Gestational diabetes mellitus (GDM) is a common pregnancy complication in 1 % to over 30 % of pregnancies worldwide(Reference McIntyre, Catalano and Zhang1). In mainland China, the prevalence of GDM is estimated at 14·8 % by a meta-analysis involving 79 064 Chinese participants in 2019(Reference Gao, Sun and Lu2). Women with GDM suffer short-term complications such as preeclampsia and shoulder dystocia, and type 2 diabetes (T2D), hypertension and CVD in later life(Reference Billionnet, Mitanchez and Weill3–Reference Fadl, Ostlund and Magnuson5). Children born to mothers with GDM are at increased risk of overweight, impaired glucose tolerance and the metabolic syndrome in later life(Reference Lowe, Lowe and Kuang6–Reference Bianco and Josefson8). To improve pregnant women and their children’s well-being, exploring modifiable risk factors to prevent GDM incidents is of considerable significance.
Epidemiological studies have indicated that diet is one of the key modifiable factors contributed to GDM risk. Plant foods such as cereal fibre, fruit and legumes are reported to protect against GDM(Reference Zhang, Liu and Solomon9–Reference Goshtasebi, Hosseinpour-Niazi and Mirmiran11). On the contrary, potatoes seem to play the opposite role in some Western populations(Reference Bao, Tobias and Hu12,Reference Halton, Willett and Liu13) . As diet is a complex combination of various components, dietary patterns rather than a single food win in considering all these components’ cumulative effect on health outcomes(Reference Schoenaker, Mishra and Callaway14,Reference Gerber15) . However, prior studies about plant-based diets’ health effects have mainly focused on vegetarians, who tend to avoid some or all animal-original foods in their dietary choices. Therefore, the overall plant-based diet index (PDI) has been developed to make up for this limitation, making it easy to evaluate the general population’s plant-based diet. The PDI positively weighs plant foods and negatively weighs animal foods and measures the plant-based diet on a continuous scale.
The high PDI has been demonstrated to protect against T2D in the general population. Prospective cohort studies suggested that higher PDI was associated with reduced longitudinal insulin resistance and lower risk of pre-diabetes and T2D(Reference Satija, Bhupathiraju and Rimm16,Reference Chen, Zuurmond and van der Schaft17) . Since PDI is associated with the risk of T2D among the general population, we propose the hypothesis that PDI also plays a vital role in GDM development among pregnant women. However, there is limited evidence to show the association between PDI and GDM risk. Only a case–control study from Iran reported the inverse association between PDI and the risk of GDM(Reference Zamani, Milajerdi and Tehrani18). However, it suffers from small sample size and sampling and informational bias(Reference Dupépé, Kicielinski and Gordon19). In the present study, we planned to investigate the association between PDI and GDM risk based on a large prospective cohort – Tongji Maternal and Child Health Cohort (TMCHC). We hoped to figure out whether adopting a plant-based diet took effect in GDM prevention in Chinese women.
Methods
Study population
In the present study, pregnant women were part of the participants in the TMCHC study. TMCHC is a prospective cohort study conducted among pregnant women and their offspring in Wuhan, Hubei province, central China. Within 8–16 weeks of gestation, pregnant women were recruited to the cohort during their first prenatal care at the participating hospital, between January 2013 and May 2016(Reference Huang, Chen and Zhang20). All participants provided written, informed consent at enrolment. The study was approved by the Ethics Review Committee of Tongji Medical College, Huazhong University of Science and Technology (no. 201302), and was registered at clinicaltrials.gov as NCT03099837.
Eligibility criteria required individuals from TMCHC to have a completed FFQ between the 13th and 28th weeks of pregnancy. The exclusion criteria were as follows: (1) multiple gestations; (2) an implausible total energy intake (<500 or >3500 kcal/d (<2092 or >14 644 kJ/d))(Reference Qiu, Frederick and Zhang21); (3) registered GDM in a previous pregnancy; (4) registered history of T2D; (5) fasting plasma glucose (PG) value in this early pregnancy ≥5·1 mmol/l; (6) data unavailable on GDM diagnosis and (7) FFQ completed after GDM diagnosis. Finally, we included 2099 pregnant women in this study (Fig. 1).
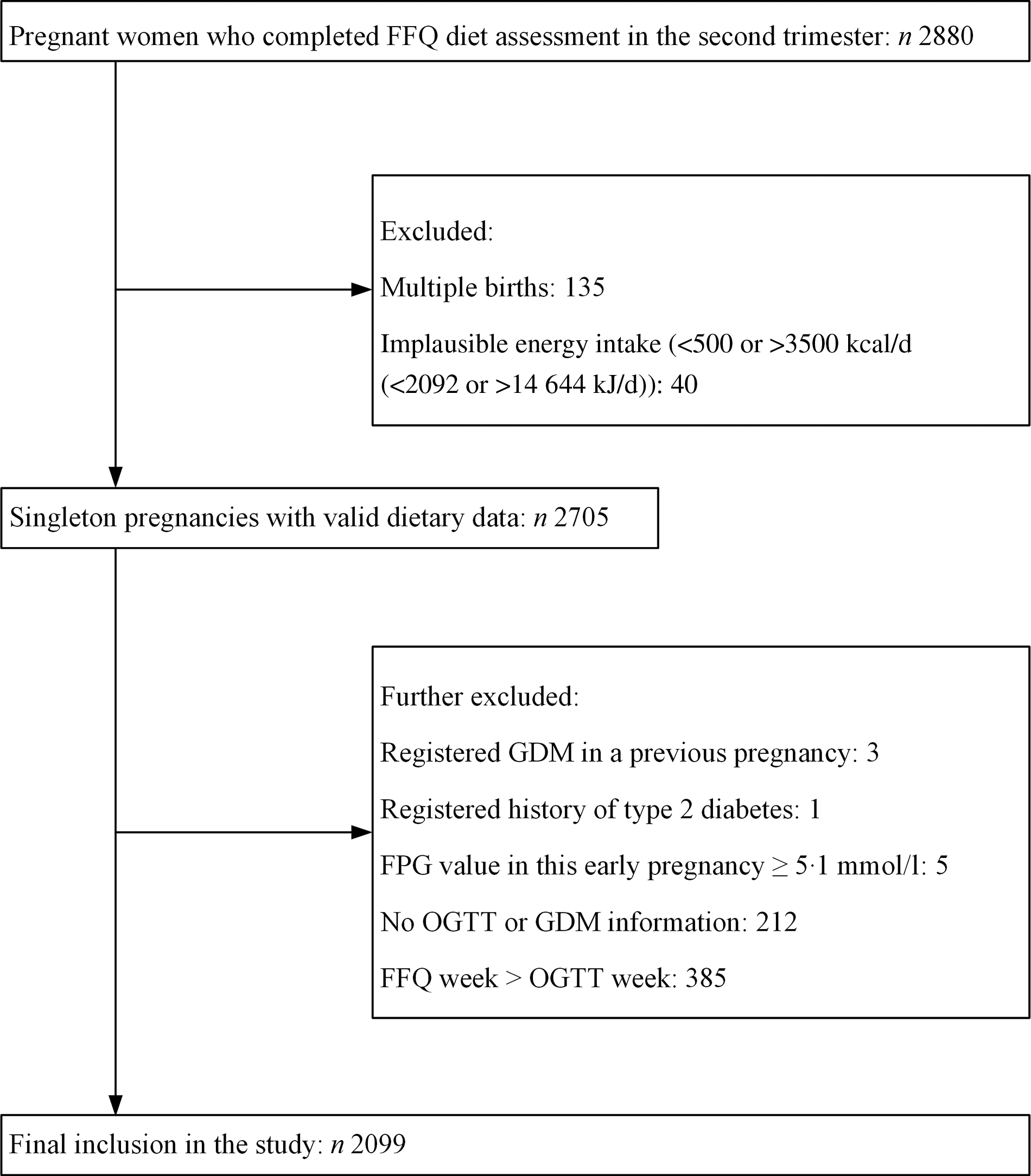
Fig. 1. Flow chart for participant selection from the Tongji Maternal and Child Health Cohort study. GDM, gestational diabetes mellitus; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test.
Dietary assessment and the overall plant-based diet index
Food intake data were collected using a semi-quantitative FFQ consisting of sixty-one food items formed based on food nutrient composition and eating habits of Chinese(Reference Zhang, Qiu and Zhong22). The intake frequency and portion size of each food item in the last month were self-reported. Food models presented a standard portion size were used to help participants more accurately estimate the amounts of food consumed. The validity and reliability of the FFQ have been described previously(Reference Zhang, Qiu and Zhong22).
The calculation method of PDI was proposed by Satija et al. (Reference Satija, Bhupathiraju and Rimm16). For each plant food group, participants received a score of 5 if they were above the highest quintile of consumption, a score of 4 if they were above the second-highest quintile but below the highest quintile, and so on, with a score of 1 for consumption below the lowest quintile (positive scores). For each animal food group, participants received a score of 1 if they were above the highest quintile of consumption, a score of 2 if they were between the highest and second-highest quintiles, and so on, with a score of 5 for consumption below the lowest quintile (reverse scores)(Reference Satija, Bhupathiraju and Rimm16).
We used Satija’s method with some modifications to calculate the PDI among Chinese pregnancies. Considering the dietary difference between Western countries and China, our calculation has included slightly different food types. For example, food items were assembled into sixteen food groups, and the lists for each food group are displayed in online Supplementary Table S1. The frequency and portion size of each consumed food were converted in g/d or ml/d. Then, the grams or millilitres of foods that belonged to each of the sixteen food groups were added up. In our population, several food groups were consumed by <20 % of the population, making it difficult to assign score according to the quintile of consumption (online Supplementary Table S2). Food groups including juices, tea and coffee, sugar-sweetened beverages and animal fat were excluded, but we further adjusted them in the later analyses. The other twelve food groups were included in the calculation of PDI. Among them, plant food groups included whole grains, refined grains, fruits, vegetables, nuts, beans, vegetable oil, sweets and desserts. Animal food groups included dairy products, eggs, meat and fish. PDI for each participant was obtained by summing the twelve food group scores, with the lowest possible score of 12 to the highest possible score of 60.
Outcome assessment
GDM was diagnosed based on the 75 g 2-h oral glucose tolerance test (OGTT) results, which included fasting PG and 1-h and 2-h PG levels. OGTT was performed during 24–28 weeks of gestation by professional laboratory technicians for each participant. According to the International Association of Diabetes and Pregnancy Study Group, GDM was diagnosed if any of the following three thresholds were met: 5·1, 10·0 and 8·5 mmol/l for fasting PG, 1 h PG and 2 h PG, respectively(Reference Metzger, Gabbe and Persson23).
Covariates
A structured questionnaire was used to collect maternal information in a face-to-face interview by trained investigators at baseline enrolment. The questionnaire included information on maternal age, ethnicity, education level, average personal income, last date of the menstrual period, family history of diabetes, parity and other pre-pregnancy information such as weight, drinking and smoking status, leisure-time physical activity and sleep quality. Education level was grouped by the number of completed schooling years: ≤12, 13–15 and ≥16 years. Average personal income was divided into three groups: <5000, 5000–9999 and ≥10 000 China Yuan per month. Pre-pregnancy BMI (kg/m2) was calculated by pre-pregnancy body weight (self-reported) and height (measured at enrolment). Weight gain before GDM diagnosis (kg) was calculated using a weight at GDM diagnosis minus the pre-pregnancy weight. Gestational weeks of OGTT (or FFQ) performed were calculated from the last date of the menstrual period to OGTT (or completed FFQ). Sleep quality was categorised into either poor sleep quality (reported insomnia frequently) or good sleep quality (reported insomnia sometimes, occasionally or never).
Statistical analysis
Continuous variables were described as medians with interquartile ranges (IQR) and compared using Kruskal–Wallis tests or Wilcoxon rank-sum tests. Categorical variables were expressed as frequencies and proportions (%) and compared using χ 2 tests.
Logistic regression models were fitted to estimate OR of GDM, with associated 95 % CI, comparing women in different PDI quartiles. In model 1, age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 China Yuan/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg) and total energy intake (kcal/d) were adjusted. In model 2, consumptions of juices (ml/d), tea and coffee (ml/d), sugar-sweetened beverages (ml/d) and animal fat (g/d) were additionally adjusted. PDI was first included in the models as a categorised variable (as quartiles). The presence of a linear trend was tested by assigning each quartile of the median value and modelling this variable as a continuous variable. If the trend test showed a linear trend between PDI and risk of GDM, PDI was then included in the model as a continuous variable (as an increment of IQR). Besides, the potential non-linear relationship between PDI (as a continuous variable) and risk of GDM was visually evaluated by cubic-restricted spline function with three knots, which were located on the 5th, 50th and 95th, respectively(Reference Desquilbet and Mariotti24). We evaluated whether associations between PDI and GDM risk were modified by age (<30 or ≥30 years), pre-pregnancy BMI (<24 or ≥24 kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), leisure-time physical activity (yes or no) or weight gain before GDM diagnosis (<8·2 or ≥8·2 kg) by stratified analyses.
Sensitivity analyses were performed based on model 2 by evaluating associations between diverse PDI versions and the risk of GDM. First, to check if the association was driven by any specific components of the PDI, we repeated our analyses by excluding each one of the twelve food groups from the PDI one by one at a time, and additionally adjusting for the excluded food groups. Second, we excluded less healthy plant food groups (refined grains, and sweets and desserts) combined from PDI and adjusted them in the model. Last, we further evaluated the associations between healthful PDI (assigning healthy plant food groups positive scores while assigning less healthy plant food groups and animal food groups reverse scores) and unhealthful PDI (assigning less healthy plant food groups positive scores while assigning healthy plant food groups and animal food groups reverse scores) with GDM risk.
All statistical tests were two-sided, and P < 0·05 was considered statistically significant. SAS version 9.4 (SAS Institute Inc.) was used for all statistical analyses.
Results
Characteristics of participants
Of the 2880 participants who completed the FFQ diet assessment in the second trimester, we first excluded those with multiple births (n 135) or implausible energy intake of <500 or >3500 kcal/d (<2092 or >14 644 kJ/d) (n 40). We further excluded those who registered GDM in a previous pregnancy (n 3), registered history of T2D (n 1), fasting PG value in this early pregnancy ≥5·1 mmol/l (n 5), without OGTT information (n 212) or FFQ completed after GDM diagnosis (n 385). Finally, a total of 2099 pregnant women were included in the present study (Fig. 1). The median age was 28·0 (IQR 26·0–30·0) years old. The median of pre-pregnancy BMI was 20·4 (IQR 18·8–22·2) kg/m2, and the median of weight gain before GDM diagnosis was 8·2 (IQR 6·1–10·3) kg. Among the 2099 participants, 2051 (97·7 %) were Han Chinese, 1769 (84·3 %) were nulliparous and 174 (8·3 %) had a family history of diabetes. The characteristics of all participants in the baseline categorised according to PDI quartiles are shown in Table 1. The PDI of included participants ranged from 21·0 to 52·0 (theoretical range: 12·0–60·0); the median was 36·0 (IQR 33·0–39·0). Those in the higher quartile of PDI were more likely to be multiparous and do leisure-time physical activity (P < 0·05 for all comparisons). There were no significant differences in other characteristics (P > 0·05 for all comparisons).
Table 1. Baseline characteristics and nutritional characteristics of 2099 participants by the quartile (Q) of overall plant-based diet index (PDI) in the Tongji Maternal and Child Health Cohort study
(Median values and interquartile ranges (IQR); numbers and percentages)

GDM, gestational diabetes mellitus; CNY, China Yuan.
* Based on the Kruskal–Wallis test for continuous data and χ 2 tests for categorical data.
† To convert kcal to kJ, multiply by 4·184.
Among the 2099 participants, 169 (8·1 %) were diagnosed with GDM. The comparisons of characteristics between GDM and non-GDM pregnant women are shown in online Supplementary Table S3. Women with GDM tended to be multiparous and not exercise in leisure-time than those without GDM (P < 0·05 for all comparisons). Moreover, women with GDM had higher pre-pregnancy BMI, more weight gain before GDM diagnosis, older age and a higher proportion of family history of diabetes at enrolment than the remaining participants (P < 0·01 for all comparisons). There were no significant differences in other characteristics between women with GDM and women without GDM (P > 0·05 for all comparisons).
Nutritional characteristics
The median consumption (g/d) of each component food group was as follows: whole grains: 15·0 (IQR 5·4–34·3); refined grains: 200·0 (IQR 150·0–250·0); fruits: 365·8 (IQR 232·9–525·8); vegetables: 311·6 (IQR 183·3–478·8); nuts: 11·4 (IQR 2·9–18·0); beans: 10·0 (IQR 2·9–20·0); sweets and desserts 0·1 (IQR 0·0–8·6); vegetable oil: 30·0 (IQR 25·0–35·0); dairy products: 185·7 (IQR 76·6–290·9); eggs: 40·0 (IQR 22·9–50·0); meat: 50·0 (IQR 25·0–75·0); fish: 22·9 (IQR 7·9–49·8). Those in the highest quartile of PDI consumed higher intake of total energy and plant foods but lower intake of animal foods compared with those in the lowest quartile (P < 0·001 for all comparisons) (Table 1). The consumption difference of each component food group between the highest and lowest quartiles of PDI (Q4 v. Q1) was as follows: whole grains: 28·6 v. 7·9 (g/d); refined grains: 225·0 v. 170·0 (g/d); fruits: 443·7 v. 270·3 (g/d); vegetables: 415·1 v. 214·3 (g/d); nuts: 14·6 v. 7·1 (g/d); beans: 15·0 v. 4·3 (g/d); sweets and desserts 5·7 v. 0·0 (g/d); vegetable oil: 30·0 v. 30·0 (g/d); dairy products: 128·6 v. 250·0 (g/d); eggs: 35·7 v. 50·0 (g/d); meat: 35·7 v. 52·9 (g/d); fish: 18·6 v. 28·6 (g/d) (P < 0·001 for all comparisons).
The association between the overall plant-based diet index and risk of gestational diabetes mellitus
In the crude logistic regression model, a higher PDI quartile was significantly associated with decreased odds of GDM (OR for the highest quartile 0·54; 95 % CI 0·34, 0·87; P for trend = 0·011) (Table 2). After adjusting for social-demographic characteristics, lifestyle factors and energy in model 1, the OR was 0·43 (95 % CI 0·24, 0·75), with P for trend 0·004 across the PDI quartiles (Table 2). The associations remained significant and similar in magnitude after accounting for the consumption of juices, tea and coffee, sugar-sweetened beverages and animal fat in model 2 (OR for the highest quartile 0·43; 95 % CI 0·24, 0·77; P for trend = 0·005) (Table 2). For each IQR increment in PDI, the OR for GDM were 0·79 (95 % CI 0·65, 0·96) in crude model, 0·71 (95 % CI 0·56, 0·90) in model 1 and 0·71 (95 % CI 0·56, 0·90) in model 2. We observed no effect modification by age, pre-pregnancy BMI, parity, family history of diabetes, leisure-time physical activity or weight gain before GDM diagnosis (P > 0·05 for all interaction terms) (Table 3). In the continuous analysis, there was an approximately linear inverse relationship between PDI and risk of GDM (Fig. 2), after adjusted for covariates (P for overall association = 0·019, P for non-linearity = 0·539). Associations between PDI and GDM risk were robust in sensitivity analyses. The estimates did not substantially change with the exclusion of each one of twelve food groups from PDI one by one at a time. The estimate also remained similar after excluding less healthy plant food groups (refined grains, and sweets and desserts) (OR 0·70; 95 % CI 0·54, 0·90) (online Supplementary Table S4). Per IQR increment of healthful PDI was reversely related to the risk of GDM (adjusted OR 0·76; 95 % CI 0·60, 0·97) (online Supplementary Table S5). There was no significant association between per IQR increment of unhealthful PDI and GDM risk (adjusted OR 0·89; 95 % CI 0·68, 1·17) (online Supplementary Table S5).
Table 2. Associations of overall plant-based diet index (PDI) with gestational diabetes mellitus (GDM) risk in the Tongji Maternal and Child Health Cohort study
(Odds ratios and 95 % confidence intervals; numbers and percentages)

IQR, interquartile range; Q1–Q4, quartiles 1–4; CNY, China Yuan.
* Logistic regression model was adopted and without adjusting for other variables.
† Multiple logistic regression model was adopted and adjusted for age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 CNY/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg) and energy (kcal/d).
‡ Multiple logistic regression model was adopted and adjusted for age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 CNY/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg), energy (kcal/d), juices (ml/d), tea and coffee (ml/d), sugar-sweetened beverages (ml/d) and animal fat (g/d).
§ IQR of PDI was 6 points.
|| Tests for trend based on logistic regression by assigning each quartile of the median value and modelling this variable as a continuous variable.
Table 3. Stratified analyses for gestational diabetes mellitus (GDM) risk in relation to per interquartile range (IQR) increase of overall plant-based diet index (PDI) in the Tongji Maternal and Child Health Cohort study*
(Odds ratios and 95 % confidence intervals; numbers and percentages)

CNY, China Yuan.
* IQR of PDI was 6 points.
† Multiple logistic regression model was adopted and adjusted for age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 CNY/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg) and energy (kcal/d), except for the corresponding stratification variable.
‡ Multiple logistic regression model was adopted and adjusted for age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 CNY/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg), energy (kcal/d), juices (ml/d), tea and coffee (ml/d), sugar-sweetened beverages (ml/d) and animal fat (g/d), except for the corresponding stratification variable.
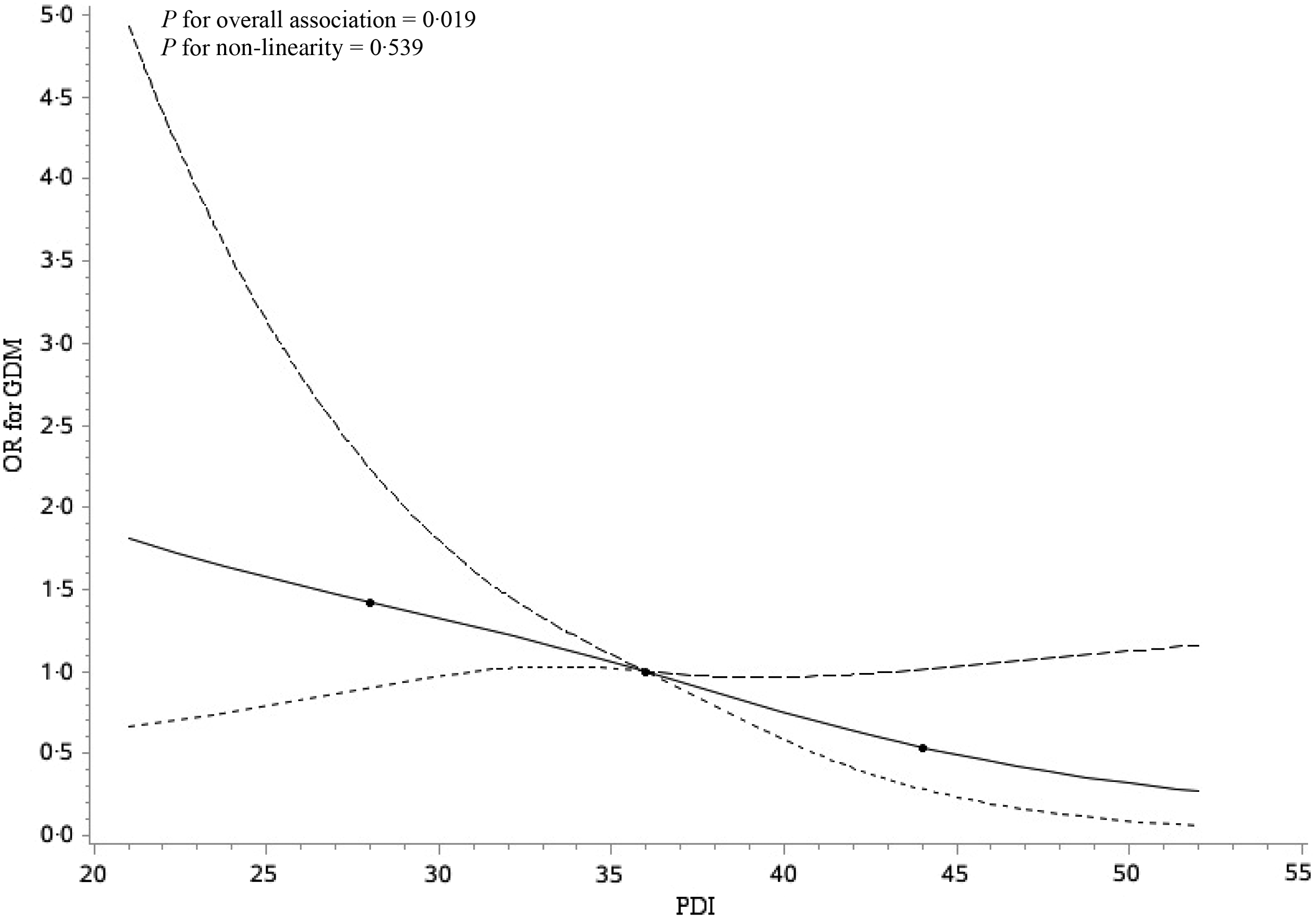
Fig. 2. Restricted cubic spline analyses illustrating the shapes of multivariable association between overall plant-based diet index (PDI) and gestational diabetes mellitus (GDM) in the Tongji Maternal and Child Health Cohort study. Adjusted for age (years), ethnicity (Han Chinese or other), education (≤12, 13–15 and ≥16 years), income (<5000, 5000–9999 and ≥10 000 China Yuan (CNY)/month), pre-pregnancy BMI (kg/m2), parity (nulliparous or multiparous), family history of diabetes (yes or no), smoking status (yes or no), drinking status (yes or no), exercise (yes or no), sleep quality (poor or good), weight gain before GDM diagnosis (kg), energy (kcal/d), juices (ml/d), tea and coffee (ml/d), sugar-sweetened beverages (ml/d) and animal fat (g/d). The reference value for PDI was chosen to the median value, that is, 36. ![]() , Estimation;
, Estimation; ![]() , lower confidence limit;
, lower confidence limit; ![]() , upper confidence limit;
, upper confidence limit; ![]() , knots.
, knots.
Discussion
In the present population-based cohort study, we found that higher PDI was associated with a lower risk of GDM in Chinese women. An IQR increment in PDI decreased the odds of GDM by 29 %. Moreover, women having the highest quartile of PDI had a 57 % decreased odds of GDM compared with those in the lowest quartile. To our knowledge, this is the first prospective cohort study to evaluate the association of maternal PDI during pregnancy and GDM risk in Chinese women.
We have addressed other conventional risk factors for GDM when evaluating the health effects of PDI. First, we excluded participants who had a previous history of GDM or T2D, for they may suffer from increased risk of developing GDM and have accepted dietary interventions(Reference Cosson, Benbara and Pharisien25). Second, after adjusting the covariates such as social-demographic characteristics, lifestyle factors and nutrition consumption information in the regression models, the reverse association between PDI and GDM risk remained statistically significant. Moreover, when conducting stratified analyses with interaction items by age, pre-pregnancy BMI, parity, family history of diabetes, leisure-time physical activity and weight gain before GDM diagnosis, we observed no modification effects of these factors on the association between PDI and risk of GDM.
Our findings support the protective role of the plant-based diet on GDM risk. Behzad et al. found that higher PDI was associated with a lower risk of GDM in Iranian pregnant women(Reference Zamani, Milajerdi and Tehrani18). We observed similar findings in our sample of Chinese women, which was in accordance with the existing evidence that the vegetable pattern was associated with a decreased risk of GDM in Chinese population(Reference He, Yuan and Chen26). Moreover, we found that the protective role was not mainly driven by a specific food group as the estimates remained similar when excluding each one of twelve food groups one by one at a time, which supports the importance of evaluating the overall plant-based diet. To figure out whether these less healthy plant food groups contributed to the observed associations, we further excluded the less healthy food groups from PDI and adjusted them in the model and the results were robust. We also observed a significant inverse association between healthful PDI and GDM risk. These findings indicate that the beneficial associations were mainly driven by increasing intakes of healthy plant foods and decreasing animal foods intakes. Due to the limited consumption of less healthy plant foods in our population, we did not observe a statistically significant effect of unhealthful PDI on GDM risk. Further studies in other populations are needed to confirm these findings.
Although there are very few studies on the association between a plant-based diet and GDM risk, numerous epidemiology studies have revealed the protective effect of a plant-based diet on the development of T2D(Reference Lee and Park27,Reference Prentice, Aragaki and Howard28) . Our findings also indicate that PDI seems to play a similar role in GDM as that in T2D. Qian et al. performed restricted cubic splines regression and found a significant inverse association between plant-based indices and T2D risk(Reference Qian, Liu and Hu29). We found the same inverse association between PDI and GDM risk. There are some similarities in pathogenesis and genetic background between GDM and T2D(Reference Kühl30,Reference Huopio, Cederberg and Vangipurapu31) . GDM is considered as a forerunner of T2D, and women with a history of GDM appear to have a nearly 10-fold higher risk of developing T2D than those with a normoglycaemic pregnancy(Reference Baz, Riveline and Gautier32,Reference Vounzoulaki, Khunti and Abner33) . Women with GDM should be a target group for interventions aimed at preventing T2D(Reference Engeland, Bjørge and Daltveit34). Therefore, adopting a plant-based diet is of significant benefit for pregnant women, as they can achieve not only lower risk of GDM during pregnancy period but also a lower risk of developing T2D in later life.
There are possible biological mechanisms that may support our findings. A plant-based diet tends to be rich in dietary fibres, antioxidants and micronutrients. Considerable experimental evidence has demonstrated that viscous dietary fibres’ addition slows gastric emptying rates, digestion and glucose absorption to benefit immediate postprandial glucose metabolism(Reference Dahl and Stewart35). Microbiota-generated fibre-derived SCFA are known to reduce hepatic glucose output, improve lipid homoeostasis and influence gut microbiota(Reference Russell, Baka and Bjorck36,Reference Weitkunat, Stuhlmann and Postel37) . Antioxidants such as polyphenols, naringenin, vitamin C are shown to improve insulin sensitivity, inflammation and oxidative stress associated with GDM(Reference Santangelo, Zicari and Mandosi38–Reference Nguyen-Ngo, Willcox and Lappas40). The supplementations of micronutrients such as Mg and Se improve glucose parameters in people with diabetes and improve insulin-sensitivity parameters in those at high risk of diabetes(Reference Veronese, Watutantrige-Fernando and Luchini41,Reference Asemi, Jamilian and Mesdaghinia42) . Simultaneously, a plant-based diet usually has less saturated fat and animal protein, which may adversely affect insulin sensitivity and risk of pre-diabetes(Reference Chen, Franco and Lamballais43,Reference von Frankenberg, Marina and Song44) . All of these biological mechanisms may help explain why adopting a plant-based diet could lower the risk of GDM.
Although our findings are from a prospective cohort study, several limitations need to be acknowledged when interpreting our findings. First, information on dietary food intake was self-report, which may lead to inevitable measurement errors. However, our FFQ has been shown to have acceptable reproducibility and reasonable validity in assessing most food and nutrient intakes among pregnant women(Reference Zhang, Qiu and Zhong22). Second, several food groups were excluded from PDI calculation due to low consumption in the studied population. To estimate these food groups’ effect, we adjusted the consumption of these food groups as continuous variables in our models and the associations between PDI and GDM risk remained robust. Third, the prevalence of GDM in this study was lower than the overall prevalence in China (8·1 % v. 14·8 %)(Reference Gao, Sun and Lu2). Previous studies have suggested that women with older age, higher pre-pregnancy BMI and a family history of diabetes are more likely to develop GDM(Reference Juan and Yang45,Reference Gao, Leng and Liu46) . However, participants in our cohort were younger, had lower pre-pregnancy BMI and less family history of diabetes than that in other populations in China(Reference Li, Wei and Ni47,Reference Hou, Li and Xia48) . These could lead to the low GDM prevalence in our study. Therefore, the generalisability of our findings to general population may be limited. Finally, each participant’s PDI was calculated based on the quintiles of food intake in the studied population. Thus, the use of sample-based scores makes it unable to infer whether there is an absolute level of a specific food group concerning GDM risk. Further research should be undertaken to investigate the optimal thresholds of intake of these foods in the context of an overall plant-based diet.
In conclusion, the results of this large prospective cohort study support the hypothesis that higher PDI is associated with a reduced risk of GDM in Chinese. Our findings suggest that adopting a plant-based diet during mid-pregnancy may be protective against GDM among the Chinese population. Further research is needed to clarify the associations between plant-based diets and the risk of GDM in other Asian populations.
Acknowledgements
We acknowledge all the participants in this study for their cooperation. We gratefully thank all members of the Tongji Maternal and Child Health Cohort study group for their valuable contributions.
This work was supported by the National Program on Basic Research Project of China (no. 2013FY114200) for Nianhong Yang.
S. W., N. Y. contributed to the conception and designed research; X. Y., L. H. and N. Y. supervised the study conduct; L H., L. L., X. C., C. Z., Q. L., N. L., D. G., X. Z., R. C., Y. Z. and B. Y. collected the data; H. W. drafted the manuscript and performed the statistical analyses; S. W., N. Y. reviewed and revised the manuscript. All authors read and approved the final manuscript.
All authors have no conflicts of interest to declare.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114521000234








