Human milk seems to have numerous benefits for infants including a reduced risk for neonatal inflammatory diseases (necrotising enterocolitis and allergic colitis) or respiratory infectionsReference Schack-Nielsen and Michaelsen1–Reference Schanler and Atkinson3. Although the components that cause these effects are not known yet human milk oligosaccharides (HMO) belong to potential candidates.
Oligosaccharides from human milk have been discussed to be growth promoting factors for the bifidobacteria dominated flora in the gut of breastfed infantsReference Kunz and Rudloff4. In addition, these carbohydrates may have a more specific effect on the colonisation of the gut by acting as soluble analogues to epithelial receptors for specific microbes and may thus prevent their adhesion to the intestinal wallReference Sharon and Ofek5. As the human gut hosts a diverse collection of microorganisms the host microbe interactions are currently an important research topicReference Fava, Lovegrade, Gitau, Jackson and Tuohy6. To better understand the interactions of HMO with various micro-organisms it is certainly necessary to characterise the specific components and to investigate the underlying mechanisms. Also, it has recently been shown that HMO inhibit leucocyte adhesion to activated endothelial cells and may influence neutrophil platelet interactionsReference Bode, Rudloff, Kunz, Strobel and Klein7, Reference Bode, Kunz, Muhly-Reinholz, Meyer, Seeger and Rudloff8.
Regarding the biosynthesis of HMO the human mammary gland is thought to be the only organ where lactose and lactose-derived oligosaccharides are synthesised. A β1·4-galactosyltransferase as housekeeping enzyme links galactose to glucose instead of N-acetyl glucosamine to form lactose which is then used for the assembly of HMOReference Brew and Hill9, Reference Charron, Shaper and Shaper10.
The amount of HMO depends on the lactational stage of the women; it is highest in colostrum (days 1–4 post-partum), but even mature milk (>day 10 postpartum) contains oligosaccharides in concentrations up to 15 g/l or moreReference Stahl, Thurl, Zeng, Karas, Hillenkamp, Steup and Sawatzki11, Reference Kunz, Rudloff, Schad and Braun12. There is also an individual variation of HMO due to the specificity of the mother's blood group and secretor statusReference Kunz and Rudloff13. In the past more than 150 HMO have been characterised which can be separated into a neutral and an acidic HMO fraction depending on the presence of one or more sialic acid (N-acetylneuraminic acid) residues.
Assuming an average milk intake of 1 litre/d, breastfed infants receive about 15 g HMO per day or more. As these components are considered to be indigestible in the infant's gastrointestinal tract, the intestine of the breastfed infant is continuously exposed to very high concentrations of HMOReference Gnoth, Kunz, Kinne-Saffran and Rudloff14–Reference Gnoth, Rudloff, Kunz and Kinne17. Due to their unique structures, milk oligosaccharides do have the potential to modulate intestinal development and functionsReference Kunz and Rudloff13, Reference Bode18–Reference Takeda, Sakata, Minekawa, Yamamoto, Hayashi, Tasaka and Murata21. The gastrointestinal tract of the newborn undergoes maturational changes in the early postnatal period to develop its full function for digestion and absorption or as a barrier and as an immunological active organ. Renewal of the intestinal villi occurs through a highly regulated process of proliferation, differentiation and apoptosisReference Sreedharan and Mehta22–Reference Studzinski and Harrison24. Human milk has been found to stimulate gastrointestinal mucosal proliferation and maturation in animal models and is thought to protect the infant from harmful environmental factors by affecting the mucosal barrierReference Ichiba, Kusuda, Itagane, Fujita and Issiki20, Reference Takeda, Sakata, Minekawa, Yamamoto, Hayashi, Tasaka and Murata21, Reference Walker25, Reference Wagner, Forsythe and Wagner26. So far, no reports focusing on the growth-related effects of HMO fractions or individual oligosaccharides within the human gastrointestinal tract have been published.
For studying the effect of oligosaccharides on intestinal growth-related events we used three epithelial cell lines: human intestinal epithelial cells (HIEC), HT-29 and Caco-2 which vary in their degree of differentiation (Fig. 1). The HIEC cell line was generated from normal human fetal intestine. The morphological und functional characterisation of HIEC cells provided evidence that they are comparable with undifferentiated cells of the cryptsReference Wagner, Forsythe and Wagner26. They express a number of crypt cell but no villus cell markers and appear to be unable to differentiate when reaching confluenceReference Desloges, Basora, Perreault, Bouatrouss, Sheppard and Beaulieu27, Reference Basora, Vachon, Herring-Gillam, Perreault and Beaulieu28. Thus, they are considered to be intestinal stem-like cellsReference Escaffit, Perreault and Jean29. HT-29 cells also represent a very low differentiated phenotype such as normal crypt colonocytes. Similar to HIEC cells they are unable to differentiate after reaching confluence. Caco-2 cells are derived from a well characterised cell line used as a model of intestinal epithelial cells. Under pre-confluent conditions, the cells reveal a moderate differentiation status; however, upon reaching confluence they spontaneously undergo a gradual villus-like enterocytic differentiation process similar to that observed in the epithelium of the intact fetal small and large bowel. Under culture conditions, cells grow as a monolayer and show typical cylindrical polarised morphology with apical microvilli and brush-border associated enzymes dependent on the degree of differentiationReference Sambuy, De Angelis, Ranaldi, Scarino, Stammati and Zucco30, Reference Tremblay, Auclair, Delvin, Levy, Ménard, Pshezhetsky, Rivard, Seidman, Sinnett, Vachon and Beaulieu31. Thus, under the conditions used in our experiments pre-confluent Caco-2 as well as HT-29 and HIEC cells comprise the proliferation compartment of the crypt/villus axis simulating the situation in the fetal and neonatal intestine. As is well known that nutritional factors are able to induce differentiation under such pre-confluent conditions by for example, modifying cell culture medium or nutrient supplyReference Graz and Cowley32, Reference Thomson, Doring, Keelan and Amstrong33. our objective was to investigate whether cell dynamics in these cell lines are affected by milk oligosaccharides.
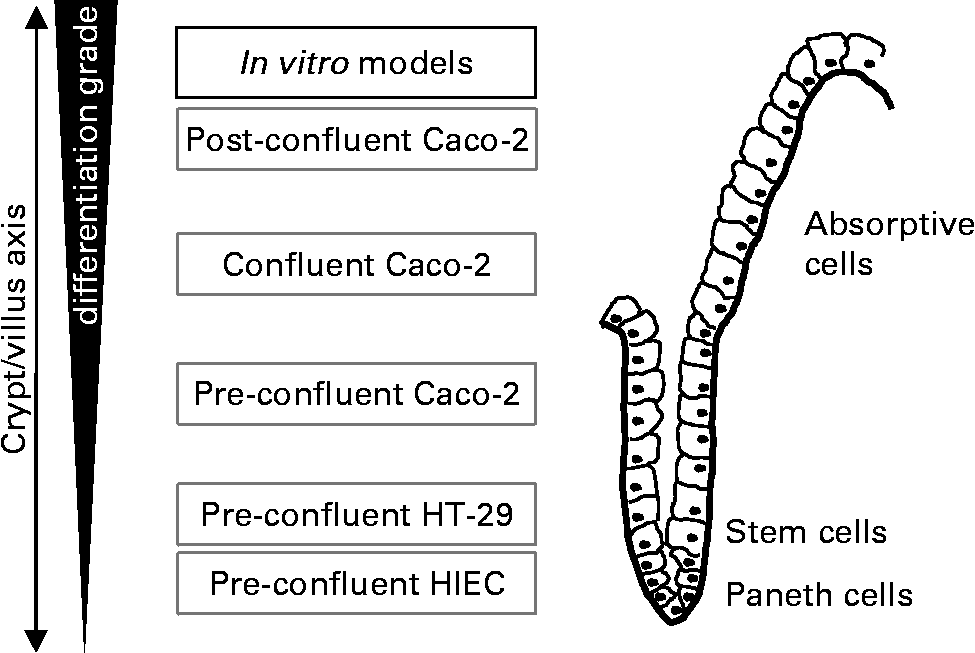
Fig. 1 Experimental in vitro model of proliferating cells which vary in their stage of differentiation. Alkaline Phosphatase activity in unstimulated pre-confluent control cells was used as a marker of differentiation. AP activity was 0·193 ± 0·023/h/106 cells in HT-29, 0·185 ± 0·005/h/106 cells in HIEC and 0·609 ± 0·013/h/106 cells in Caco-2.
Experimental methods
Isolation of human oligosaccharides from human milk and human milk oligosaccharide preparation
Oligosaccharides were isolated from human milk as described previouslyReference Kunz, Rudloff, Hintelmann, Pohlentz and Egge34, Reference Rudloff, Obermeier, Borsch, Pohlentz, Hartmann, Brösicke, Lentze and Kunz35. Briefly, after centrifugation, the lipid layer was removed, and proteins were precipitated from the aqueous phase using ice-cold ethanol. Lactose was removed by gel filtration on Sephadex G-25 (Pharmacia Biotech, Uppsala, Sweden). The total oligosaccharide fraction was further separated into an acidic fraction (aHMO) and a neutral fraction (nHMO) using HPLC anion-exchange chromatography on a Resource Q column (Pharmacia Biotech).
Oligosaccharide analysis
Oligosaccharide analysis was performed by high pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a CarboPac PA1 column (Dionex, Sunnyvale, CA, USA) using the conditions described previouslyReference Bode, Kunz, Muhly-Reinholz, Meyer, Seeger and Rudloff8.
Oligosaccharide standards
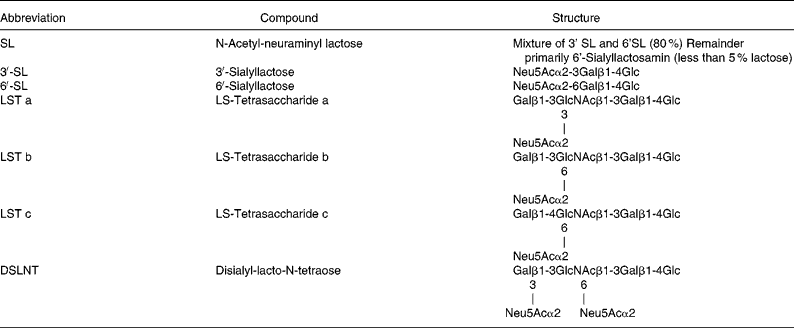
Oligosaccharide standards shown in Tables 1 and 2 were obtained from Dextra Laboratories (Reading, Berks, UK).
Table 1 Structures of selected neutral oligosaccharides for cell culture studies

Table 2 Structures of selected acidic oligosaccharides for cell culture studies
Oligosaccharide concentrations used for cell culture studies
Neutral and acidic HMO fractions for cell culture studies were used at 5, 7·5 and 15 mg/ml for differentiation and apoptosis, whereas the dose dependent effect on proliferation has been investigated with concentrations between 0 to 15 mg/ml; individual oligosaccharides have been tested at 1 mg/ml.
Cell culture
To investigate effector-mediated growth and differentiation events, HIEC, HT-29 and Caco-2 cells were used in the exponential growth phase under pre-confluence conditionsReference Tremblay, Auclair, Delvin, Levy, Ménard, Pshezhetsky, Rivard, Seidman, Sinnett, Vachon and Beaulieu31, Reference Pageot, Perreault, Basora, Francoeur, Magny and Beaulieu36.
HT-29 cells
The human colon carcinoma cell line HT-29 was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and was used between passages 40 and 50Reference Fogh, Trempe and Fogh37.
Caco-2 cells
Caco-2 cells were a gift from Professor R. K. H. Kinne at the Max-Planck-Institute of Molecular Physiology (Dortmund, Germany). They were used between passages 35 and 50.
HIEC cells
The human small intestinal epithelial crypt cells of fetal origin (HIEC) were generously donated by J.F. Beaulieu (Department of Anatomy and Cell Biology, Faculty of Medicine, Université de Sherbrooke, Sherbrooke, Quebec, Canada). HIEC cells were used between passages 15 and 20Reference Perreault and Beaulieu38.
All cell lines were cultured in 75 cm2 tissue culture flasks (Renner, Dannstadt, Germany) in RPMI 1640 (Roswell Park Memorial Institute media) for HT-29 and HIEC cells or Dulbecco's Modified Eagle Medium (Caco-2) supplemented with 10 % fetal calf serum and 2 mm glutamine (Invitrogen, Karlsruhe, Germany). The cultures were maintained in a humidified atmosphere of 5 % CO2 at 37°C. Cells were passaged at pre-confluent densities by using 0·05 % trypsin and 0·5 mm-EDTA (Invitrogen, Karlsruhe, Germany).
Measurement of cell proliferation with -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay
Proliferation of cells was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay. Regarding the different duplication times of the cell lines the initial seeding density of Caco-2 and HT-29 were 1500 and 2500 cells per well for HIEC on ???96-well plates. After initial seeding they were grown in media with supplements and allowed to adhere for 24 h. Thereafter, medium was replaced and cells were exposed to variable concentrations of oligosaccharides dissolved in cell culture medium (pH 7·4) and cells were allowed to grow for another 72 h. Relative cell numbers were determined by incubating the cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (Calbiochem, Bad Soden, Germany) for 3 h. The resulting intracellular purple formazan was solubilised in a solution of 10 % Na dodecyl sulfate and 0·1 M HCl and quantified by spectrophotometry (absorbance was measured at wavelength of 550 nm) after 5 h using an ELISA microplate reader (Asys, Eugendorf, Austria). Cell numbers were determined based on a calibration curve using cell counts between 500 and 30 000.
Determination of alkaline phosphatase activity as a marker of differentiation
After having reached 30–40 % confluency on 25 cm2-culture flasks (Renner, Dannstadt, Germany), cells were incubated for 72 h in the presence or absence (control) of oligosaccharides (pH 7·4). Cell numbers were determined after cells had been washed twice with PBS and trypsinised; harvested cells were pelleted at 1 500 g for 10 min. Pellets were resuspended in 1 m diethanolamine buffer with 0·5 mm MgCl2 (pH 9·8) and homogenised. The homogenate was mixed with 10 μm p-nitrophenyl phosphate (Calbiochem, Bad Soden, Germany) in diethanolamine buffer and alkaline phosphatase activity as a marker for differentiation was determined by the release of p-nitrophenol using the microplate reader (Asys, Eugendorf, Austria) at a wavelength of 405 nm. Alkaline phosphatase activity was measured as ΔE/h/106 cells; the control was set to 100 %.
Determination of caspase-3-like activity as an early marker of apoptosis
Caspase-3-like activity was assessed as describedReference Kuntz, Wenzel and Daniel39–Reference Nicholson, Ali and Thornberry41 and served as an early apoptosis marker. Briefly, all cell lines were seeded at a density of 50 000 cells per well on 6-well plates and allowed to adhere for 24 h. Cells were then incubated for 24 h in the absence (control) or presence of oligosaccharides. Subsequently, cells were trypsinised, cell numbers were determined and then the cells were centrifuged at 400 g for 3 min. Cytosolic extracts were prepared according to Nicholson et al. Reference Nicholson, Ali and Thornberry41 and finally the cytosolic supernatant fraction was incubated with the colorimetric caspase-3 tetrapeptide-substrate N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Calbiochem, Bad Soden, Germany) at a final concentration of 20 μm. Cleavage of the caspase-3 substrate was determined by using the microplate reader (Asys, Eugendorf Austria) at a wavelength of 405 nm; the apopain activity was measured as ΔE/h/106 cells and the control was set to 100 %.
Statistical analysis
We applied a nonlinear approximation model, using the least square method, to derive the ???IC50 values for growth inhibition; ???IC50 was defined as the concentration by which the cell growth is reduced by half times in comparison to the end point. This model was based on a competition curve using 1 component. Data were evaluated by one-way ANOVA and statistical differences were tested by Bonferroni's post hoc test. For each variable at least three independent experiments (three replications) were carried out and the results were expressed as mean values with their standard errors (sem). All analyses were carried out with the GraphPad Software Prism 3 (San Diego, CA, USA); differences were considered significant at *P < 0·05 and **P < 0·01.
Results
Fractions of neutral and acidic oligosaccharides were isolated from human milk and further separated by HPAEC-PAD as shown in Fig. 2. Based on the quantitative abundance of specific compounds after HPAEC-PAD oligosaccharide standards were selected (Tables 1 and 2) to determine their effects on intestinal cell proliferation, differentiation and apoptosis.

Fig. 2 HPAEC-PAD of oligosaccharide fractions separated by HPLC anion exchange. Oligosaccharides were prepared according to the procedure described in material and methods. The chromatogram represents the separation of the neutral and the acidic HMO fraction. Individual components have been identified by standard components (for abbreviations see TABLE 1 and 2).
Influence of oligosaccharide fractions and isolated compounds on proliferation
Neutral and acidic oligosaccharide fractions exerted a pronounced effect on intestinal cell proliferation in all cell lines in a dose-dependent manner after 72 h of exposure (Figs. 3 (A) and (B)). Compared with control experiments, neutral oligosaccharides reduced proliferation rates with IC50 values of 2·33 (sem 0·01) mg/ml, 0·34 (sem 0·01) mg/ml and 0·20 (sem 0·00) mg/ml in HT-29, Caco-2 and HIEC cells, respectively. When the cells were exposed to the highest neutral HMO concentration (15 mg/ml), the potency of growth inhibition was reduced to 41·73 (sem 8·18) % in HT-29, to 62·49 (sem 3·54) % in Caco-2 and to 77·32 (sem 4·03) % in HIEC cells. In the case of acidic HMO fractions a similar dose-dependent effect was observed. The IC50 values for acidic HMO varied between the intestinal cell lines, i.e. cell growth was inhibited at a IC50 values of 0·29 (sem 0·01) mg/ml, 0·71 (sem 0·01) mg/ml and 1·62 (sem 0·02) mg/ml for HT-29, Caco-2 and HIEC cells, respectively. At the highest concentration of acidic HMO (15 mg/ml), cell growth was reduced to 31·56 (sem 4·62) % for HT-29, to 53·80 (sem 8·38) % for Caco-2 and to 65·68 (sem 3·98) % for HIEC cells with differences in potency. These growth inhibition effects were not due to cytotoxic side-effects, because nonviable cells, as determined by trypan blue staining, were counted on a haemocytometer after 4 h of incubation and the percentage of nonviable cells in all groups was below < 5 % of controls.
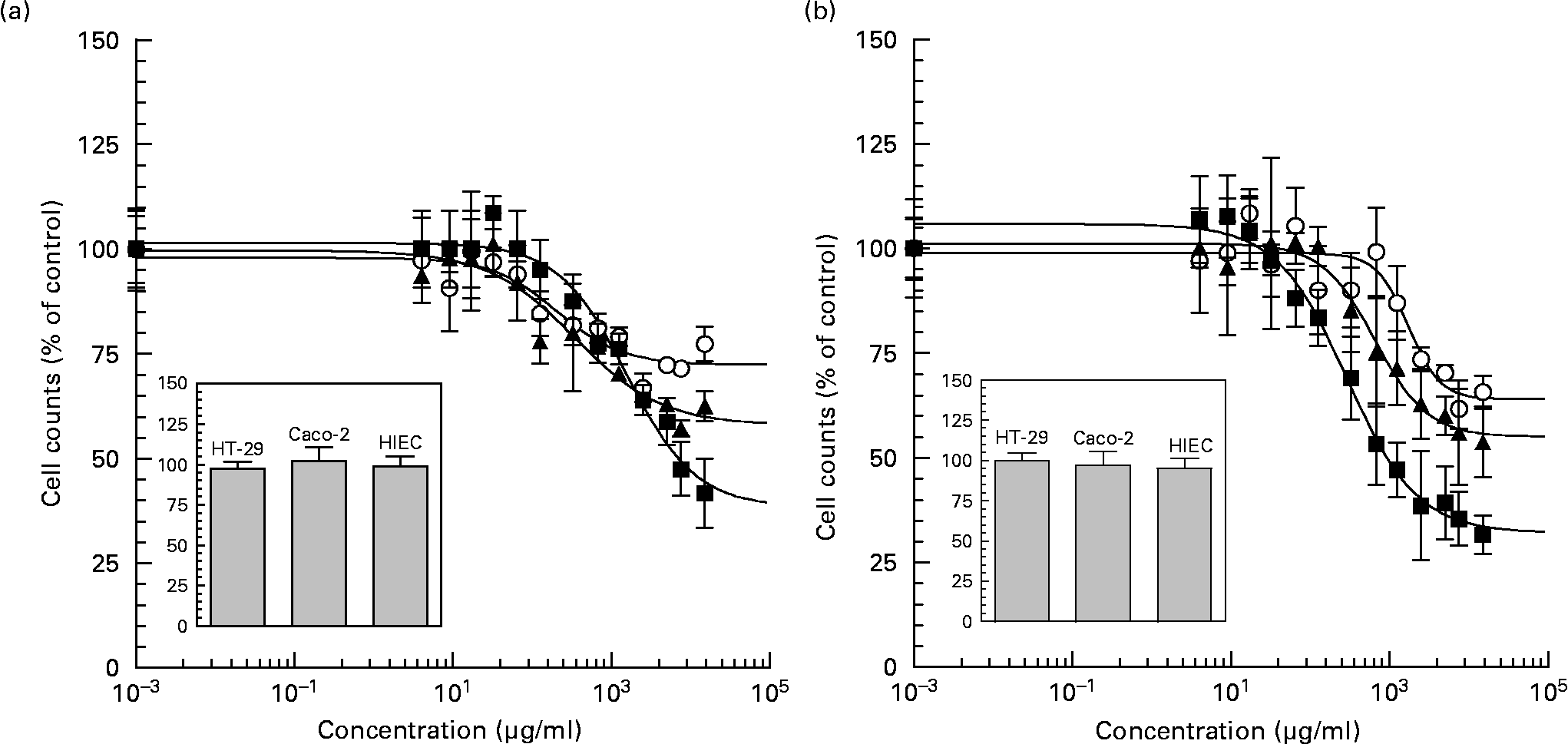
Fig. 3 Dose dependent inhibition effects of neutral (a) and acidic (b) oligosaccharide fractions on proliferation of cultured HT-29 (■), Caco-2 (▲) and HIEC (○) cells. HT-29, Caco-2 (1,500 per well) and HIEC (2,500 per well) cells were incubated 24 h to allow the cells time to attach. The cells were then left untreated (control) or treated with concentrations between 0-15 mg/ml of neutral and acidic HMO fractions for 72 h. After 72 h incubation the proliferation was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay. The concentration that induced a 50 % inhibition of cell proliferation compared with controls was calculated with a non-linear approximation model, using the least square method to derive the IC50 values for growth inhibition. FIG. 3 (inset). Cytotoxicity was measured after 4 h of incubation with 15 mg/ml oligosaccharides using the trypan blue exclusion test. Results were expressed as % of control (untreated) and each value represents the mean ± SEM from three independent experiments.
In order to identify responsible components for the observed growth inhibition, we selected neutral and acidic oligosaccharides occurring in human milk (Fig. 2;Tables 1 and 2) and used those as standards although in a much lower concentration in the same proliferation assay as has been described for oligosaccharide fractions. The growth inhibition at 1 mg/ml is shown in Table 3. Growth of HT-29 cells was inhibited by all neutral and acidic oligosaccharides except for the two fucosyllactoses. LNDFH II, revealing the most pronounced effect in all cell lines, reduced the cell growth to 28·82 (sem 8·55) % for HT-29 and to 63·72 (sem 5·09) % for Caco-2 cells; the effects in HIEC cells were less pronounced. Also, LNDFH I was able to reduce HT-29 and Caco-2 cell growth, but no significant effects have been observed in HIEC cells. In case of LNFP I and II only HT-29 and Caco-2 cell growth was influenced; again, HIEC cells remained unaffected. For LNT and LNH, an inhibition of cell growth was observed only in HT-29 cells.
Table 3 Effects of selected neutral and acidic oligosaccharides on proliferation of HT-29, Caco-2 and HIEC cells1, 2, 3
(Mean values with their standard errors)

1 Each value represents the mean ± SEM from three independent experiments (n 3),
2 The growth inhibition at 1 mg/ml is giving as % of control,
3 Means differ from control (*P < 0·05 and **P < 0·01).
For abbreviations see Table 1 and 2.
With regard to growth inhibition, single acidic compounds also seem to be very efficacious and similar to the observed effect of most of the neutral oligosaccharide standards. All acidic compounds tested reduced the proliferation of HT-29 cells with 3′-SL, LST′a and DSLNT showing the highest impact. Also, HIEC cell proliferation was influenced by acidic compounds with the largest effects observed for LST a, LST b and DSLNT (**P < 0·01) followed by 3′-SL, 6′-SL and LST c (*P < 0·05). In contrast, the growth of Caco-2 cells was only influenced by DSLNT and 6′SL.
Influence of oligosaccharide fractions and isolated compounds on alkaline phosphatase activity as a marker of differentiation
Incubation of intestinal cells with neutral HMO fractions increased AP activity statistically significant only at the highest concentration tested (15 mg/ml) in HT-29 and HIEC cells although a tendency towards increased ???AP activity can already be observed at 7·5 mg/ml (Fig. 4 (A)). The induction of AP by neutral HMO at 15 mg/ml was 121·26 (sem 5·48) % for HT-29 and 119·32 (sem 5·50) % for HIEC cells compared with controls (non-supplemented cells). In contrast, acidic oligosaccharides were able to stimulate differentiation statistically significant already at 7·5 mg/ml and reached a maximum of 145·53 (sem 3·71) % in HT-29 cells and 171·00 (sem 7·78) % in HIEC cells at 15 mg/ml (Fig. 4 (B)). In Caco-2 cells, neither neutral nor acidic oligosaccharides fractions were able to induce differentiation.
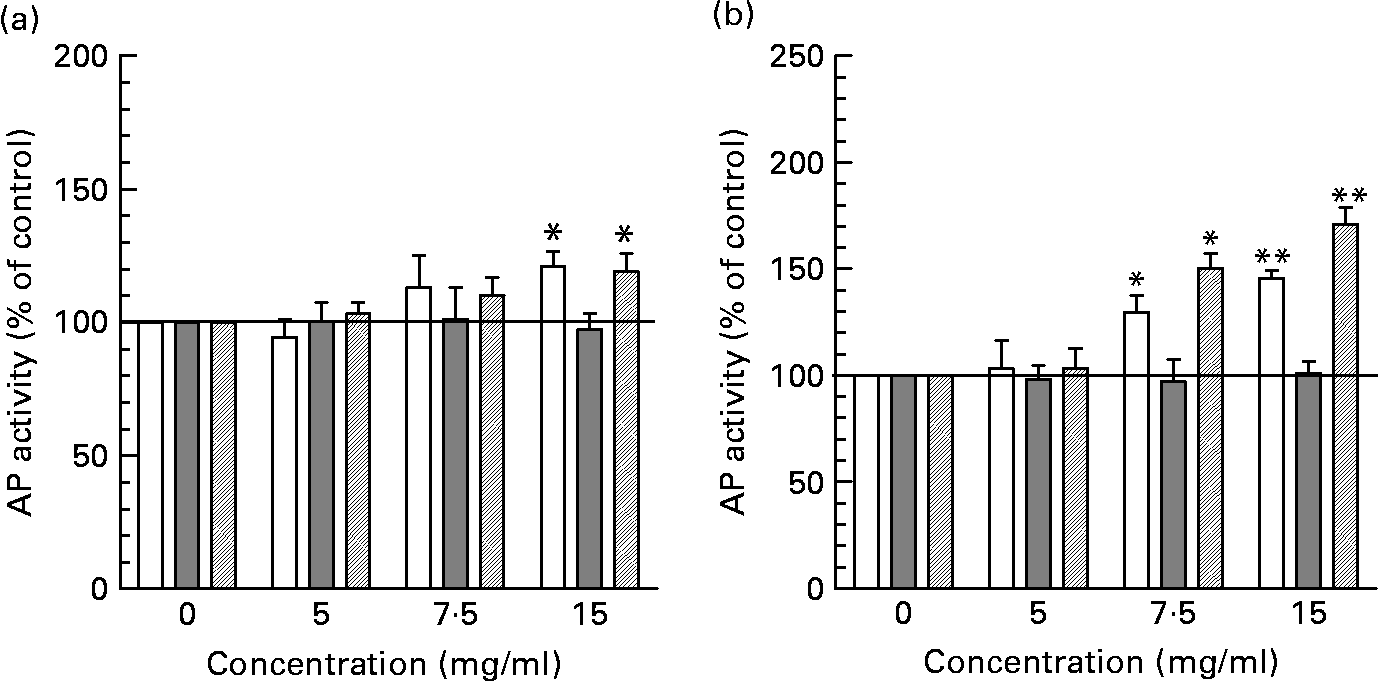
Fig. 4 Determination of differentiation in intestinal cells after incubation with neutral (a) and acidic (b) HMO fractions. Alkaline phosphatase (AP) activity of intestinal HT-29, Caco-2 and HIEC cells exposed to 0, 5, 7·5 and 15 mg/ml neutral or acidic HMO was determined after 72 h of incubation as indicated in materials and methods. AP activity in control cells was 0·193 ± 0·023/h/106 cells (HT-29), 0·185 ± 0·005/h/106 cells (HIEC) and 0·609 ± 0·013/h/106 cells (Caco-2) and was set to 100 %. Values (% of control) are given as mean ± SEM (n 3; *P < 0·05 and **P < 0·01).
Under the same experimental conditions, individual neutral oligosaccharides were tested at a concentration of 1 mg/ml (Table 4). Only LNDFH II was able to induce AP activity in HT-29 cells, whereas all other standards were ineffective in the three cell lines. Among acidic oligosaccharides, sialyllactoses (3′-SL, 6′-SL or a mixture of both) exerted a moderate but significant AP stimulating effect in HT-29 cells and on HIEC cells, but not on Caco-2 cells.
Table 4 Effects of selected neutral and acidic oligosaccharides (1 mg/ml) on differentiation of HT-29, Caco-2 and HIEC cells1, 2, 3
(Mean values with their standard errors)

1 Each value represents the mean ± SEM from three independent experiments (n 3).
2 The growth inhibition is given as % of control.
3 Means differ from control (*P < 0·05 and **P < 0·01).
Concentrations used were the same as in Table 3.
For abbreviations see Table 1 and 2.
Effect of human milk oligosaccharides on early marker of apoptosis (caspase-3 like activity)
As shown in Fig. 5, only HT-29 cells and HIEC but not Caco-2 cells were sensitive to neutral HMO at the highest concentration of 15 mg/ml. The induction of apoptosis was almost 3-fold in HT-29 cells and about 2-fold in HIEC cells. In contrast, the treatment with acidic HMO fractions did not change caspase-3 activity in comparison to control cells (data not shown). Also, when screening for individual compounds none of the oligosaccharide standards showed an effect on caspase-3 (data not shown).
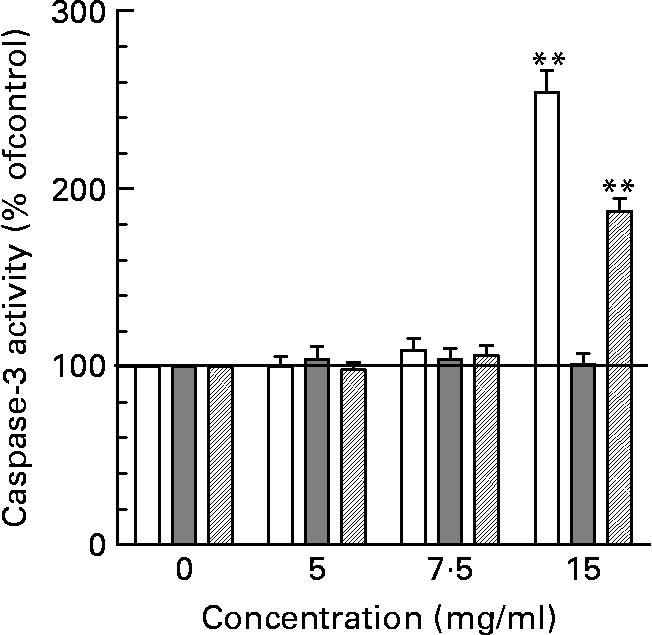
Fig. 5 Apoptotic effects of oligosaccharides in intestinal cells. Intestinal cells were cultured with 0, 5, 7 and 15 mg/ml of neutral HMO over 24 h and the degree of treatment-induced apoptosis was assessed using the caspase-3 assay as described in the materials and methods. Apopain activity in control cells was 0·056 ± 0·005/h/106 cells (HT-29), 0·046 ± 0,004/h/106 cells (HIE) and 0·049 ± 0·007/h/106 cells (Caco-2) and was set to 100 %. Data points are given as mean ± SEM of three independent experiments performed in triplicates (**P < 0·01).
Discussion
As gut maturation occurs during the last trimester of intra-uterine life and continues after birth, the first weeks of the postnatal period are of extraordinary interest with regard to intestinal developmentReference Sreedharan and Mehta22–Reference Walker25. Morphogenic changes are directly related to physiologic functions of the gut and are influenced by various dietary factorsReference Donovan19, Reference Takeda, Sakata, Minekawa, Yamamoto, Hayashi, Tasaka and Murata21. The aim of the present study was to investigate a possible direct impact of HMO on the growth of intestinal cells by using different cell lines representing different stages of intestinal cell development (Fig. 1), all of which are well established as in vitro models.
We demonstrated a concentration-dependent inhibition of growth of neutral and acidic HMO fractions as well as of some single oligosaccharides present in human milk in HT-29, Caco-2 and HIEC cells. However, the effects varied between cell lines. HT-29 and Caco-2 cells were more sensitive to growth inhibition than HIEC cells (Fig. 3).
This difference was observed at all concentrations tested with a threshold at about 7·5 mg/ml but was mostly pronounced at the highest concentration (15 mg/ml).
At the highest level, we also observed a stimulatory effect of both neutral and acidic HMO fractions on cell differentiation in HT-29 and HIEC cells but not in Caco-2 cells (Fig. 4). AP activity as a marker of cell differentiation was significantly enhanced in HT-29 and HIEC cells after they had been exposed to 7·5 or 15 mg/ml acidic HMO. A similar tendency for both concentrations has been observed for neutral HMO fractions, although statistically significant only at 15 mg/ml.
Thus, the reason for the higher sensitivity of HT-29 to growth inhibition compared with HIEC cells could be that HIEC cells are more susceptible to an induction of differentiation. In the case of Caco-2 cells, the failure to enhance differentiation was expected since these cells already represent a more differentiated phenotype. To confirm the postulated differentiation-dependent effect we compared the basal AP activity of the different cell lines and determined activities of 0·193 (sem 0·023), 0·185 (sem 0·005) and 0·609 (sem 0·013) ΔE /h/106 cells for HT-29, HIEC and Caco-2, respectively. These differences in the cell differentiation phenotype support our hypothesis and were also observed by othersReference Comalada, Bailon, de Haro, Lara-Villoslada, Xaus, Zarzuelo and Galvez42–Reference Gamet, Daviaud, Denis-Pouxviel, Remesy and Murat45. The influence of acidic oligosaccharide fractions on differentiation was confirmed by individual acidic oligosaccharides. The strongest effect was found for 3′- and 6′-sialyllactose. As sialyllactose is the major acidic oligosaccharide in human and bovine milk considerations of supplementing infant formula with such components is supported by this observationReference Wang, Bing and Karim46.
With regard to apoptosis, it was shown that all cell lines were non-susceptible to acidic HMO fractions. This observation, i.e. a failure of apoptosis induction, may be explained by the more differentiated phenotype (Caco-2) or the induction of differentiation (HT-29 and HIEC cells) by acidic oligosaccharides. Neutral HMO, however, were able to induce a marked increase of caspase-3 activity in HT-29 and HIEC cells supporting the hypothesis of differentiation-associated processes (Fig. 5); again, no effect was seen in Caco-2 cells.
The limited capacity to influence apoptosis or differentiation processes in Caco-2 cells could be due to the different interaction of oligosaccharides with for example, cell surface receptors and, hence, the generation of a different intracellular signalling response. We assume that the interaction of oligosaccharides with cell surface receptors is differentially regulated in the three cell lines. As the involvement of oligosaccharides in growth factor signaling is well knownReference Cai, He, Zhu, Lu and Vlassara47 our data support a link between oligosaccharide cell surface interaction and growth related effects. Furthermore, a cell surface interaction could induce differences in cell signalling. Mediators of cell cycle events such as cylins, cyclin-dependent kinases (CDK) and their counterparts, the CDK-inhibitors, participate in these processes. When interacting with growth factor receptors it is conceivable that oligosaccharide-mediated activation of CDK-inhibitors and G2-M cell cycle arrest is required for the induction of differentiation and apoptosis in these intestinal cellsReference Tremblay, Auclair, Delvin, Levy, Ménard, Pshezhetsky, Rivard, Seidman, Sinnett, Vachon and Beaulieu31, Reference Dydensborg, Herring, Auclair, Tremblay and Beaulieu48.
When differentiated cells like Caco-2 cells express lower levels of cyclins or CDK they should be less responsive to apoptosis induced by oligosaccharides compared with HT-29 and HIEC cells expressing higher levels of such cell cycles regulators. This observation is an accepted mechanism comparing cancer and non-cancer cellsReference Gomez, de Las Pozas, Reiner, Burnstein and Perez-Stable49–Reference Benitez, Pozo-Guisado, Alvarez-Barrientos, Fernandez-Salguero and Castellón51.
The specific differences found between the less differentiated HT-29 and HIEC and the more differentiated Caco-2 cells suggest that oligosaccharides act through different mechanisms possibly depending on upon their differentiation phenotypeReference Hinnebusch, Ma, Henderson, Siddique, Archer and Hodin52, Reference Pshezhetsky, Fedjaev and Ashmarina53.
To identify individual components for the observed growth-related effects of neutral and acidic HMO, the same experiments were repeated with standard oligosaccharides. The use of individual components, however, did not completely confirm the observed effects of neutral and acidic oligosaccharide fractions. In the case of HT-29, all tested compounds, except for 2′FL and 3-FL, led to a dose-dependent growth inhibition but without distinguishable structure-function relationship. Also, several acidic oligosaccharides inhibited the growth of HIEC and Caco-2 cells, whereas among neutral oligosaccharides only LNDFH II had an effect.
While the inhibition of growth can in part be attributed to several selected substances, this was not possible for differentiation and apoptosis. In HT-29, the use of LNDFH II as neutral oligosaccharide induced cell differentiation, but neither Caco-2 nor HIEC differentiation was influenced by any of the tested neutral oligosaccharides. The enhancement of caspase-3 activity in HT-29 and HIEC cells by neutral HMO (Fig. 5) was not influenced by the selected single compounds at indicated concentration. As there are many different oligosaccharides in human milk, it is easily conceivable that other components not tested in the present study might be responsible for the observed effects.
In summary, we observed a pronounced growth inhibition effect on intestinal epithelial cells by both, neutral and acidic milk oligosaccharide fractions. Effects on differentiation in Caco-2 and HIEC cells were mostly pronounced for acidic and neutral oligosaccharide fractions at 15 ml/ml with a threshold at about 7·5 mg/ml. Regarding neutral HMO fractions growth inhibition was associated with induction of differentiation and apoptosis in undifferentiated cells such as HT-29 and HIEC cells. In addition, we identified some single neutral compounds responsible for these effects. On the other hand, growth arrest in HT-29 and HIEC cells induced by acidic oligosaccharides was associated with effects on differentiation but not on apoptosis. Thus, HMO were shown to induce growth inhibition in different intestinal cells through two different mechanisms, (i) by suppressing cell cycle progression through induction of differentiation and/or (ii) by influencing apoptosis. Based on these observations, oligosaccharides at physiologically relevant concentrations may serve as modulators of maturational intestinal changes.
Acknowledgements
The authors would like to thank Ms Nadine Metz for her excellent technical assistance. The present study was supported by Wyeth Nutrition, 500 Arcola Road, Collegeville, PA 19 426 USA. The authors declare no conflict of interest.











