Breast cancer is the most common cancer and the second leading cause of cancer deaths among females in the USA(1). For decades, Mediterranean countries, such as Italy, Greece, and Spain, have had lower incidence of breast cancer than the USA(Reference Schwingshackl and Hoffmann2). The traditional diet of Mediterranean countries is characterised by high consumption of fruit, vegetables and olive oil, a low intake of red meat and dairy products and a moderate intake of red wine during meals(Reference Bloomfield, Kane and Koeller3,Reference Schwingshackl, Morze and Hoffmann4) . Prior meta-analyses suggest that Mediterranean diets overall are associated with a decreased risk of breast cancer(Reference Bloomfield, Koeller and Greer5–Reference Schwingshackl, Schwedhelm and Galbete7). While the Mediterranean diet varies by country, olive oil is the main source of dietary fat(Reference Linseisen, Welch and Ocké8,Reference Gaforio, Visioli and Alarcón-de-la-Lastra9) and has been of particular interest due to its potentially beneficial MUFA profile(Reference Gorzynik-Debicka, Przychodzen and Cappello10). Additionally, extra virgin olive oil is high in polyphenols, which have been shown to have antioxidant and anti-inflammatory properties in in vitro studies(Reference Gorzynik-Debicka, Przychodzen and Cappello10). Further, greater olive oil consumption has been associated with improvements in inflammatory biomarkers in meta-analyses of intervention and observational studies(Reference Schwingshackl, Morze and Hoffmann4). Oleocanthal, a specific polyphenol, is an agent reported to suppress cancer cells(Reference Pang and Chin11). A few prior reviews and meta-analyses have suggested a potential reduction in breast cancer risk with increasing intake of olive oil(Reference Escrich, Moral and Solanas12–Reference Xin, Li and Sun15); however, they have often included studies that have examined monounsaturated fat intake, other vegetable/liquid oils and studies of dietary patterns with olive oil as a component, which may increase misclassification or be confounded by other foods. Additionally, if olive oil is found to decrease breast cancer risk, it is relatively easy to increase exposure levels in women as supplementation of extra virgin olive oil in a diet has been shown to have high adherence(Reference Vitolins, Vitolins and Blackwell16). We therefore conducted a meta-analysis to summarise the association of olive oil intake specifically and breast cancer risk, as well as examine the potential of a dose–response relationship.
Methods
Search strategy
We performed a systematic search to identify relevant original research studies examining the association between olive oil and breast cancer risk in the following databases: PubMed, Web of Science, CINAHL and Cochrane Central Register of Controlled Trials through June 2020 with no initial search restrictions using the keywords ‘olive oil’ combined with ‘breast neoplasms’ or ‘breast cancer’. Additionally, to identify studies not captured by our search strategy, we examined the reference lists of relevant articles. This review was not registered in the International Prospective Register of Systematic Reviews.
Inclusion and exclusion criteria
We used commercially available software to remove duplicate records and to screen titles and abstracts for inclusion(Reference Ouzzani, Hammady and Fedorowicz17). Studies were included in the present meta-analysis if they met the following inclusion criteria: (i) if they were an observational study (cohort, case–control, cross-sectional) or randomised control trial, (ii) conducted amongst human participants, (iii) had olive oil as a separate exposure of interest, (iv) had breast cancer risk as the outcome of interest and (v) reported risk estimates (relative risks, OR or hazard ratios) and 95 % CI or sufficient information for these to be estimated for three or more categories of olive oil intake. We additionally excluded: articles not in English, conference abstracts and studies of other exposures (e.g. liquid oils, monounsaturated fat), combination of oils (e.g. olive and canola oils) or dietary patterns that include olive oil as a component (e.g. olive oil and salad vegetable pattern). For studies with an overlapping study population, we used the study with the most complete information. Two authors, N. S. and S. C. H., independently identified articles meeting inclusion criteria from search results, and any disagreements were resolved by discussion or consultation with a third reviewer (S. E. H.).
Data extraction and risk of bias
One investigator (N. S.) abstracted the following information from selected studies, which was reviewed by a second investigator (S. C. H.): last name of the first author, year published, study location, study population characteristics (e.g. age, menopause status), study design, follow-up duration, number of cases, number of participants, exposure assessment, outcome assessment, exposure cut points for each category of intake and corresponding risk estimates and 95 % CI for the maximally adjusted model and covariates included. As breast cancer is a relatively rare event, OR and hazard ratio estimates were considered equivalent. For studies that presented risk estimates graphically only, we attempted to contact the study authors for precise risk estimates. Additionally, for articles that provided stratified results by menopause status, we abstracted risk estimates and 95 % CI for menopause strata. For each study, we additionally (S. C. H. and S. E. H.) assessed the risk of bias using the Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) tool assigning each bias domain as low, moderate, serious, critical or unclear(Reference Sterne, Hernán and Reeves18).
Statistical analysis
Assuming study heterogeneity a priori, risk estimates for the highest v. lowest intake category were combined across studies to estimate an overall OR and 95 % CI using a random effects model(Reference DerSimonian and Laird19,Reference DerSimonian and Laird20) . In cases where the reference category reported was not the lowest intake category, we recalculated the OR using the method by Hamling et al. (Reference Hamling, Lee and Weitkunat21). We additionally estimated a postmenopausal breast cancer specific summary OR and 95 % CI, for studies that reported stratified estimates by menopause status or if the study had been conducted among postmenopausal women only.
Heterogeneity among studies was assessed using Higgins I 2 statistic, where 25, 50 and 75 % were considered low, medium and high heterogeneity, respectively(Reference Higgins and Thompson22). To examine potential sources of heterogeneity among case–control studies, we conducted subgroup analyses by study location (Italy, Spain, Greece v. other country), source of controls (hospital based v. population based), number of cases (<500 v. ≥500), exposure assessment (quantity consumed v. frequency consumed) and whether energy intake was controlled for. We did not assess heterogeneity among prospective studies since there were only two studies. Our analysis including the Prevención con Dieta Mediterránea (PREDIMED) trial by Toledo et al. was conducted using only the per protocol analysis, breaking the randomisation and allowing for its characterisation as a prospective study, not a randomised control trial(Reference Toledo, Salas-Salvadó and Donat-Vargas23). This was done because the per protocol analysis examined the effect of olive oil specifically rather than the intention to treat analysis which examined the effect of the Mediterranean diet plus olive oil compared with a Mediterranean diet plus nuts or low fat diet (control arm). We assessed publication bias visually using a funnel plot. Analyses were repeated omitting one study at a time and estimating the overall OR, to evaluate the influence of a single study. Lastly, as a sensitivity analysis, we additionally estimated the OR using an inverse-variance fixed effects model.
Dose–response
The dose–response meta-analysis using random effects generalised least-squares regression described by Greenland & Longnecker(Reference Greenland and Longnecker24) and Orsini et al.(Reference Orsini, Li and Wolk25) were used to estimate the OR and 95 % CI for breast cancer associated with a 14 g/d (one tablespoon) increase in olive oil intake. For studies that reported frequency of consumption, a 14 g (one tablespoon) portion was assumed per instance. Second, to examine potential non-linear associations, we used restricted cubic spline models with three fixed knots at the 25th, 50th and 75th percentiles of the total reported intake distribution.
All statistical analyses were conducted using RevMan 5.3 (Nordic Cochrane Center) and SAS 9.3 (SAS Institute Inc.). All tests were two-sided, and P value <0·05 was considered statistically significant.
Grading the evidence and summary of findings
We assessed the overall quality and strength of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool(Reference Atkins, Best and Briss26). S. C. H. independently assessed the quality of evidence which was then confirmed by a second investigator (S. E. H.).
Results
Search results
From our search strategy, we identified 757 records through June 2020 (Fig. 1). After removing 219 duplicate records and screening titles and abstracts of the remaining 538 records, we excluded 484 records that were not relevant. After reviewing reference lists of relevant articles, we identified an additional two articles not captured by our search strategy, leading to fifty-seven articles for full-text review. From these we excluded forty-seven articles that did not meet our criteria for inclusion; seventeen were reviews, editorials, or conference abstracts; four were not in English; nine did not assess olive oil specifically; ten reported olive oil only as part of a dietary pattern; two studies were excluded due to overlapping study populations; two did not provide both effect estimates and CI and three had continuous estimates or less than three categories. Therefore, ten articles were eligible for inclusion in the present meta-analysis(Reference Toledo, Salas-Salvadó and Donat-Vargas23,Reference Richardson, Gerber and Cenée27–Reference Pervaiz, Tosun and Besim35) .
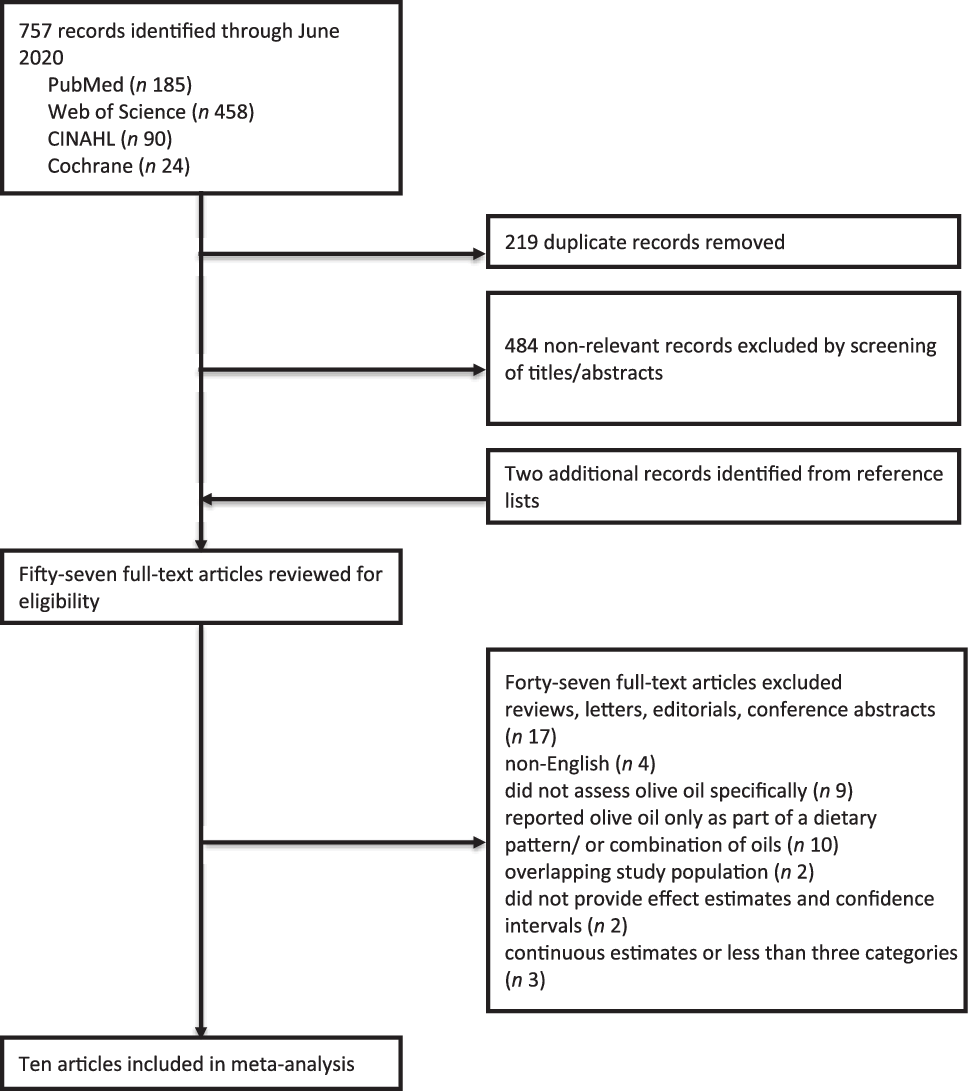
Fig. 1. Flow chart of study selection.
Characteristics of studies
Characteristics of the ten included studies are presented in Table 1. The meta-analysis included a total of 7030 cases of breast cancer among 81 436 participants. Three of the ten studies(Reference Toledo, Salas-Salvadó and Donat-Vargas23,Reference Buckland, Travier and Agudo34,Reference Pervaiz, Tosun and Besim35) were conducted only among postmenopausal women, whereas the ages in the other studies ranged from 18 to 85. Except for two studies conducted in Kuwait(Reference Saleh, Reno and Ibrahim32) and Turkey(Reference Pervaiz, Tosun and Besim35), the majority of the studies were conducted in the European countries: Spain, Greece, Italy and France(Reference Toledo, Salas-Salvadó and Donat-Vargas23,Reference Richardson, Gerber and Cenée27–Reference García-Segovia, Sánchez-Villegas and Doreste31,Reference Bessaoud, Daurès and Gerber33,Reference Buckland, Travier and Agudo34) . Eight of the studies were case–control studies(Reference Richardson, Gerber and Cenée27–Reference Bessaoud, Daurès and Gerber33,Reference Pervaiz, Tosun and Besim35) and two were prospective(Reference Toledo, Salas-Salvadó and Donat-Vargas23,Reference Buckland, Travier and Agudo34) . Two studies were considered to have low risk of bias(Reference Toledo, Salas-Salvadó and Donat-Vargas23,Reference Buckland, Travier and Agudo34) , others ranged from moderate to critical(Reference Richardson, Gerber and Cenée27–Reference Bessaoud, Daurès and Gerber33,Reference Pervaiz, Tosun and Besim35) (online Supplementary Table S1).
Table 1. Characteristics of selected studies
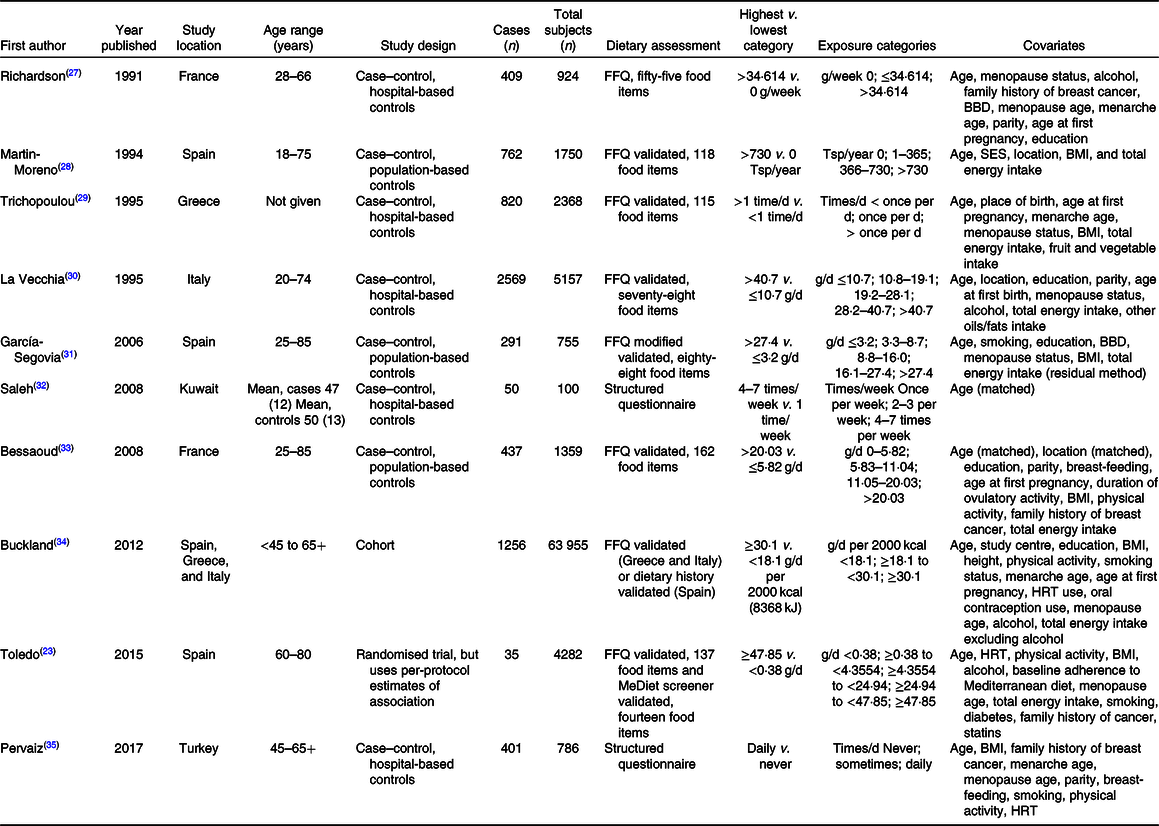
BBD, benign breast disease; Tsp, tablespoon; SES, socio-economic status; HRT, hormone replacement therapy.
Overall analyses
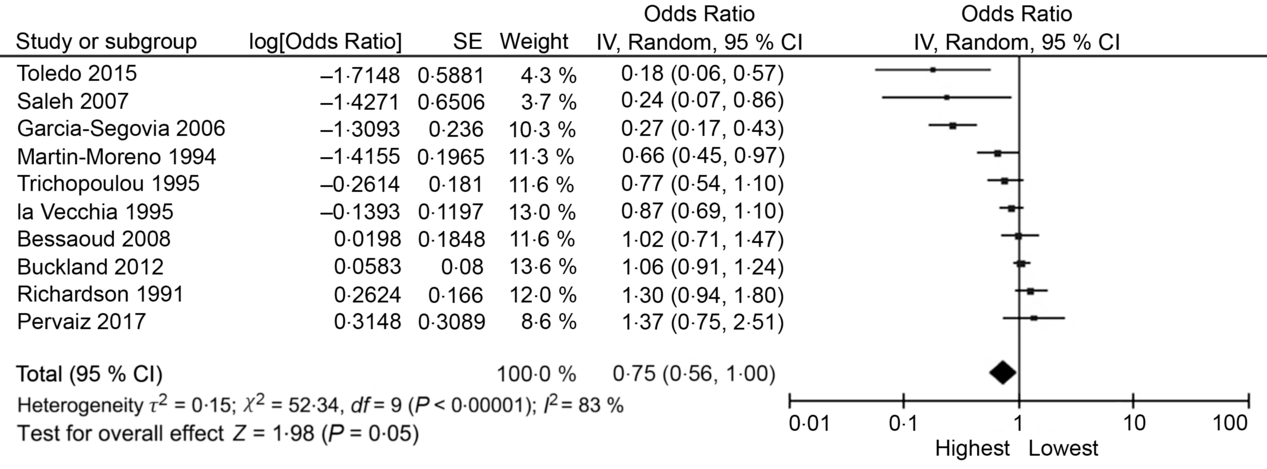
The random effects summary OR for breast cancer was 0·48 (95 % CI 0·09, 2·70) for prospective studies and 0·76 (95 % CI 0·54, 1·06) in case–control studies, comparing women with the highest intake to those with the lowest intake category of olive oil (Fig. 2). Heterogeneity between the studies was high (prospective I 2 = 89 %, case–control I 2 = 82 %). Visual inspection of the funnel plot suggested evidence of publication bias (Fig. 3). Influence analyses omitting one study at a time suggested moderate influence on the findings; the summary OR ranged from 0·69 (95 % CI 0·51, 0·94) when Richardson et al. (Reference Richardson, Gerber and Cenée27) was omitted to 0·89 (95 % CI 0·71, 1·10) when García-Segovia et al. (Reference García-Segovia, Sánchez-Villegas and Doreste31) was omitted (Table 2). The results were attenuated when we used a fixed effects method (OR 0·91, 95 % CI 0·82, 1·00). As a secondary analysis, we additionally repeated our case–control random effects model using the spline estimates in the study by Bessaoud et al. (OR 0·72, 95 % CI 0·51, 1·02)(Reference Bessaoud, Daurès and Gerber33), rather than the quintile estimates we had abstracted, as both models were fully adjusted. For postmenopausal breast cancer, the random effects summary OR was 0·94 (95 % CI 0·71, 1·24; I 2 = 74 %; P for heterogeneity = 0·008) overall, 0·48 (95 % CI 0·09, 2·70; I 2 = 89 %; P for heterogeneity = 0·003; n 2) for prospective studies and 1·00 (95 % CI 0·70, 1·42; I 2 = 43 %; P for heterogeneity = 0·19; n 2) for case–control studies for highest v. lowest olive oil intake (Fig. 4).
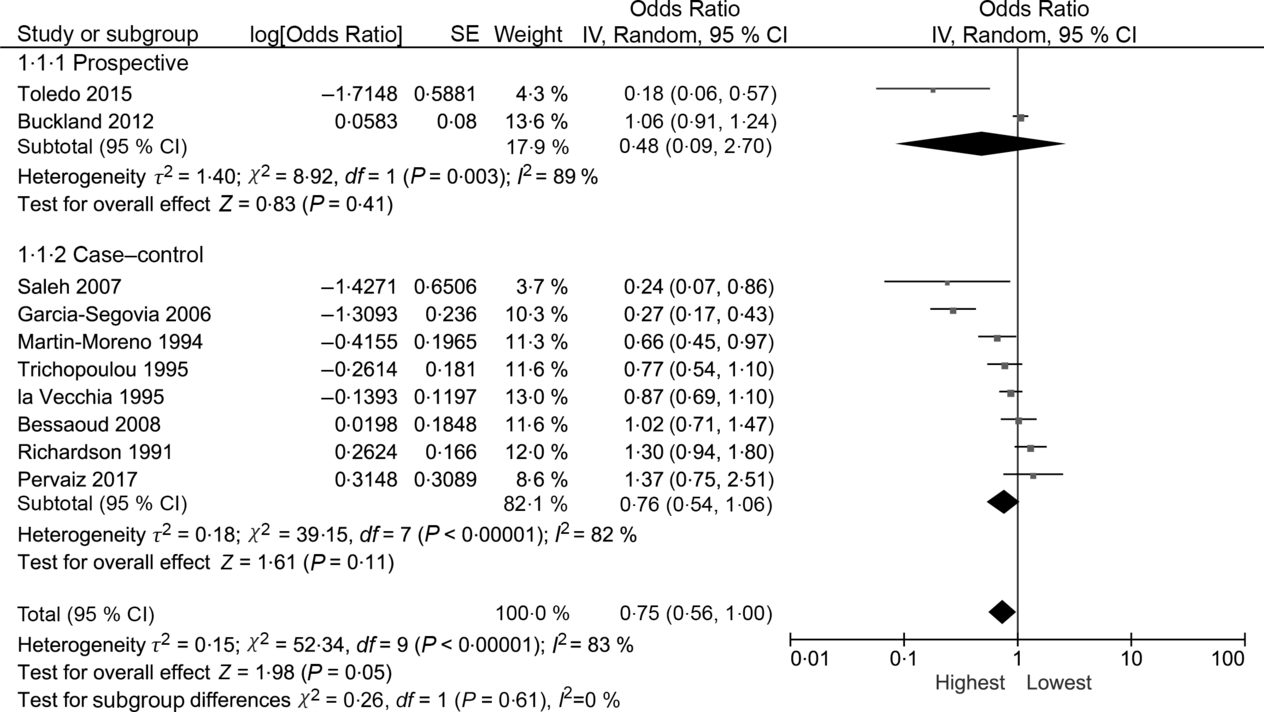
Fig. 2. Forest plot of studies examining the association between the highest v. lowest category of olive oil intake and breast cancer risk. IV, inverse variance.
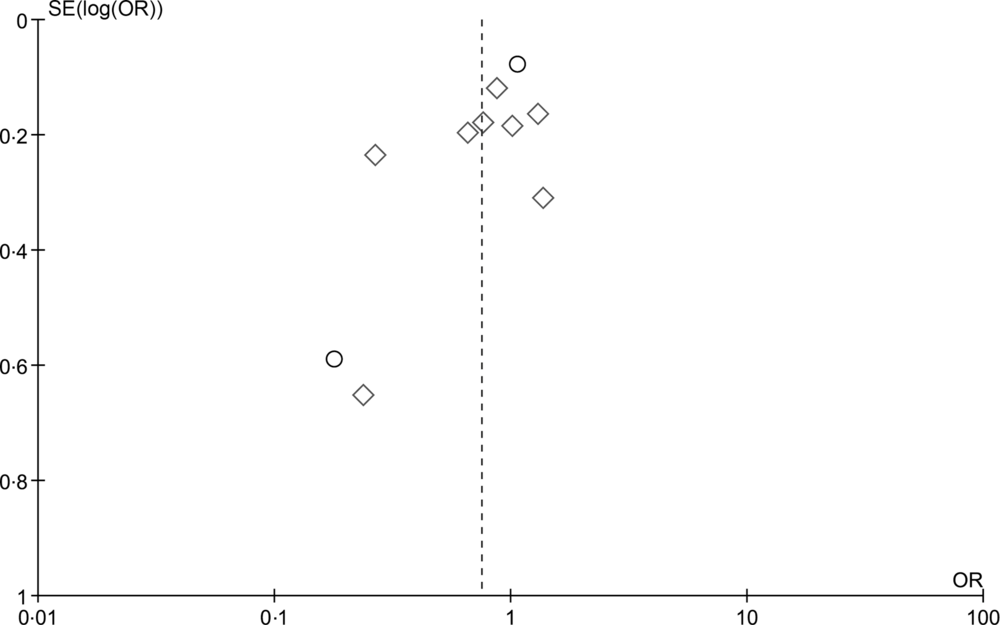
Fig. 3. Funnel plot for detection of publication bias for the highest v. lowest category of olive oil intake and breast cancer risk. ○, Prospective; ◊, case–control.
Table 2. Influence of individual studies removed one at a time on the summary estimate
(Odds ratios and 95 % confidence intervals)

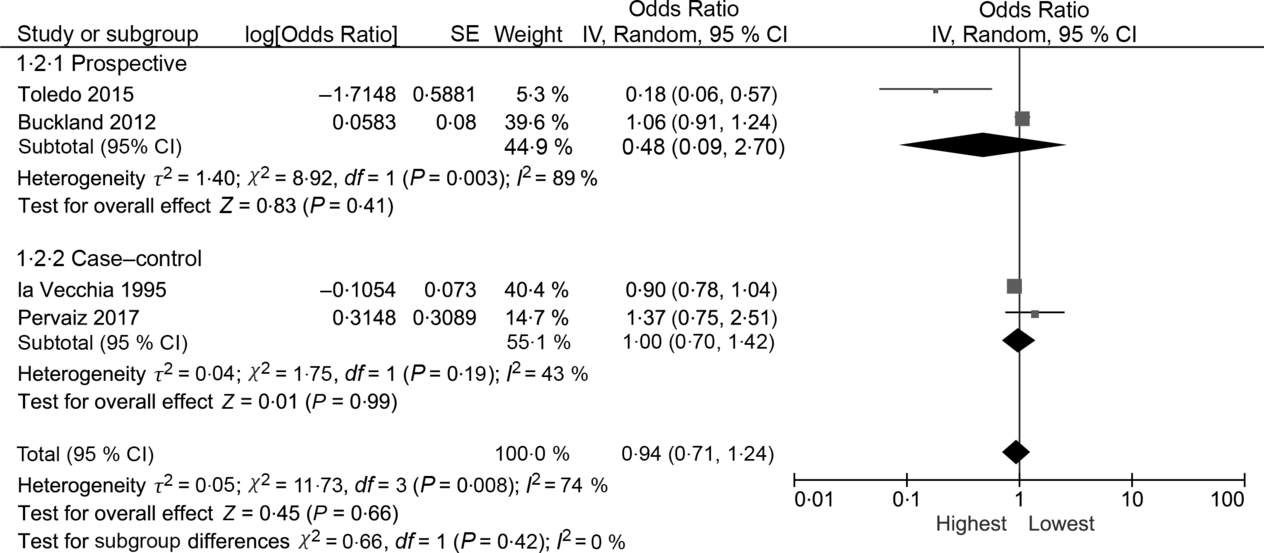
Fig. 4. Meta-analysis of the association between the highest v. lowest category of olive oil intake and breast cancer risk among postmenopausal women. IV, inverse variance.
Subgroup analyses
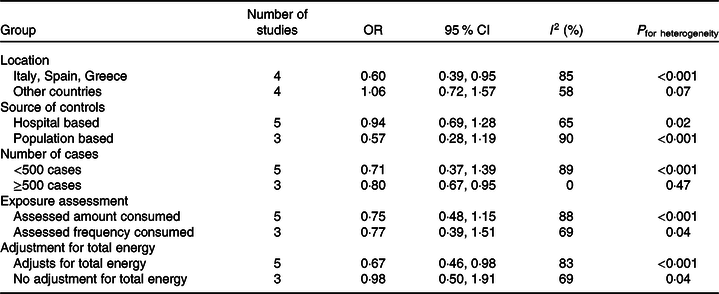
In subgroup analyses of case–control studies by study location, source of controls, exposure assessment, and adjustment for total energy (Table 3), heterogeneity remained moderate to high for all subgroups. Among case–controls studies with 500 or more cases there was low heterogeneity; however, there were only three studies in this subgroup. Significant inverse associations were seen among studies that were conducted in traditional Mediterranean countries (i.e. Italy, Spain and Greece), for studies with 500 or more cases, and for studies that adjusted for total energy intake. Estimates were similar to the overall estimate for case–control studies for both studies that assessed the amount consumed and those that only assessed frequency of olive oil consumption.
Table 3. Subgroup analyses for case–control studies of olive oil and breast cancer
Dose–response analyses
The OR for breast cancer in the dose–response meta-analysis with a 14 g/d increase in olive oil intake was 0·93 (95 % CI 0·83, 1·04). Since we made assumptions in the proportion of olive oil consumed (i.e. one time = 14 g) for studies that only reported frequency of consumption, when we restricted the analysis to exclude these studies the estimates were similar (OR 0·93; 95 % CI 0·83, 1·04). There was no evidence of non-linear associations in the cubic spline analyses (Fig. 5), P = 0·27 and P = 0·09, respectively.
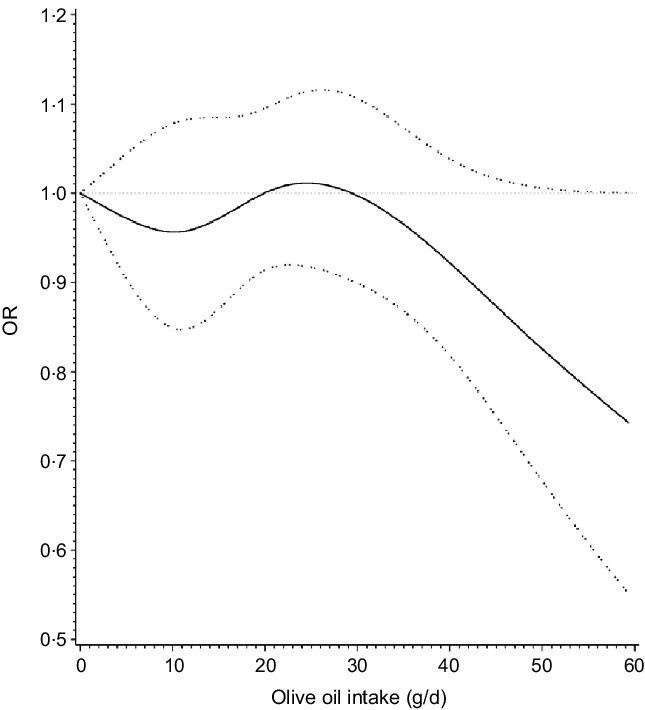
Fig. 5. Dose–response relationship between olive oil intake and breast cancer.
GRADE assessment
The overall quality and strength of the evidence were judged to be very low due to the limited number of studies and wide CI, some inconsistency and study heterogeneity, lack of dose–response association, high risk of bias in some of the included studies and possible publication bias.
Discussion
The present meta-analysis of ten studies, eight of which are case–control studies, including 81 436 individuals, examined the association between olive oil intake and breast cancer risk. Comparison of women in the highest category of olive oil intake compared with the lowest was suggestive of an inverse association with breast cancer, but not significant in either prospective or case–control analyses. There was no evidence of an association in dose–response analyses. Additionally, there was a limited number of studies and evidence of study heterogeneity and publication bias.
As there were only two prospective studies with substantial heterogeneity, the ability to draw conclusions from these is greatly limited. The prospective cohort that was larger but did not distinguish types of olive oil was null; whereas, the per protocol analysis of a smaller randomised trial that examined extra-virgin olive oil specifically multiple times during follow-up observed a significant strong inverse association.
When we use fixed effects models, the estimates were attenuated compared with the random effects models. One reason for this may be that the largest study included in the meta-analysis was null(Reference Buckland, Travier and Agudo34), which could then attenuate the results in fixed effect models due to the greater weight of large studies(Reference Greenland36). As we saw evidence of publication bias, a second possibility is that the random effect model may be more sensitive to the lack of published non-significant smaller studies, as a greater weight is applied to smaller studies in random effects models than in fixed effects(Reference Greenland36).
Our findings were similar to a meta-analysis by Psaltopoulou et al. (2011) that examined five olive oil and breast cancer studies conducted between 1990 and March 2011 and found that those with the highest category of olive oil consumption compared with the lowest was associated with lower odds of cancer (0·64; 95 % CI 0·46, 0·89)(Reference Psaltopoulou, Kosti and Haidopoulos14). A more recent meta-analysis (through December 2014) that examined all vegetable oils, not limited to olive oil, and breast cancer risk comparing highest v. lowest consumption (n 16 studies) finding an OR of 0·88 (95 % CI 0·77, 1·01), and a dose–response meta-analysis among six studies found per 10 g of vegetable oil/d (OR 0·98, 95 % CI 0·95, 1·01)(Reference Xin, Li and Sun15). In a subgroup analysis of olive oil (n 12 studies), the authors reported an OR of 0·74 (95 % CI 0·60, 0·92), suggesting that olive oil may be driving the overall association(Reference Escrich, Moral and Solanas12). Both previous meta-analyses included studies that included dietary patterns such as ‘olive oil and salad vegetables’ which may be one potential reason for the differences between our results. Additionally, due to differences in fatty acid profiles and phenolic compounds, the effects of olive oil on breast cancer risk may differ from other vegetable oils. As our results were non-significant, we cannot exclude the possibility that the significant inverse associations seen previously may be due to factors other than olive oil.
Olive oil did not appear to be associated with postmenopausal breast cancer in our analysis of studies that reported postmenopausal specific estimates. Of the two studies that stratified by menopause status(Reference Trichopoulou, Katsouyanni and Stuver29,Reference La Vecchia, Negri and Franceschi30) , Trichopolou et al. found a stronger association among postmenopausal (estimates not given)(Reference Trichopoulou, Katsouyanni and Stuver29), whereas La Vecchia et al. did not find that the association between olive oil and breast cancer varied by menopausal status(Reference La Vecchia, Negri and Franceschi30).
One of the included studies in the meta-analysis was the PREDIMED study, a randomised trial conducted in Spain of the Mediterranean diet, comparing (1) Mediterranean diet plus extra-virgin olive oil and (2) Mediterranean diet plus nuts to a low-fat diet (control group)(Reference Toledo, Salas-Salvadó and Donat-Vargas23). While limitations have been noted with the study’s original randomisation(Reference Estruch, Ros and Salas-Salvadó37), the estimates included in the meta-analysis were from a per protocol analysis v. the intention-to-treat analysis. The per protocol found similar directions of effects compared with the intention-to-treat analysis which found that the hazard was 0·38 (95 % CI 0·16, 0·87) for a Mediterranean diet plus extra virgin olive oil v. control, 0·62 (95 % CI 0·29, 1·36) for Mediterranean diet plus nuts v. control and 0·49 (95 % CI 0·25, 0·94) when both experimental arms (e.g. olive oil, nuts) were merged together v. control diet(Reference Toledo, Salas-Salvadó and Donat-Vargas23). When the analysis was done using the olive oil intention-to-treat findings v. per protocol, similar summary estimates were observed. While randomised trials are the gold standard in epidemiological research as they can eliminate the potential for confounding if done correctly, we are unable to fully distinguish whether the observed association is due to the Mediterranean diet or the olive oil. Given the Mediterranean olive oil arm had a significantly reduced risk and stronger magnitude than the nut arm, this suggests that olive oil is driving the association. The per protocol analysis, while better able to examined the effect of olive oil specifically, does not have the benefit of the randomisation. Though taken together, the results indicate that olive oil may reduce breast cancer risk.
The chemopreventive properties of olive oil have been hypothesised to be due to the antioxidant activity of its polyphenolic (e.g. hydroxytyrosol, tyrosol) and secoiridoid derivatives (e.g. oleuropein, oleocanthal)(Reference Gorzynik-Debicka, Przychodzen and Cappello10). However, polyphenol concentrations in olive oil vary widely due to agricultural factors, processing and extraction methods, and storage(Reference Gorzynik-Debicka, Przychodzen and Cappello10). Refined olive oil has a very low polyphenol concentration (approximately 0–5 mg/kg), followed by common olive oil (approximately 10–100 mg/kg), with extra virgin olive oil having the highest polyphenol content (approximately 15–400 mg/kg)(Reference Gorzynik-Debicka, Przychodzen and Cappello10). Except for the PREDIMED study, which provided extra-virgin olive oil to trial participants and saw the largest reduction in risk(Reference Toledo, Salas-Salvadó and Donat-Vargas23), others studies do not distinguish between types of olive oil. If the chemopreventive action is greater for, or limited to extra-virgin olive oil, potential associations may be diluted by inclusion of refined olive oil intake in the exposure assessment.
One strength of the meta-analysis is that we examined the effect of olive oil alone, rather than including dietary patterns including olive oil, where it is unclear whether the association is due to olive oil or some other food in the dietary pattern. Additionally, we were able to examine the potential for a dose–response. However, as the studies included in the meta-analysis were observational studies (or used per-protocol analyses) and primarily case–control studies, misclassification of olive oil intake and residual confounding are probably present. Further, small numbers of published papers, potential publication bias and considerable heterogeneity among all studies probably due to variation in study designs and study population characteristics were present and thus the estimates should be treated with caution. Heterogeneity still persisted even in the subgroup analyses, though the subgroups were limited by small number of studies in each sub-group. Only one study examined tumour subtypes, limiting the ability to examine oestrogen receptor status. Further, the subgroup analyses indicated that the estimates may not be generalisable to non-Mediterranean countries. Lastly, the study was not registered in International Prospective Register of Systematic Reviews; however, we did not find a relevant protocol on the topic in International Prospective Register of Systematic Reviews.
In summary, olive oil may reduce breast cancer risk; however, as the certainty level is very low, additional prospective studies are needed before public health recommendations can be made. In addition to additional large prospective studies, more detailed exposure assessment information is needed, such as type of olive oil and cooking method, or biomarkers of olive oil intake.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conception and design: S. C. H., S. E. H.; Analysis and interpretation of the data: N. S., S. C. H., S. E. H.; Writing, review and/or revision of the manuscript: N. S., S. C. H., S. E. H.
There are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003499