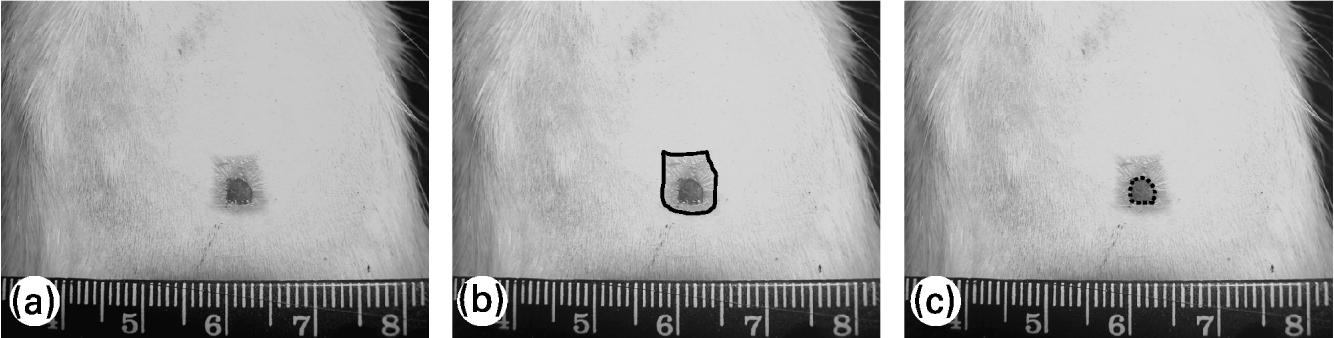
Fig. 1 Evaluation of wound contraction and re-epithelialization. (a) Original photo of wound area; (b) — shows total wound area; (c) ––- shows non re-epithelialized wound area (still uncovered by a neo-epidermis). The difference between the two areas represents the re-epithelialized wound area.
Wound tissue processing and staining
Rats were killed 21 d after injury (control group n 5 and diet group n 10) in a CO2 chamber. The lesions and the adjacent skin were removed, formol-fixed and paraffin-embedded. Retroperitoneal fat was removed and weighed.
Sections (5 μm) were stained with haematoxylin-eosin to analyse general aspects, inflammatory infiltrate and re-epithelialization; with Sirius Red for collagen fibre analysis; with 0·2 % toluidine blue for mast cells evaluation.
The amount of polymorphonuclears was evaluated in haematoxylin-eosin-stained tissue sections. Ten random fields (0·02 mm2) were analysed using a × 100 objective (Olympus CBA; Olympus Optical Co. Ltd, Tokyo, Japan) and the average cell count per field was calculated for each animal.
The number of mast cells was evaluated in toluidine blue-stained tissue sections. Ten random fields (0·12 mm2) were analysed using a × 40 objective (Olympus BH-2; Olympus Optical Co. Ltd) and the average cell count per field was calculated for each animal. The percentage of degranulating mast cells was also evaluated.
All analysis was done twice and blindly without any significant difference among measurements and data are expressed as means and standard deviations.
Immunohistochemistry and quantification
For assessment of myofibroblasts and blood vessels, a mouse monoclonal antibody against α-smooth muscle actin (DAKO, Carpinteria, CA, USA) was used. Briefly, sections (5 μm) were digested with 0·1 % trypsin (Difco Laboratories, Detroit, MI, USA), incubated in 3 % H2O2 in methanol to block endogenous peroxidase activity and incubated with primary antibody. To localize proliferating cells, a mouse monoclonal antibody against the proliferating cell nuclear antigen (PCNA) (DAKO) was used. Briefly, sections were incubated in citrate buffer (pH 6·0) for antigen retrieval, in 3 % H2O2 in methanol for endogenous peroxidase blocking and then with primary antibody. For revelation EnVision system® (DAKO) was used for both primary antibodies. Diaminobenzidine was used as chromogen and the nuclei were stained with Delafield's haematoxylin. Negative controls were performed replacing primary antibody by non-immune serum and no labelling was observed.
The distribution of blood vessels and myofibroblasts (expressing α-smooth muscle actin) in the granulation tissue was evaluated by a stereological method (test system with cycloid arcs). Blood vessels were identified by the presence of smooth muscle cells expressing α-smooth muscle actin in the wall and by the presence of blood cells in the lumen (Amadeu & Costa, Reference Amadeu and Costa2006). Since stereological methods are precise tools for obtaining information about three-dimensional structures based mainly on observations made in two-dimensional sections (Baddeley et al. Reference Baddeley, Gundersen and Cruz-Orive1986; Gundersen et al. Reference Gundersen, Bendtsen and Korbo1988), the quantitative distribution of myofibroblasts and of superficial and deep vessels was evaluated using this method. To distribution of myofibroblasts, ten random fields were analysed in granulation tissue in each specimen using a × 40 objective and to evaluate the distribution of blood vessels, eight random fields were analysed in superficial and deep regions of granulation tissue using a × 20 objective. For assessment, a videomicroscopic system (Axiophot ZEISS microscope, JVC video-camera and Sony Trinitron monitor; Sony, Pencoed, UK) was used. The test system was displayed on the screen of the monitor and calibrated with a micrometer. Volume density (Vv), surface density and length density were estimated in both superficial and deep regions. Vv, surface density and length density results are shown as means and standard deviations.
The number of PCNA-positive cell nuclei was counted in neo-epidermis and granulation tissue. In neo-epidermis, all basal cells were counted and the result is expressed as a percentage of PCNA-positive basal cells. In granulation tissue, ten random fields were counted with a × 40 objective using a two-dimensional test frame area of 3·636 μm2 and data are expressed as PCNA-positive cells/mm2 in the granulation tissue. For counting, a video-microscopic system (Axiophot ZEISS microscope, JVC video-camera, and Sony Trinitron monitor) was used. The data are expressed as means and standard deviations. All analysis was done twice and blindly without significant difference among measurements.
Statistical analysis
One-way ANOVA with Tukey post test was used to analyse body weight. Unpaired t test Welch corrected was used to analyse retroperitoneal fat, glucose, blood pressure, lesion area, inflammatory cells and cellular proliferation. Non-parametric Mann-Whitney test was used to analyse mast cells, myofibroblasts and blood vessel stereology. The software Graph Pad Instat version 3.01 (GraphPad Software Inc., San Diego, CA, USA) was used and values of P < 0·05 were considered statistically significant.
Results
Body weight, fat depot, blood pressure and glucose
Rats fed with a high-fat diet exhibit a bimodal pattern in body weight gain similar to that observed in man. Two-thirds of the rats gained weight rapidly when compared with chow-fed rats, whereas one-third gained body weight at a rate similar to that of the chow-fed animals. This model allows us to dissociate the factors related to a high-fat diet and increased body fat content (Lauterio et al. Reference Lauterio, Bond and Ulman1994). For this reason, the ten animals with the greatest weight gain after 15 weeks of diet were selected to continue the experiment.
The initial mean body weight of the groups did not present a difference between them (139·2 (sd 7·2) g in control and 141·7 (sd 5·3) g in the diet groups). At week 3, the diet group had significantly higher (P < 0·05) body weight than the control group and the body weight remained higher throughout the 18-week dietary period. On the day the lesion was performed (day 0), body weight was 25·7 % higher in the diet group when compared with the control group and 18·6 % 21 d after wounding (P < 0·001 for both) (Fig. 2(a)). In terms of body weight gain, in the diet group it was significantly greater than in the control group (249·1 (sd 21·1) g v. 190·3 (sd 15·7) g; P < 0·001). Retroperitoneal fat mass was higher in the diet group when compared with the control group after 18 weeks of diet (10·3 (sd 1·9) g v. 2 (sd 0·7) g; P < 0·0001) (Fig. 2(b)). The high-fat diet caused a 5-fold increase in the amount of retroperitoneal fat. The Obesity Index of Lee (the equivalent of BMI for human subjects) significantly increased in the diet group 4 weeks after the beginning of the diet and it was still higher at 15 (6·9 %; P < 0·001) and at 18 (5 %; P < 0·05) weeks after the beginning of the diet compared with the control group, reflecting the increased body fat content (Fig. 2(c)). Although the body weight of the diet group was greater, the animals were not obese. To be considered obese, animals should have a mean overweight of 57 % (Cattaneo et al. Reference Cattaneo, De Gennaro Colonna, Zoli, Muller and Cocchi1997), but the rats presented a mean overweight of 18·6 % after 18 weeks of the diet and as such they should be considered overweight (Cattaneo et al. Reference Cattaneo, De Gennaro Colonna, Zoli, Muller and Cocchi1997).
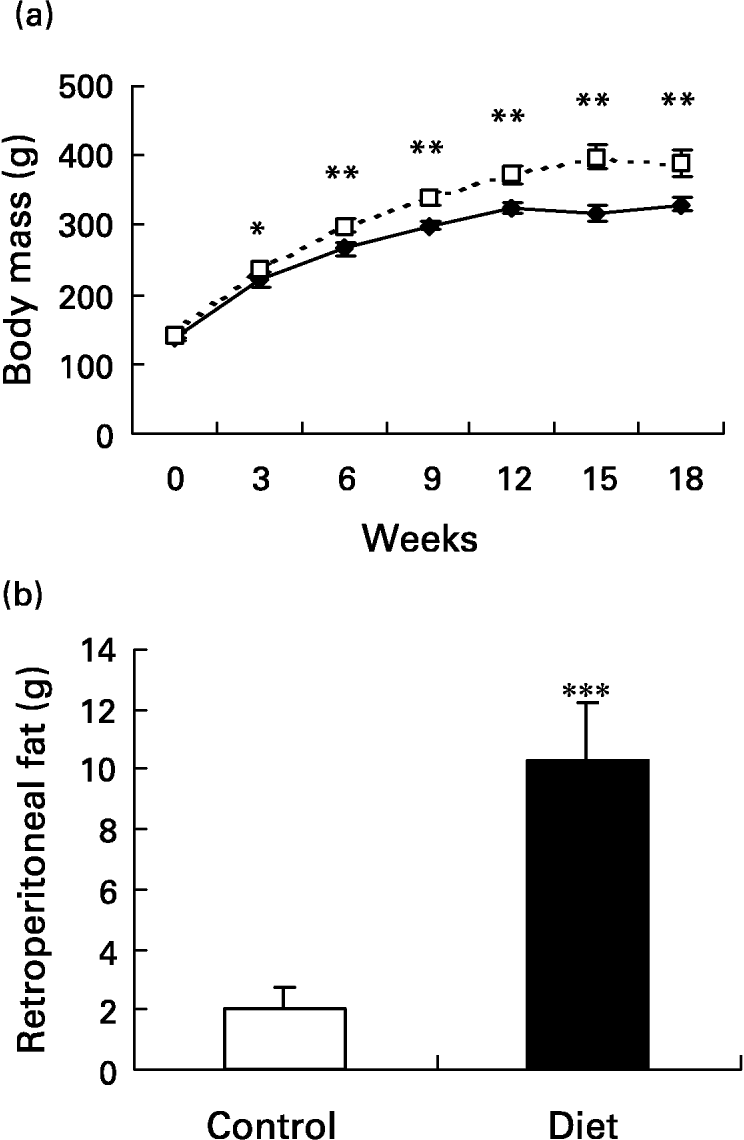
Fig. 2 Evaluation of body and of retroperitoneal fat mass. Mean values and standard deviations for five animals in the control group and ten animals in the diet group. For details of diets and procedures, see p. 1070. (a) Changes in body weight over 18 weeks on diets (–♦–, control; - - -□- - -, diet group). Mean values were significantly higher than those for the control group after 3 weeks of diet: *P < 0·05; **P < 0·001. (one-way ANOVA with Tukey post test). (b) Retroperitoneal fat mass after 18 weeks of diet. The high-fat diet promoted an increase in retroperitoneal fat depot. Mean value was significantly different from that of the control group: ***P < 0·0001. (unpaired t test Welch corrected). (c) Comparison of obesity index. The diet group (- - -□- - -) presented an increased index after 4, 15 and 18 weeks of diet. Mean values were significantly higher than those for the control group: *P < 0·05, **P < 0·01, ***P < 0·001. (–♦–, control group). (one-way ANOVA with Tukey post test).
The baseline systolic blood pressure (106·2 (sd 4·3) mmHg in control and 106·3 (sd 4·5) mmHg in the diet group) and blood concentration of glucose (6·5 (sd 0·2) mmol/l in control and 6·8 (sd 0·3) mmol/l in the diet group) were not different between the two groups throughout the experiment, indicating that the high-fat diet did not induce the development of hypertension nor diabetes.
Wound contraction and re-epithelialization
Rats are loose-skinned animals, with the wound healing occurring primarily by first wound contraction and then by re-epithelialization via keratinocyte migration and proliferation (Davidson, Reference Davidson1998). The control and diet groups presented a progressive reduction in wound area during the experiment. It was found that 14 d after wounding, the wound area in the diet group was 19 % (P < 0·05), higher than in the control group and 32 % (P < 0·01) higher at 21 d after wounding, indicating less contraction (Fig. 3(a)).

Fig. 3 Evaluation of wound contraction and formation of neo-epidermis in control (□) and diet (■) groups. Mean values and standard deviations for five animals in the control group and ten animals in the diet group. For details of diets and procedures, see p. 1070. (a) Animals fed the high-fat diet presented delayed wound contraction. Mean values were significantly different from that of control: *P < 0·05, **P < 0·01. (b) animals fed the high-fat diet presented delayed re-epithelialization. Mean values were significantly different from that of control: *P < 0·05, ***P < 0·0001 (unpaired t test Welch corrected).
Neo-epidermis formation progressively covered the wound area. The re-epithelialization was not complete in both groups 21 d after wounding. The re-epithelialization rate was 40 % (P < 0·0001) smaller in the diet group when compared with the control group and 14 and 11 % (P < 0·05) smaller 21 d after wounding (Fig. 3(b)), thus showing a delayed keratinocyte migration in rats fed a high-fat diet.
Epithelial cell proliferation was smaller in the control group when compared with the diet group 21 d after wounding (Fig. 4(a)). In the control group, PCNA-positive cells were limited to basal layer (Fig. 4(b)) and in the diet group PCNA-positive cells were also found in supra-basal layers (Fig. 4(c)). This result shows that in the diet group the epithelial cells were still in intense proliferation 21 d after wounding, but the small rate of re-epithelialization is probably due to a disturbance in keratinocyte migration.

Fig. 4 (a) Percentage of proliferating cell nuclear antigen (PCNA)-positive basal epithelial cells in wound area of control and diet groups. Values are means and standard deviations. Bar 10 μm. For details of diets and procedures, see p. 1070. Mean value was significantly different from that of control: **P < 0·01 (unpaired t test Welch corrected). Diet group (c) presented a larger number of proliferating cells in epidermal basal layer 21 d after wounding compared with control group (b).
Inflammatory infiltrate and granulation tissue formation
It was found that 21 d after wounding, the control group presented a small amount of inflammatory cells in the wound area and a greater amount of ‘fibroblast-like’ cells (Fig. 5(a)) when compared with the diet group (Fig. 5(b)). The diet group presented a higher amount of inflammatory cells localized mainly in the superficial area of lesion.
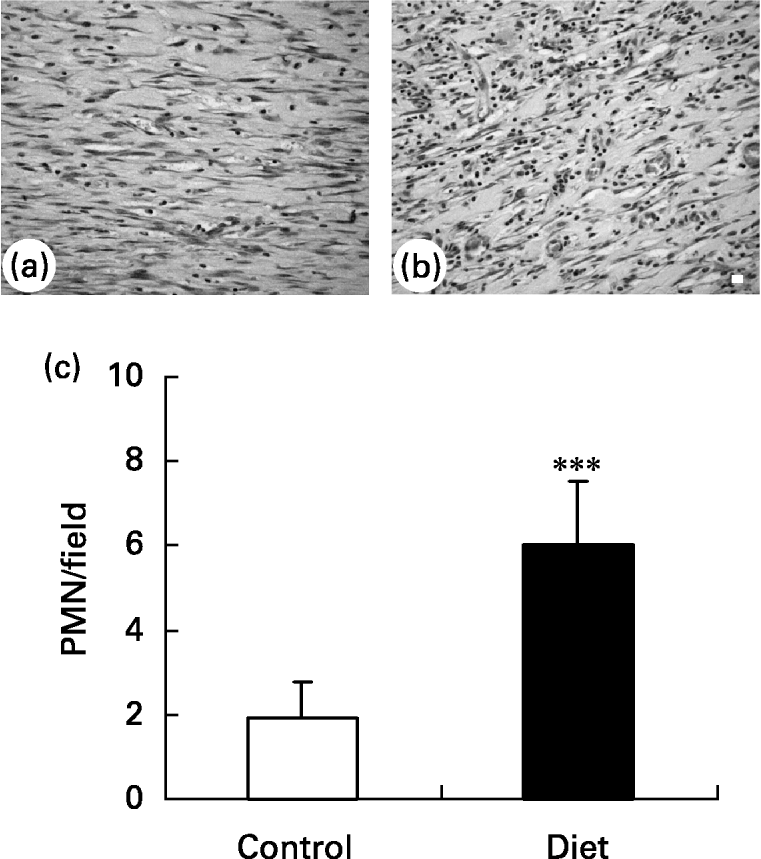
Fig. 5 Granulation tissue in (a) control and (b) diet groups 21 d after wounding. Distribution of inflammatory infiltrate in wound area. (a) Control group presented a high amount of fibroblastic cells in granulation tissue; (b) in diet group an increase in inflammatory infiltrated was observed. Bar 10 μm. (c) Quantification of polymorphonuclear (PMN) leukocytes/field in granulation tissue of control and diet groups. Mean values and standard deviations for five animals in the control group and ten animals in the diet group, ten fields were analysed in each animal. For details of diets and procedures, see p. 1070. Mean value was significantly different from that of the control group: ***P < 0·0001 (unpaired t test Welch corrected).
Quantification of polymorphonuclear leukocytes (Fig. 5(c)) in the wound area showed that 21 d after wounding the amount of polymorphonuclear leukocytes was smaller in the control group than in the diet group. This suggests that wound healing is impaired in the diet group due to prolongation of the inflammatory phase.
In both groups, mast cells were localized mainly in the deep area of granulation tissue. The majority of them were ovoid and localized near to blood vessels. The amount of mast cells was not different 21 d after wounding comparing the control and the diet groups (data not shown). Concerning the percentage of degranulating mast cells, there was also no difference between the control and the diet groups (82 (sd 16) % and 92 (sd 10) %).
The analysis of myofibroblast showed that the Vv of myofibroblast was smaller (P < 0·0001) in the granulation tissue of the control (0·6 (sd 1·9) %) compared with the diet group (4·6 (sd 5·1) %) (Fig. 6(a)). It was found that 21 d after wounding, the Vv of myofibroblast in the control group was reduced and limited in the lesion edges (Fig. 6(b)); the diet group still presented a larger amount of myofibroblasts with homogeneous distribution along the granulation tissue (Fig. 6(c)).

Fig. 6 Stereological analysis of α-smooth muscle actin expressing cells in wound area of control and diet groups. (a) Volume density (Vv) of myofibroblasts in granulation tissue. Mean values and standard deviations for five animals in the control group and ten animals in the diet group. For details of diets and procedures, see p. 1070. Mean value was significantly different from that of the control group: ***P < 0·0001 (Mann-Whitney test). Diet group (c) still presented a larger amount of myofibroblasts 21 d after wounding compared with control group (b). Bar 10 μm.
PCNA-positive connective tissue cells were homogeneously distributed in the wound area of the control and the diet group. Quantification of PCNA-positive connective tissue cells showed that the diet group presented a higher (P < 0·001) number of proliferating cells 21 d after wounding (Fig. 7(a)). It was found that 21 d after wounding, the control group (Fig. 7(b)) presented a small amount of connective tissue proliferating cells compared with the diet group (Fig. 7(c)). This result indicates that lesions of animals fed a high-fat diet presented an intense proliferative response 21 d after wounding, a period when apoptosis occurs during normal wound repair, suggesting a delay in the proliferation of connective tissue cells.

Fig. 7 (a) Number of proliferating cell nuclear antigen (PCNA)-positive connective tissue cells/mm2 in wound area. Mean values and standard deviations for five animals in the control group and ten animals in the diet group, ten fields were analysed in each animal. For details of diets and procedures, see p. 1070. Mean value was significantly different from that of the control group: ***P < 0·0001 (unpaired t test Welch corrected). Control group (b) presented smaller amount of proliferating cells in granulation tissue than diet group (c). Bar 10 μm.
Concerning collagen organization, 21 d after wounding the control group presented thick yellow-red collagen fibres arranged similarly to normal skin in the superficial and deep regions of the granulation tissue; the diet group showed a prevalence of thick red-yellow collagen fibres in the edge of the lesion and a lower amount of collagen fibres in the centre, always parallel to surface. The density of collagen fibres was higher in the control group than in the diet group and this indicates a delay in the synthesis and remodelling of extracellular matrix in the high-fat diet group (data not shown).
Neovascularization of the granulation tissue
The Vv of vessels was calculated to evaluate the volume occupied by vessels in both groups (Fig. 8(a)). In the diet group, the Vv of blood vessels was higher in both superficial and deep regions of the granulation tissue 21 d after wounding, when compared with those of the control group. The volume occupied by vessels in the diet group was 55 % (P < 0·05) higher in the superficial region and 70 % (P < 0·01) higher in the deep region when compared with those of the control group (Fig. 8(a)).

Fig. 8 Stereological analysis of blood vessels 21 d after wounding in control and diet groups. Mean values and standard deviations for five animals in the control group and ten animals in the diet group, sixteen fields were analysed in each animal (eight in superficial region and eight in deep region). For details of animals and procedures, see p. 1070. (a) Volume density (Vv). Mean value was significantly different from that of control in superficial region: *P < 0·05. Mean value was significantly different from that of control in deep region: **P < 0·01. (b) Surface density (Sv). Mean value was significantly different from that of control in superficial region: **P < 0·01. Mean value was significantly different from that of control in deep region: ***P < 0·001. (c) Length density (Lv). Mean values were significantly different from those of the control group in superficial and deep region: ***P < 0·0001 (Mann-Whitney test).
The surface density of vessels was calculated to determine the surface occupied by vessels in both groups (Fig. 8(b)). An increase in the surface density of vessels indicates that they are dilated. In the diet group, the surface occupied by vessels was higher than in the control group 21 d after wounding. The comparison of the control and diet groups showed an increase of 54 % (P < 0·01) of the surface occupied by vessels in the superficial region and 84 % (P < 0·001) in the deep region of the diet group (Fig. 8(b)).
The length density was used to indicate the winding of blood vessels (Fig. 8(c)). The length density of blood vessels increased in both the superficial and deep regions of the granulation tissue of the diet group when compared with the control group. The winding of blood vessels was 217 % (P < 0·0001) higher in the superficial region and 277 % (P < 0·0001) higher in the deep region 21 d after wounding in the diet group, when compared with the control group (Fig. 8(c)).
The present result shows that 21 d after wounding there is still a great amount of blood vessels in the granulation tissue of the diet group, indicating a delay in healing maturation.
Discussion
In the present study, overweight was induced by a high-fat diet in Wistar rats and its effects were investigated on wound contraction, re-epithelialization, inflammatory infiltrate, granulation tissue formation and neovascularization. Our study showed that overweight impairs excisional cutaneous wound healing by delaying wound contraction, re-epithelialization and granulation tissue formation by increasing the duration of inflammatory phase and by disturbing neovascularization and vessel morphology.
Overweight and obesity are problems of epidemic proportions (World Health Organization, 2002) and are associated with the increased incidence of many chronic diseases (Burton et al. Reference Burton, Foster, Hirsch and Van Itallie1985) and chronic wounds (Wilson & Clark, Reference Wilson and Clark2004). Moreover, overweight is also associated with a variety of dermatoses (Khimich, Reference Khimich1997; Dowling et al. Reference Dowling, Steele and Baur2001; Hills et al. Reference Hills, Hennig, McDonald and Bar-Or2001; Kiess et al. Reference Kiess, Galler, Reich, Muller, Kapellen, Deutscher, Raile and Kratzsch2001). The increased body mass reflects an increase in the adipose mass as evidenced by the increase in the obesity index and in the retroperitoneal fat mass. The present data agree with a previous study (Dobrian et al. Reference Dobrian, Davies, Prewitt and Lauterio2000) that showed that, similar to human subjects, not all rats fed with a high-fat diet became overweight. Rats submitted to a high-fat diet present a bimodal distribution in body mass, that is, not all animals became overweight. The animals in the present study were not obese, neither did they present hypertension, contradicting data presented in previous studies (Aoyama et al. Reference Aoyama, Fukui, Takamatsu, Hashimoto and Yamamoto2000; Barnes et al. Reference Barnes, Lapanowski, Conley, Rafols, Jen and Dunbar2003). This difference can be related to the start time of the high-fat diet (Hirsch, Reference Hirsch1972); in the present study, the diet started 40 d after birth and in the study of Aoyama et al. (Reference Aoyama, Fukui, Takamatsu, Hashimoto and Yamamoto2000) the diet started soon after weaning (21 d after birth). Obesity has been positively correlated with changes in the cardiovascular system leading to the development of hypertension (Zhang et al. Reference Zhang, Thakur, Morse and Reisin2002). However, the mechanism by which obesity and hypertension are linked is still a question of great discussion. In the present work, our animals did not develop hypertension probably because they were not obese and a high-fat diet also did not induce diabetes. This result agrees with the literature in which high-fat feeding led to insulin resistance but not to diabetes in Wistar rats (Chalkley et al. Reference Chalkley, Hettiarachchi, Chisholm and Kraegen2002).
Re-epithelialization begins just after injury. At the beginning, keratinocytes migrate rather than proliferate to resurface the epidermal defect; when neo-epidermis is complete, keratinocytes proliferate to reconstruct the epidermis (Metze & Luger, Reference Metze and Luger2001). Overweight, induced by a high-fat diet, impaired re-epithelialization, mainly inhibiting keratinocyte migration, since the diet group presented a small re-epithelialized wound area, with an increase in the amount of proliferating keratinocytes in basal and supra-basal layers. Leptin is an adipocyte-derived polypeptide hormone (Ahima & Flier, Reference Ahima and Flier2000) and its secretion is directly proportional to the adipose tissue mass (Fain et al. Reference Fain, Madan, Hiler, Cheema and Bahouth2004). Recently, it was shown that leptin is a strong mediator of keratinocyte proliferation during wound healing in vivo (Frank et al. Reference Frank, Stallmeyer, Kampfer, Kolb and Pfeilschifter2000) and this allows us to suggest that adipose tissue enlargement and the consequent increase in leptin concentration may lead to an increase in keratinocyte proliferation.
In rodents, wound contraction is the main mechanism of wound closure (Davidson, Reference Davidson1998). The present data showed a reduction in wound contraction 14 and 21 d after wounding in animals fed with a high-fat diet. Adipose tissue excess has been viewed as an inductor of the inflammatory process (Dandona et al. Reference Dandona, Weinstock, Thusu, Abdel-Rahman, Aljada and Wadden1998; Grimble, Reference Grimble2002; Black, Reference Black2003) and recent attention has been focused on cytokines such as TNF-α and IL6 (Chan et al. Reference Chan, Cheung, Stehouwer, Emeis, Tong, Ko and Yudkin2002). Although inflammation is important to induce myofibroblastic differentiation, the high degree of inflammation disturbs myofibroblastic differentiation. Therefore, it may be speculated that impaired wound contraction induced by overweight may be related to an increased inflammatory phase and to a delayed myofibroblastic differentiation caused by adipose tissue-derived pro-inflammatory cytokines.
During the early phases of wound healing, inflammatory cells are important in tissue debridement and bacterial killing and also because they release several cytokines and growth factors. While cytokines and growth factors seem to be necessary for wounds to heal, an excessive amount of inflammatory cytokines were detected in non-healing wounds (Garner et al. Reference Garner, Karmiol, Rodriguez, Smith and Phan1993; Cooney et al. Reference Cooney, Iocono, Maish, Smith and Ehrlich1997; Trengove et al. Reference Trengove, Bielefeldt-Ohmann and Stacey2000). In this study, overweight increased inflammatory infiltrate in wound area. Overweight is associated with a chronic, low grade pro-inflammatory state as indicated by leukocytosis (Perfetto et al. Reference Perfetto, Mancuso and Tarquini2002), increased plasma levels of pro-inflammatory cytokines (IL-6 (Yudkin et al. Reference Yudkin, Kumari, Humphries and Mohamed-Ali2000) and TNF-α (Dandona et al. Reference Dandona, Weinstock, Thusu, Abdel-Rahman, Aljada and Wadden1998)), C-reactive protein and acute phase proteins (Visser et al. Reference Visser, Bouter, McQuillan, Wener and Harris1999; van Dielen et al. Reference van Dielen, van't Veer, Schols, Soeters, Buurman and Greve2001). Overweight is also associated with increased levels of endothelial cell dysfunction and activation markers (Ferri et al. Reference Ferri, Desideri, Valenti, Bellini, Pasin, Santucci and De Mattia1999). The stimuli for cytokine production are not known, but it is assumed that the TNF-α in chronic low-grade systemic inflammation originates in the adipose tissue (Coppack, Reference Coppack2001). These findings may explain the increase, 21 d after wounding, of the infiltration of polymorphonuclear leukocytes observed in the diet group.
In granulation tissue formation, the myofibroblast differentiation is important since myofibroblasts are responsible for extracellular matrix deposition and wound contraction (Gabbiani, Reference Gabbiani2003; Desmouliere et al. Reference Desmouliere, Chaponnier and Gabbiani2005). It was found that 21 d after wounding, a high-fat diet increased myofibroblastic Vv, but decreased collagen fibre density and organization. This suggests that myofibroblastic differentiation was delayed, also delaying extracellular matrix deposition. Therefore, adipose tissue excess probably delays myofibroblastic differentiation due to the prolongation of the inflammatory phase.
Angiogenesis is closely related to the formation of granulation tissue. It depends on the interaction of multiple factors, such as vascular endothelial growth factor produced by a variety of cells (Dvorak et al. Reference Dvorak, Detmar, Claffey, Nagy, van de Water and Senger1995; Mick et al. Reference Mick, Wang and McCormick2002; Silha et al. Reference Silha, Krsek, Sucharda and Murphy2005). Conversely, angiogenesis has to cease when the wound defect is filled with granulation tissue. These sequential events reflect temporal changes in the balance between pro-angiogenic and anti-angiogenic factors. The present data have shown that 21 d after wounding the increase of the adipose tissue by a high-fat diet increased the volume occupied by blood vessels in superficial and deep regions of granulation tissue. These alterations may be explained by the increase of leptin levels, a circulating hormone secreted by adipocytes in quantities directly proportional to the adipose tissue mass (Fain et al. Reference Fain, Madan, Hiler, Cheema and Bahouth2004). Leptin has been implicated in stimulation of angiogenesis (Bouloumie et al. Reference Bouloumie, Drexler, Lafontan and Busse1998; Sierra-Honigmann et al. Reference Sierra-Honigmann, Nath and Murakami1998).
In summary, the current study demonstrated that overweight induced by a high-fat diet exerts negative effects on rat cutaneous wound healing, mainly due to prolongation of the inflammatory phase. The present data showed that a high-fat diet impairs wound contraction and re-epithelialization, increases inflammatory cell migration and delays myofibroblastic differentiation, collagen deposition, epithelial and connective cell proliferation and angiogenesis. Therefore, it is important not only to pay attention to the nutritional status of the patients, but also to the quality of the diet, since both can interfere in the wound-healing process.
Acknowledgements
This study was partially supported by CNPq and FAPERJ. A.P. Nascimento holds a postgraduate fellowship from CNPq.











