Dementia is a syndrome characterised by deterioration of cognitive function that affects memory, language, learning capacity, orientation, comprehension, social interactions and the ability to perform daily activities(1,Reference Livingston, Sommerland and Orgeta2) , and its most common cause is Alzheimer’s disease(1–4). Worldwide, approximately 50 million people had dementia in 2019 and the total number is projected to reach 152 million by 2050(1,4–Reference Livingston, Huntley and Sommerlad6) , decreasing the quality of life of a lot more people with dementia, and also their families and carers(1,3) . Currently, there is no effect treatment or cure for dementia; however, increasing evidence suggests that lifestyle modification may delay the onset(3,Reference Martinez-Lapiscina, Clavero and Toledo7) , reducing projected dementia prevalence(Reference Livingston, Sommerland and Orgeta2,Reference Kivipeltoa, Solomona and Ahtiluotob8) .
Cognitive impairment and dementia have a multifactorial aetiology, in which both genetic and environmental factors interact(Reference Kivipeltoa, Solomona and Ahtiluotob8). Although age is the strongest risk factor for dementia(1,3,4) , dementia is not an unavoidable consequence of ageing(1,Reference Livingston, Sommerland and Orgeta2,3,4) , and many cases may theoretically be preventable(Reference Livingston, Sommerland and Orgeta2,Reference Livingston, Huntley and Sommerlad6,Reference Ngandu, Lehtisalo and Solomon9) . For example, recent studies have shown that lifestyle risk factors increase the risk of cognitive decline and dementia(1–4,Reference Livingston, Huntley and Sommerlad6,Reference Ngandu, Lehtisalo and Solomon9–Reference Radd-Vagenas, Duffy and Naismith11) , and approximately 30 % of dementia cases are attributable to modifiable vascular risk factors and depression(Reference Norton, Matthews and Barnes12). Dementia shares risk factors with CVD such as hypertension, hypercholesterolaemia, diabetes, overweight/obesity, physical inactivity, smoking, unhealthy diet and excessive alcohol consumption(1–4,Reference Livingston, Huntley and Sommerlad6,Reference Ngandu, Lehtisalo and Solomon9,Reference Radd-Vagenas, Duffy and Naismith11,Reference Richard, Jongstra and Soininen13) . Therefore, interventions that improve modifiable risk factors related in part to lifestyle might prevent or delay the onset of cognitive impairment and dementia(Reference Livingston, Sommerland and Orgeta2,4,Reference Livingston, Huntley and Sommerlad6,Reference Ngandu, Lehtisalo and Solomon9,Reference Abbatecola, Russo and Barbieri10,Reference Richard, Jongstra and Soininen13) . Diet modification is one such lifestyle intervention that has been proven to reduce CVD risk factors, and recent studies have shown that specific dietary interventions may also reduce the risk of dementia. This paper will describe the rationale and design of the Nutrition Module in the Maintain Your Brain (MYB) randomised controlled trial (RCT). The Nutrition Module is one out of four intervention modules designed to target modifiable risk factors for Alzheimer’s disease and dementia.
The rationale for the Nutrition Module in MYB is based on a wealth of observational as well as some previous empirical data. The WHO guidelines for risk reduction of cognitive decline and dementia(4), a systematic review(Reference Chen, Maguire and Brodaty14) and observational studies(Reference Radd-Vagenas, Duffy and Naismith11,Reference Scarmeas, Stern and Mayeux15–Reference Psaltopoulou, Sergentanis and Panagiotakos18) have found that the Mediterranean eating pattern might slow cognitive decline and protect against dementia(Reference Kivipeltoa, Solomona and Ahtiluotob8–Reference Radd-Vagenas, Duffy and Naismith11). Furthermore, the largest RCT to date implementing the Mediterranean Diet (MedDiet): PREDIMED (Prevención con Dieta Mediterranea) reported that an intervention with the MedDiet supplemented with either extra virgin olive oil (EVOO) or mixed nuts resulted in a significant improvement in cognition compared with the control group low-fat diet(Reference Martinez-Lapiscina, Clavero and Toledo7) in 522 older adults living in Spain.
More generally, our systematic review of MedDiet and cognition interventions(Reference Radd-Vagenas, Duffy and Naismith11) in 1888 participants from five RCT found that the MedDiet intervention differed significantly between studies. The diets used during the RCT interventions varied considerably from the elements and quantities of the ‘traditional’ MedDiet(Reference Radd-Vagenas, Duffy and Naismith11,Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh19) , and in all studies, the intervention group received dietary counselling sessions. However, the effect sizes (ranging from 0·32 to 1·66) for individual trial levels provided evidence that cognitive outcomes including global cognition (1 of 5 studies), working memory (4/5 studies), verbal and visual memory (5/5 studies), visuospatial (1/5 studies), language (2/5 studies) and executive function (2/5 studies) domains were superior in the MedDiet group compared with controls(Reference Radd-Vagenas, Duffy and Naismith11). Additionally, the RCT with the most robust design reported that the MedDiet might reduce cognitive decline or improve cognition despite 4 years of ageing(Reference Radd-Vagenas, Duffy and Naismith11).
In addition to these emerging cognitive benefits, the MedDiet has also been associated with a range of other health benefits(Reference Abbatecola, Russo and Barbieri10,Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh19–Reference Valls-Pedret, Sala-Vila and Serra-Mir27) in observational and clinical trials, including a reduction in the risk of cancer, CVD, inflammatory bowel disease and type 2 diabetes(Reference Abbatecola, Russo and Barbieri10,Reference Davis, Bryan and Hodgson20) . The protective effect of the MedDiet has been theorised to be related to its anti-inflammatory and antioxidant properties(Reference Martinez-Lapiscina, Clavero and Toledo7), and effects on the microbiome have been implicated as well(Reference Pistollato, Sumalla Cano and Elio28,Reference Cani and Van Hul29) . Therefore, mounting evidence suggests that this dietary pattern benefits cognition as well as many risk factors related to cognitive decline, and thus translation of such knowledge to at-risk cohorts is critical at this stage.
The most effective methods to change dietary behaviours relevant to cognitive protection are unknown. The MedDiet interventions for cognition identified in our systematic review of RCT(Reference Radd-Vagenas, Duffy and Naismith11) were all administered in person, either individually or via group sessions. These trials used various ways to assess adherence, which ranged from a reported 80–93 % of the prescribed diet or an approximately 2-point improvement in the Mediterranean Diet Adherence Screener (MEDAS) score from a baseline of 8·6/14 to 10·47/14 and 8·3/14 to 10·68/14 in groups supplemented with EVOO and mixed nuts, respectively. As the number of dietary education sessions in these trials ranged from only one to twenty-six sessions, the reach may have been limited by the need for face-to-face delivery by dietitians. Scaling-up interventions to benefit large portions of the vulnerable population requires a more cost-effective approach.
Internet dissemination and telehealth programmes could potentially facilitate the delivery of preventative programmes that might improve self-management and contribute to lifestyle risk factors reduction for CVD as well as dementia(Reference Richard, Moll van Charante and Hoevenaar-Blom30). The Healthy Aging Through Internet Counseling in the Elderly RCT is an interactive internet intervention that promotes self-management of cardiovascular risk factors to reduce the risk of CVD and dementia in 2398 older adults living in the Netherlands, Finland and France. Its authors suggest that health programmes delivered by the internet may increase the opportunity to disseminate prevention programmes efficiently(Reference Richard, Jongstra and Soininen13,Reference Heffernan, Andrews and Fiatarone Singh31) and reach individuals in geographically isolated areas. However, to date, no internet delivery of a MedDiet intervention without including face-to-face consultation for the specific purpose of preventing dementia has yet to be published. The MYB trial (trial registration: Australian New Zealand Clinical Trials Registry ACTRN 12618000851268) is an internet-delivered multidomain RCT designed to target modifiable risk factors for Alzheimer’s disease and dementia(Reference Heffernan, Andrews and Fiatarone Singh31). The MYB trial is one of the largest online cognitive decline-prevention trials and has recruited 6236 Australians aged 55–77 years with multiple dementia risk factors but no dementia diagnosis(Reference Walton, Lampit and Boulamatsis32). Participants were recruited into MYB from the Sax Institute’s 45 and Up Study. Recruitment methods, response rates and ethics approvals are described in detail elsewhere(Reference Heffernan, Andrews and Fiatarone Singh31,Reference Banks and Redman33) . Up to four preventative lifestyle-based modules could be administered depending on the person’s individual risk factor profile: a Brain Training System Module for those with an inactive cognitive history or current lifestyle, a Physical Activity Module for participants who are insufficiently physically active or have chronic diseases/risk factors for dementia known to benefit from exercise (e.g. diabetes, obesity, CVD, hypertension and frailty), a Nutrition Module for those reporting dietary intake that does not indicate adherence to a Mediterranean-type cuisine or those who have chronic diseases/risk factors for dementia known to benefit from this type of diet (e.g. obesity, metabolic syndrome, CVD, diabetes and excess alcohol consumption) and a Peace of Mind Module for those with chronic stress or current anxiety/depression-based symptoms or past history of depression or anxiety, see Heffernan et al. (Reference Heffernan, Andrews and Fiatarone Singh31) for the trial protocol, and more details on the trial’s inclusion criteria and outcomes measures. The trial is currently on-going, with 3-year outcomes anticipated to be available in 2022. The aim of this paper is to describe how the MYB Nutrition Module was designed.
Ethics
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the University of New South Wales Human Research Ethics Committee, Ethics Number 16 252, and NSW Population and Health Services Ethics Committee, Ethics Number 2016/03/636. The conduct of the 45 and Up Study was approved by the University of New South Wales Human Research Ethics Committee. Written informed consent was obtained electronically from all subjects/patients. The trial was registered with the Australian New Zealand Clinical Trials Registry (trial registry number ACTRN 12618000851268, http://www.anzctr.org.au).
Design
Nutrition Module overview
The MYB Nutrition Module is a fully online intervention promoting the adoption of the ‘traditional’ MedDiet pattern. The ‘traditional’ MedDiet is defined as high in unprocessed plant foods including grains, vegetables, fruits, legumes, nuts/seeds and EVOO, moderate in fish/shellfish and wine and low in meat, dairy, eggs, animal fats and discretionary foods(Reference Martinez-Lapiscina, Clavero and Toledo7,Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh19) . The MYB MedDiet also incorporated general elements of the DASH (Dietary Approaches to Stop Hypertension) diet in the material provided for hypertension promoting the intake of the low amount of Na in the diet as well as moderate intake of foods rich in K, Ca and Mg, and the Australian Dietary Guidelines(34) as a guide for serving sizes. Furthermore, the Nutrition Module included elements relating to cuisine and eating habits such as intake of home-cooked meals; use of moist, lower temperature and cooking methods; eating main meals with company and reduced snacking occasions(Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh19). The overall diet was offered to all participants eligible for the Nutrition Module only, but there was scope for flexibility to allow for the Australian context, food preferences, financial circumstances, special dietary requirements, allergies, intolerances and ethnic backgrounds, which may not make the full MedDiet feasible or suitable for all participants.
Participants who were eligible for the Nutrition Module were assigned to one of the three diet streams according to their medical history and adherence to the MedDiet at baseline (Table 1). The three diet streams were: Main, Malnutrition and Alcohol group. Information about nutrition and the recommended diet was provided in a variety of online formats for participants including fact sheets, shopping lists, sample meal plans, videos, recipe library and downloadable recipe cards. A dietitian and/or a nutritionist was available online in the Nutrition Module portal on the MYB digital platform, by email or telephone for consultation. The Nutrition Module was delivered over 10 weeks during the first 12 months of each eligible participant’s enrolment in MYB. This may have been in any quarter of the year, depending on which other modules they were assigned to. After these initial 10 weeks of intensive nutritional intervention, booster sessions were delivered monthly for the remainder of the 3-year follow-up. A short dietary questionnaire, MiniCul, was designed specifically for this trial and was administered weekly during the first 10 weeks and then monthly to monitor whether participants adopted or maintained the MedDiet pattern during the intervention(Reference Heffernan, Andrews and Fiatarone Singh31).
Table 1. Nutrition Module eligibility and selection criteria

MediCul, Mediterranean Diet and Culinary Index(Reference Radd-Vagenas, Fiatarone Singh and Inskip35); MEDAS, Mediterranean Diet Adherence Screener(Reference Schroder, Fito and Estruch36); ANU-ADRI_SF, ANU Alzheimer’s Disease Risk Index Short Form(Reference Kim, Cherbuin and Anstey60); HAQ, Harvard Alumni Questionnaire Physical Activity(Reference Pereira, FitzerGerald and Gregg61); IPAC, International Physical Activity Questionnaire(Reference Hagströmer, Oja and Sjöström62).
Nutrition Module eligibility
The eligibility for the Nutrition Module (Table 1) was based on participants’ health conditions and risk factors identified in MYB’s baseline assessments or adherence to the MedDiet assessed using the Mediterranean Diet and Culinary Index (MediCul) tool(Reference Heffernan, Andrews and Fiatarone Singh31,Reference Radd-Vagenas, Fiatarone Singh and Inskip35) . MediCul is a novel index tool with fifty questions created to assess adherence to a ‘traditional’ MedDiet pattern and some aspects of cuisine within a Western population; it scores from 0 to 100 where higher score represents higher adherence to a ‘traditional’ MedDiet(Reference Heffernan, Andrews and Fiatarone Singh31,Reference Radd-Vagenas, Fiatarone Singh and Inskip35) . The MediCul not only assessed participants’ nutritional risk for non-compliance to the traditional MedDiet at baseline but it was also completed annually during the 3-year follow-up as the primary outcome in terms of whether their nutritional behaviour/risk profile had changed. From MediCul, a MEDAS(Reference Schroder, Fito and Estruch36) score was derived, with a score of 9 or less indicating sub-optimal adherence to the MedDiet pattern. MEDAS was selected as the criterion to determine eligibility, as it had been used in PREDIMED, the MedDiet RCT that has been associated with beneficial cognitive outcomes(Reference Martinez-Lapiscina, Clavero and Toledo7,Reference Valls-Pedret, Sala-Vila and Serra-Mir27,Reference Estruch, Ros and Salas-Salvado37) .
Risk factors and medical conditions defining eligibility for the Nutrition Module included high cholesterol, high blood pressure, diabetes, CVD, stroke, transient ischaemic attack, peripheral vascular disease, high or low BMI, high waist circumference and history of/current excessive alcohol consumption. To be allocated in the Nutrition Module, participants had to have at least one of the eligibility criteria mentioned above or a low MEDAS score. Table 1 shows the eligibility criteria and the assessment tools used to define them. If participants reported a high alcohol consumption at baseline, they were allocated to the Alcohol stream subgroup; on the other hand, if participants were underweight, they were allocated to the Malnutrition stream subgroup. Participants in the Malnutrition stream were reallocated to the Main stream if goals were achieved after the 10-week intervention or monthly during the 3-year follow-up; Main stream participants could be reallocated in the Malnutrition stream after the 10-week intervention or monthly during the 3-year follow-up if they reported a BMI below 18·5 kg/m2 for participants less than 62 years old, or BMI below 23 kg/m2 for participants equal or older than 62 years old. The reallocation for participants in the Alcohol stream occurred only after the annual follow-up assessments, to ensure sustained risk factor change. Online Supplementary Figures S1 and S2 show participants allocation to the Nutrition Module streams.
Nutrition Module intervention
The Nutrition Module intervention is fully online, and it is part of the MYB multi-module intervention digital platform(Reference Ginige, Boulamatsis and Heffernan38). Participants randomised to the MYB trial were allocated to a minimum of one module and a maximum of four modules and allocated to the intervention (Coaching) group or the control (Information) group. Randomisation occurred after baseline assessments had been completed and therefore after module eligibility had been determined(Reference Heffernan, Andrews and Fiatarone Singh31). Those randomised to the Coaching group received weekly activities during the 10-week intervention or monthly during the 3-year follow-up, while the Information group received monthly static information. Both groups received activities unique to their assigned modules and were completed via the online MYB platform. For example, Information participants (control) who were eligible for the Nutrition Module received basic nutrition information based on the Australian Dietary Guidelines. The MedDiet or its components were not mentioned in any of the materials for the Information group or in any other module. On the other hand, participant in the Nutrition Module (coaching group) only have access to material designed for this module; there was not common material between modules.
If one of the participant’s eligible coaching modules was the Nutrition Module, participants were given access to the Nutrition Module homepage on MYB digital platform. When a participant logged into the MYB system as a Nutrition participant for the first time, there was a pop-up menu with an introductory video followed by a help/introduction video to the Nutrition Module (online Supplementary Fig. S3); after these videos, participants entered the Nutrition homepage to start the module (online Supplementary Fig. S4). On the Nutrition Module homepage, participants who logged for the first time were able to see the ideal Mediterranean food pyramid (Fig. 1).

Fig. 1. Maintain Your Brain ideal Mediterranean diet food pyramid.
The interactive MedDiet food pyramid designed specifically for the MYB trial was sub-categorised into ten food groups. The hierarchy and quantities for the ten pyramid food groups were determined by taking into consideration research findings on MedDiet and foods(Reference Radd-Vagenas, Duffy and Naismith11,Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh19) , MedDiet pyramids and educational tools(Reference Bach-Faig, Berry and Lairon39–Reference Willett, Sacks and Trichopoulou41), usual Australian population intakes(42,Reference O’Leary, Grech and Sui43) , existing high profile diet index tools, which assess adherence to the MedDiet, such as MEDAS(Reference Schroder, Fito and Estruch36). Table 2 shows the food groups hierarchy list.
Table 2. Food groups hierarchy list

Each food group had available resources in the resource library including fact sheets, recipes, videos, shopping and health tips. Food group fact sheets and shopping tips were diet stream specific, while recipes and health tips had the same content across the three diet streams. Participants had access to these resources at any time by clicking on the food group in the pyramid (Fig. 2) on the Nutrition Module homepage. Recommended quantities were based on the MedDiet eating plan and the MedDiet checklist fact sheets (online Supplementary Figs S5 and S6), which was developed taking into account multiple authoritative recommendations for consuming a MedDiet and focusing on the ‘traditional’ diet.
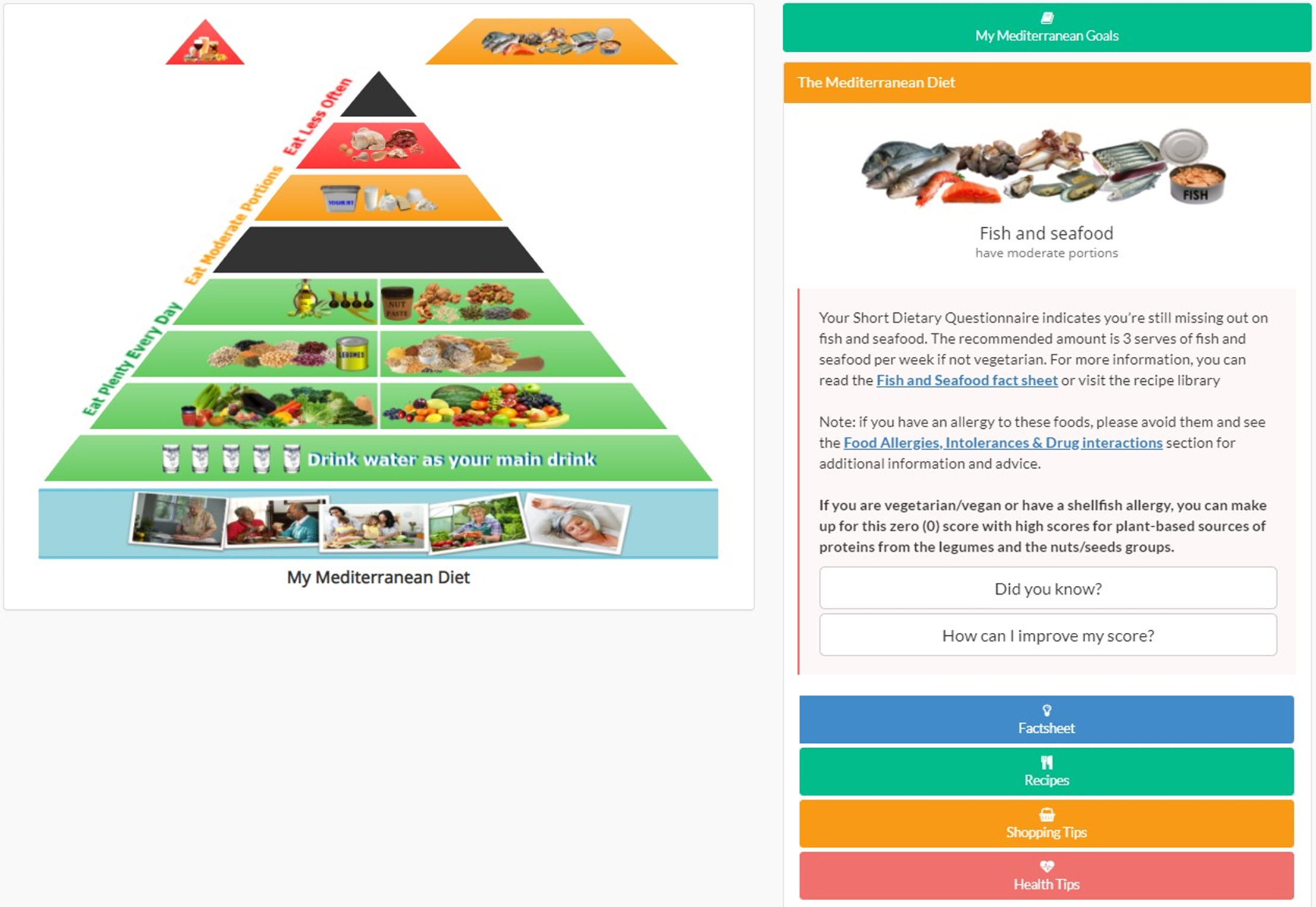
Fig. 2. Screenshot of Maintain Your Brain Mediterranean diet food pyramid resources.
To track adherence to the recommended diet, participants reported their food intake through the MiniCul (online Supplementary Fig. S17), a screener form of the validated MediCul(Reference Radd-Vagenas, Fiatarone Singh and Inskip35). The MiniCul is a short dietary questionnaire validated during MYB pilot study, derived from the MediCul(Reference Radd-Vagenas, Fiatarone Singh and Inskip35) with only ten questions, and a maximum score of 40 indicating optimal intakes of MedDiet recommendations. It was designed as a behavioural tool to monitor MedDiet adherence, and it was used weekly during the first 10 weeks, then monthly for the remainder of the 3-year follow-up. The maximum score per question is 2 (except for the question related to fats, which can score 4), meaning high adherence to the Mediterranean recommendation for that specific food. In contrast, the minimum score is zero, reflecting poor adherence to the MedDiet recommendations (online Supplementary Fig. S17 shows MiniCul scoring). In the question related to fats, scoring 4 indicates that EVOO is the main fat in the participant’s diet, and zero means that no fats are used, or other fat consumption is higher than EVOO. If a participant scores less than 2 for any of the ten food groups in the MiniCul (except for the fats question where the cut-off is less than 4), that food group piece will be missing in the participant’s food pyramid (Fig. 2), and they receive a dietary message called ‘Feedback’ for that food group. Feedback is an important component of the Nutrition Module because it supports the long-term adherence to a dietary change. Direct feedback reinforces dietary habits and behaviour, increases participant’s self-efficacy or belief in their ability to succeed and encourages motivation to continue performing the desired dietary behaviours(Reference Forster, Walsh and O’Donovan44,Reference Wright, Sherriff and Dhaliwal45) .
A maximum of four missing pieces in the pyramid and four dietary messages per week were sent to study participants, or per month during the 3-year follow-up. If, after receiving feedback for a given food group, the participant did not improve their intake (according to the weekly/monthly food intake reported in the MiniCul) they continued to receive feedback for another week/month on the same food group. However, the second feedback message for the same food group/s was worded slightly differently to motivate more urgent action by providing new facts and solutions to potential participant’s perceived barriers for change. Where participants did not make any improvement in their diet, feedback was sent for a maximum of 2 weeks on each food group according to the hierarchy list order (Table 2). All dietary messages were linked back to their corresponding resource library for specific food groups from the MedDiet food pyramid to support dietary changes. Online Supplementary Fig. S7 shows the workflow in the Nutrition Module.
After feedback was given, participants were also encouraged to fill out a weekly/monthly action plan (online Supplementary Fig. S8) to achieve the MedDiet goal suggested to improve their adherence to the MedDiet. Each goal corresponded to a ‘missing’ piece on the food pyramid and participants could type how they would achieve their goals in the text boxes. Participants were able to save and edit their action plan, and there was no limit to the number of times a participant could edit their action plan strategies during the same week throughout the initial module or during the month in the follow-up period. When the week/month was over, participants received a new set of goals according to their MiniCul answers at that timepoint. Saved goals and action plan strategies could be reviewed in the Nutrition Module homepage to allow participants to track their changes (Fig. 3).

Fig. 3. Screenshot of My Mediterranean goals.
In addition to the action plan and the Mediterranean goals, participants were able to review their progress adopting and/or maintaining the MedDiet in the ‘My Progress’ section on the Nutrition homepage (Fig. 4). The first pyramid was always the ideal food pyramid; afterwards, pyramids were shown in reverse chronological order, and the MiniCul total score was displayed under each corresponding pyramid as ‘Med Diet Score’. This provided a changing, visual feedback of progress as the ‘empty’ black pieces representing food groups that were sub-optimal would gradually be replaced with coloured pieces representing achievement of optimal intake in that domain.

Fig. 4. Screenshot of My Progress. This graph is an example of the possible intake change over time of a participant. The first pyramid in the graph is the ideal Mediterranean pyramid; afterwards, pyramids are shown in reverse chronological order from right to left. My Progress # refers to the MiniCul #, that is, My progress 1 means MiniCul #1. Med Diet Score is the total score on the MiniCul questionnaire out of 40. The arrow with the points below Med Diet Score indicates if a participant increases or decreases, their MiniCul score compared with the previous one. The pyramid is colour coded based on an individual’s intake compared with recommendations. Black pieces represent food groups that are not consistent with the MedDiet recommendation, and the other pieces indicate ideal intake has been reported by the participant in these food groups.
As mentioned, feedback messages, action plans and goals were based on participants’ most recent answers to the MiniCul. If the MiniCul was not completed within the allotted time frame (7 d), the system would copy the dataset from the previous week/month to the current session. If a participant never completed a MiniCul, the feedback will be based on their baseline MediCul until a new MiniCul was completed.
During the baseline assessments and annual follow-ups, participants were asked to self-report their weight, height and waist circumference; weight and height data were used by the system to calculate participants’ BMI. In addition, participants in the Nutrition Module were asked to self-report their weight and waist circumference weekly during the 10-week module and then monthly during the 3-year follow-up. Videos with instructions of how-to self-measure weight, height and waist circumference were uploaded onto the MYB platform and were available for participants to review as many times as needed.
Nutrition Module resources tabs
Education and information materials were available for participants on the Nutrition homepage. These materials were designed by qualified dietitians and nutritionists (Nutrition Module staff) and could be accessed as many times as the participant wished. All materials could be updated, removed and new materials could be uploaded by the Nutrition Module staff(Reference Heffernan, Andrews and Fiatarone Singh31). The materials were organised in thirteen tabs located on the Nutrition Module homepage. The resources tabs included materials related to MedDiet, behavioural change, chronic conditions, scientific literature, shopping list, menu plans, food allergies, intolerances and drug interaction, recipes, information on how to use the Nutrition Module homepage and frequently asked questions. Participants could access the fact sheets by clicking on the desired tab, and a pop-up menu would show them the resources available in that tab (online Supplementary Fig. S9). In addition to the fact sheets, the Nutrition Module staff delivered new information to participants through the module newsletter (online Supplementary Fig. S10). The newsletter was uploaded on the Nutrition Module portal and sent by email to all participants in the module periodically. Newsletters not only contained supplementary information related to MedDiet and health outcomes but also promoted participant engagement with the Nutrition Module homepage or had additional activities such as a recipe contest or sharing successful behavioural change stories.
The MedDiet resources included information for each food group clarifying serve size, health tips, shopping tips and recipes (recipe cards and videos). Additionally, participants could find information about healthy cooking methods, ideas for healthy snack and meals as well as recommendations of how to include traditional Mediterranean practices such as home-cooked meals, fasting and eating main meals with company. Fact sheet examples can be reviewed in online Supplementary Figs 11–16. All materials, including videos, were available for download to personal computers and printing if desired. The number of times that participants performed an action on the Nutrition Module, such as when viewing a fact sheet or how a resource is downloaded, is counted as markers of engagement over time. View counts are also available for videos as additional markers from the video hosting provider.
The information in the behavioural change fact sheets was based on Social Cognitive Theory and the Transtheoretical Model(Reference Clark, Blissmer and Greene46–Reference Armitage and Conner50). These behavioural theories promote change through self-efficacy, defined as the individual’s capacity to organise and perform actions necessary to achieve a specific outcome(Reference Clark, Blissmer and Greene46,Reference Locher, Bales and Ellis47,Reference Nigg, Burbank and Padula49) . The Social Cognitive Theory uses elements such as self-efficacy and outcome expectancy as determinants of behaviour change(Reference Armitage and Conner50). Some components of the Social Cognitive Theory incorporated in the Nutrition Modules include goal setting, self-monitoring, self-control, positive reinforcement, feedback, managing barriers and knowledge. The Transtheoretical Model has been effectively applied to behaviour change in older adults(Reference Nigg, Burbank and Padula49,Reference Clark, Nigg and Greene51) . This theory integrates stages of change (pre-contemplation, contemplation, preparation, action and maintenance)(Reference Prochaska and Velicer48,Reference Armitage and Conner50) with elements of decisional balance, process of change and self-efficacy(Reference Clark, Nigg and Greene51,Reference Carvalho de Menezes, Bragunci Bedeschi and dos Santos52) . As the Nutrition Module is a fully online intervention, and dietary changes are influenced by social and behavioural factors(Reference Tapsell53), resources that promoted self-efficacy, self-management and process of change were important elements to be included in the module development.
The Nutrition Module aimed to establish clear behavioural goals, action plans, problem-solving strategies as well as enhance self-efficacy for successful adherence to the MedDiet. In addition, it is a unique online programme providing individually tailored, dynamic and interactive behavioural dietary change advice and support based on participants’ medical history, characteristics and dietary eating patterns and habits. Details of the behavioural principles used are summarised in online Supplementary Table S1.
Ticket system
The Nutrition Module online trainers were qualified dietitians and a nutritionist who could be contacted by participants through a ticket system on the MYB digital platform. Tickets are the in-house messaging system that MYB participants could use to communicate with MYB trainers. Participants could create a ticket with questions or issues related to the module by clicking on the ‘Contact us’ button in the Nutrition Module homepage or on the ‘I need help’ button on the participant’s ‘To do list’ page. When a new ticket was raised, the trainers received an email notification. To resolve a ticket, trainers had access to participants’ medical history, baseline MediCul, MiniCul and Nutrition Module feedback and goals records. Tickets could be replied to directly via the online interface or outside the system, for example, by phone or email. Trainers could assign a ticket to another researcher as needed (i.e. other module trainers, module leader/physician or information technology specialist).
Medical events
Every 3 months, participants were reminded by an on-screen ‘pop-up’ to report any medical event(s) in both a check box and text box format, which was followed by specific advice to continue/start dietary changes or not, or to see their health care provider if indicated. All participants could also report adverse events at any time of the trial (ad hoc reporting). Adverse events during the Nutrition Module that were potentially related to the intervention (e.g. bloating, diarrhoea, allergic reactions or vomiting) were dealt with via fact sheets and instructions within the Module. These logged events were triaged by the Nutrition Module leader and trainers as appropriate for follow-up, referral to GP or study physicians or reporting to the Ethics Committee(Reference Heffernan, Andrews and Fiatarone Singh31).
During the first year of intervention, participants eligible for the Nutrition Module who had not started the Module yet could be reallocated in the Malnutrition subgroup if they reported weight loss greater or equal to 5 % during last year and BMI below 18·5 kg/m2 on their adverse event report.
Technologies used for the implementation of the Nutrition Module
The Nutrition Module was implemented as a subsystem within the MYB system(Reference Ginige, Boulamatsis and Heffernan38), using the same technology stack: with Laravel 5.1(54) as the base framework, PHP 5.6 for the custom coding of the backend system and MariaDB(55) an open-source fork of MySQL(56), as the relational database management system. The MediCul and MiniCul were implemented with the use of LimeSurvey(57) along with backend algorithms to calculate the MEDAS score. PDF files and image content of the virtual resource library of the Nutrition Module were hosted using a third-party cloud-based platform named Cloudinary(58). The video content was hosted via the Vimeo(59) platform that allowed content developers to produce and manage the content independent of the MYB IT team. This separation of the virtual resource library from the other parts of the Nutrition Module facilitated the efficient on-demand delivery of content to module participants.
Discussion
The MYB Nutrition Module was designed to assess the adherence to, and the effects of, the MedDiet on modifiable risk factors for cognitive decline and dementia in older adults using a web-based intervention. There are several notable strengths in its design. This novel intervention is extremely unique among internet-based health promotion interventions, as it is able to individualise the dietary advice according to both the medical and dietary history of each participant. In addition, it is the only programme to our knowledge that is responsive to both the behavioural changes made in dietary intake patterns and anthropometric data captured over extended follow-up, all without face-to-face counselling. The computerised delivery is also potentially a major strength as it allows the Nutrition Module to be used on a broad population level if scaled-up and could become a cost-effective and efficient way to prevent dementia and cognitive decline in older adults.
However, we acknowledge two potential limitations in the design as well. First, the use of self-reported anthropometric measurements could introduce slight imprecision, which will be quantified in a validation study (in preparation) which was conducted before the trial began. Second, people need computer literacy and internet availability, which could impact generalisability.
In conclusion, as one of the largest trials of its kind to date, the results from this unique intervention will contribute substantively to the evidence that links the Mediterranean-type diet with cognitive function and the prevention of dementia and will increase our understanding of the benefits of a MedDiet in a Western country. This novel trial will help identify the cognitive benefits of MedDiet and/or other lifestyle modifications incorporated through an entirely web-based platform. Moreover, the Nutrition Module could provide a model for online dietary interventions in older adults to reduce the risk of CVD and other chronic conditions and might facilitate implementation of dietary interventions as a telehealth initiative in geographically isolated areas, thus increasing the number of people who may benefit.
Acknowledgements
This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: Heart Foundation; NSW Ministry of Health; NSW Department of Communities and Justice; and Australian Red Cross Lifeblood. We thank the many thousands of people participating in the 45 and Up Study. The Nutrition Module team thanks Signs Publishing for a non-exclusive license to use traditional Mediterranean recipes from Food as Medicine: Cooking for Your Best Health by Sue Radd-Vagenas and Adventist Media Network for production and use of cooking videos.
This work was funded by National Health and Medical Research Council (NHMRC) of Australia Dementia Team Grant APP1095097. National Health and Medical Research Council (NHMRC) of Australia had no role in the design, analysis or writing of this article.
H. B., P. S. S., M. V., K. J. A., J. A. G. and M. A. F. S.: formulated the research question; H. B., P. S. S., M. H., Y. N., S. R.-V., Y. M., V. M. F., F. O., M. V., K. J. A., K. D., J. A. G., J. C. S. J., T. C. and M. A. F. S.: designed the study; C. A. R., Y. N., S. R.-V., Y. M., V. M. F., F. O., M. H., M. V., J. A. G., J. C. S. J., T. C., S. G. R. and M. A. F. S.: carried out the study; C. A. R.: wrote the article and all the authors: read the article, provided critical review and approved the final manuscript.
All authors except Sue Radd-Vagenas have no conflict of interests. Sue Radd-Vagenas is the author of Food as Medicine: Cooking for Your Best Health. Permission was granted by the publisher to freely use a select number of Mediterranean recipes from this cookbook; however, the study participants were not required to purchase the volume.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521001859









