Despite a wealth of research in both human subjects(Reference Yamada, Hosoya, Nishimura, Tanaka, Kajimoto, Nishimura and Kajimoto1, Reference Keenan, Zhou and McCutcheon2) and various animal model species(Reference Toden, Bird, Topping and Conlon3, Reference Mentschel and Claus4), the relevance of resistant starch (RS) to human health and disease prevention remains unclear. Much of this uncertainty likely stems from the exceptional diversity that is encountered among RS varieties. Although the heterogeneous nature of traditional dietary fibre supplements is well documented, it is equally important to recognise that RS varieties originating from different plant sources and manufactured with alternative processing technologies will possess unique physiochemical properties(Reference Champ5). RS is generally classified into four distinct types: physically entrapped, inaccessible starch; native granular starch; retrograded starch; chemically modified starch(Reference Englyst and Englyst6).
Due to its physiochemical attributes, RS is a particularly useful functional food additive to manage weight loss(Reference Higgins, Higbee, Donahoo, Brown, Bell and Bessesen7), improve insulin sensitivity(Reference Robertson, Bickerton, Dennis, Vidal and Frayn8) and reduce dyslipidaemia(Reference de Deckere, Kloots and van Amelsvoort9). Furthermore, RS consumption is believed to improve intestinal health and function by increasing micronutrient absorption and favourably altering bacterial fermentation patterns in the large intestine(Reference Yonekura and Suzuki10, Reference Hylla, Gostner, Dusel, Anger, Bartram, Christl, Kasper and Scheppach11). However, as literature reports do not often stress the importance of starch variety in contributing to physiological responses, it is not clear how nutrient digestion and fermentation characteristics are affected by different RS varieties. Thus, before the potential health benefits of RS can be realised, food technologists and nutritionists need clarification on how specific RS varieties influence intestinal function and systemic health.
Therefore, our objectives were to investigate changes in the efficiency of nutrient utilisation and intestinal fermentation in response to the consumption of three different varieties of RS in the pig model. To further evaluate the nutritional significance of RS, the physiological responses were compared with those associated with the consumption of guar gum (GG) and cellulose, which are well-characterised, conventional soluble and insoluble fibre supplements, respectively.
Materials and methods
Diets
In an effort to reasonably approximate a typical North American high-fat diet, a basal diet (control) was formulated with poultry meal (45 %), casein (4 %) and an exogenous animal and vegetable fat blend (15 %, Table 1). The experimental diets were formulated by replacing the normal corn starch in the basal diet with 10 % granular high amylose corn starch (GCS), granular potato starch (GPS; Avebe), retrograded high amylose corn starch (RCS), GG (SUPERCOL®-Guar Gum; Hercules Incorporated Aqualon Division) or cellulose. As the amount of starch deemed resistant to enzymatic hydrolysis in vitro differed between the starch varieties (see Results), different concentrations of the RS preparations were supplemented into the respective diets to ensure that the final inclusion of ‘resistant’ starch was 10 %. Chromic oxide was included (0·3 %) as an indigestible marker to measure distal ileal and total tract nutrient digestibility. Commercial mineral and vitamin premixes were added to the diets to meet or exceed the concentrations recommended by the National Research Council(12).

Con, Control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % resistant starch content); GPS, granular potato starch (71·51 % resistant starch content); RCS, retrograded high amylose corn starch (41·45 % resistant starch content); GG, guar gum (as food grade G2-S SUPERCOL®-Guar Gum provided by Hercules Incorporated Aqualon Division. GG properties: average molecular weight of 1·5 million; granulation of medium coarse; viscosity on as-is basis reported at 4500 centi poise with the GG dispersed in cold water to form a 1 % solution measured at 25°C after by using a Brookfield RVT viscometer at 20 rpm); Cell, cellulose (powdered cellulose; Solka-Flock, International Fibre Corporation).
* On as-fed basis.
† Details of the mineral, vitamin and salt premixes were published in a previous publication(Reference Rideout, Fan, Cant, Wagner-Riddle and Stonehouse20).
‡ Fisher Scientific.
§ Calculated values based on National Research Council (1998). Does not include digestible energy (DE) contributions from fibre supplements.
‖ Analysed values.
¶ For details of animals and procedures, see Materials and methods.
Animals and experimental design
The experimental procedures for the care and treatment of the pigs were reviewed and approved by the Animal Care Committee at the University of Guelph. The pigs used in this experiment were cared for in accordance with the guidelines established by the Canadian Council of Animal Care(Reference Olfert, Cross and McWilliam13). Thirty-six Yorkshire pigs, with an average initial body weight of 30 kg, were acquired from the Arkell Swine Research Station at the university. The pigs were placed in an environmentally controlled room (20°C) and randomly assigned to individual stainless steel metabolic crates (height 83 cm; length 145 cm; width 86 cm). For the next 3 d, the pigs were fed a typical grower ration and acclimatised to the environment and research staff. During the first 14 d of each experimental period, the pigs were fed their respective diets at 09.00 and 16.00 hours at close to ad libitum intake (5·5 % body weight). During days 10–14, faecal and urine samples for the mass balance experiments were collected. During days 15–16, the pigs were surgically fitted with a distal ileal cannula according to previously established procedures(Reference Sauer, Jorgensen and Berzins14). Following a post-surgical recovery period (days 17–20), the pigs resumed their previous feeding schedule for the duration of the experiment. On days 30–34 of the experiment, distal ileal effluent samples were collected according to the procedures outlined later. On the last day (day 36) of the experiment, the pigs were anaesthetised with isoflurane (2·5 %) in O2 (2·5 litres/min) and killed with an intracardial injection of sodium pentobarbital (50 mg/kg body weight; Schering) for collection of caecal digesta samples.
The experiment was carried out according to a randomised complete block design with six replications (blocks) and a total of thirty-six animals, according to the following model(Reference Kuehl15):
where μ is the general mean, ti is the treatment effect, ρj is the block effect, and ɛij is the experimental error.
Sample collection and processing
Fresh faecal samples were collected at 2 h intervals in the daytime of the collection period, placed in containers with sealed lids and immediately stored at 4°C for the duration of the period. The distal ileal digesta samples were collected from the distal ileal cannula into plastic bags (length 10 cm; internal diameter 1·5 cm) containing 5 ml formic acid (2·86 m) to minimise bacterial fermentation. Digesta were immediately frozen at − 20°C.
Urine samples were obtained using a funnel-shaped metal tray secured to the base of the webbed floor of the metabolic crate. Urine flowed from the metal tray into a collection container placed in an electronic cooler beneath the metabolic crate. The coolers were maintained at a temperature of 4°C to reduce microbial activity and urea degradation and NH3 emission loss during the collection period. The total volume of urine collected each day was recorded. A representative urine sample (about 5 % of the daily collection) from each day was pooled and stored at 4°C for the remainder of the experimental period.
At the conclusion of the experiment, representative faecal and digesta samples from each pig were freeze-dried, ground into a homogenous mixture and stored at 4°C in sealed containers. Fresh caecal digesta samples were collected and stored in sealed plastic centrifuge tubes at − 20°C until analyses. Dietary samples were ground and stored in a similar manner.
Chemical analyses
Analyses of DM were carried out according to Association of Official Analytical Chemists methods(16). Chromic oxide concentration in diet, distal ileal digesta and faecal samples was analysed with an atomic absorption spectrometer (SpectrAA-10/20; Varian) at 375 nm with a slit width of 0·5 nm according to the procedures outlined by Saha and Gilbreath(Reference Saha and Gilbreath17). Total crude protein (CP) content (N × 6·25) in dietary, distal ileal digesta and faecal samples was analysed according to the combustion method (Dumas procedure) on a Leco FP-428 Nitrogen analyser (Leco Corporation). Urine samples were first diluted (5 × ) with sulfuric acid (0·20 m) to prevent NH3 vaporisation, then analysed using sucrose (0·1 g) as the carrier compound. Total Ca and P contents in diet, distal ileal effluent, faecal and urine samples were analysed according to previously published procedures(Reference Rideout and Fan18).
RS content in starch varieties and diet samples was measured following incubation with pancreatic α-amylase and amyloglucosidase for 16 h at 37°C with a commercial kit (Megazyme International Ltd.) according to an Association of Official Analytical Chemists method(Reference McCleary and Monaghan19). Total starch content in diet, ileal digesta and faecal samples was measured enzymatically with a commercial assay kit (Megazyme International Ltd.). GG content in diet, ileal digesta and faecal samples was measured with a commercial galactomannan assay kit (Megazyme International Ltd.). Cellulose content in diet, ileal digesta and faecal samples was measured according to official Association of Official Analytical Chemists methods(16) in an ANKOM fibre analyser (ANKOM Technology). Caecal SCFA were determined by GC-MS analyses according to previously established procedures(Reference Rideout, Fan, Cant, Wagner-Riddle and Stonehouse20).
Differential scanning calorimetry
Thermal analyses of selected starches were performed using a differential scanning calorimeter (2920 Modulated DSC; TA Instruments) equipped with a refrigerated cooling system. Samples of starch (12 mg) were weighed into high-volume pans. Distilled water was added using a micropipette to make suspensions with 70 % moisture content. Pans were sealed and equilibrated overnight at room temperature before heating in the differential scanning calorimeter. The measurements were carried out at a heating rate of 10°C/min from 5 to 190°C. The instrument was calibrated using indium and an empty pan as the reference. The change in enthalpy (ΔH) of phase transitions was measured from the endotherm of differential scanning calorimetry thermograms using software (Universal Analysis, version 2.6D; TA Instruments) based on the mass of dry solid. Onset and peak temperatures of phase transitions were also measured from differential scanning calorimetry thermograms.
Calculations and statistical analyses
The efficiency of digestive and post-absorptive utilisation, including digestibility and retention, of CP, Ca and P was calculated according to previously established equations(Reference Rideout and Fan18, Reference Rideout, Fan, Cant, Wagner-Riddle and Stonehouse20). Data were analysed with the mixed model of SAS according to a completely randomised block design. Multiple comparisons between treatments were made using Tukey's test. Treatment differences from the control were further analysed using Dunnett's test. Correlations between starch thermal properties and nutrient utilisation and fermentation characteristics were conducted using Pearson partial correlation analyses. Differences were considered significant at P < 0·05.
Results
The pigs remained healthy and had similar feed intake (P = 0·55–0·89) for the duration of the experimental periods. The fraction of starch analysed to be resistant to in vitro enzymatic hydrolysis was different among the selected starches, i.e. normal corn starch 0 (sem 0·01) %, GCS 45·04 (sem 0·29) %, GPS 71·5 (sem 2·51) % and RCS 41·45 (sem 0·39) %. As expected, the conventional starch in the control, GG and cellulose diets was almost completely digested by the end of the distal ileum (Table 2). The distal ileal starch concentrations (g/100 g DM) and recoveries (% RS intake) were higher (P < 0·05) in pigs consuming the RS varieties compared with the control group (Table 2). Furthermore, faecal starch concentrations and RS recoveries (0·11–0·17 % RS intake), reflecting an almost complete fermentation in the hindgut, did not differ (P = 0·37–0·92) among the RS varieties (Table 2). The distal ileal recovery of GG (62·26 (sem 5·27)) was higher (P < 0·05) than that of cellulose (37·91 (sem 6·73)) and all three of the RS varieties (Table 2).
Table 2 Distal ileal and faecal starch characteristics following the consumption of different varieties of resistant starch (RS) and conventional fibres in the pig‡ (Values represent least squares means with their standard errors of the mean)

Con, control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % RS content); GPS, granular potato starch (71·51 % RS content); RCS, retrograded high amylose corn starch (41·45 % RS content); GG, guar gum; Cell, cellulose.
a,b,c Values within a row with unlike superscript letters are significantly different (P < 0·05) as analysed with Tukey's multiple comparison test using PROC MIXED model of SAS.
Values are significantly different from the Con group (*P < 0·05) as analysed with Dunnett's test using PROC MIXED model of SAS.
† Recoveries for the RS have been corrected for the contribution of the conventional starch measured from the control group. Values are % of dietary RS content (GCS, GPS and RCS diets) and % of dietary total starch content (GG and Cell diets).
‡ For details of animals and procedures, see Materials and methods.
The efficiency of digestive and post-absorptive utilisation of CP in response to the consumption of RS, GG and cellulose is presented in Table 3. Consumption of the granular corn starch varieties (GCS and GPS) depressed (P < 0·05) apparent total tract CP digestibility and increased (P < 0·05) total tract CP loss in comparison with the control group. However, only the GCS variety reduced (P < 0·05) apparent CP retention in comparison with the control group. The urinary:faecal CP ratio was decreased (P < 0·05) in the pigs consuming GCS, GPS and GG in comparison with the control group.
Table 3 Efficiency of digestive and post-absorptive utilisation of crude protein (CP) following the consumption of resistant starch (RS) and conventional fibres in the pig† (Values represent least squares means with their standard errors of the mean)
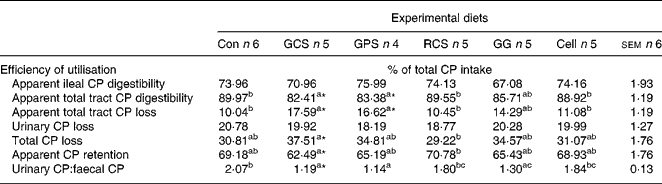
Con, control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % RS content); GPS, granular potato starch (71·51 % RS content); RCS, retrograded high amylose corn starch (41·45 % RS content); GG, guar gum; Cell, cellulose.
a,b,c Values within a row with unlike superscript letters are significantly different (P < 0·05) as analysed with Tukey's multiple comparison test using PROC MIXED model of SAS.
Values are significantly different from the Con group (*P < 0·05) as analysed with Dunnett's test using PROC MIXED model of SAS.
† For details of animals and procedures, see Materials and methods.
The efficiency of digestive and post-absorptive utilisation of Ca in response to the consumption of RS, GG and cellulose is presented in Table 4. Consumption of GPS reduced (P < 0·05) total tract Ca digestibility, increased (P < 0·05) total tract Ca excretion and reduced (P < 0·05) whole-body Ca retention in comparison with the control group. Consumption of RCS increased (P < 0·05) total tract Ca digestibility and reduced total tract Ca loss but did not significantly affect (P = 0·42) whole-body Ca retention in comparison with the control group. Among the three RS preparations, the pigs consuming RCS had the highest (P < 0·05) total tract Ca digestibility and whole-body Ca retention.
Table 4 Efficiency of digestive and post-absorptive utilisation of Ca following the consumption of resistant starch (RS) and conventional fibres in the pig† (Values represent least squares means with their standard errors of the mean)
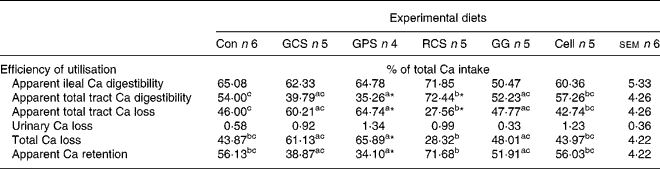
Con, control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % RS content); GPS, granular potato starch (71·51 % RS content); RCS, retrograded high amylose corn starch (41·45 % RS content); GG, guar gum; Cell, cellulose.
a,b,c Values within a row with unlike superscript letters are significantly different (P < 0·05) as analysed with Tukey's multiple comparison test using PROC MIXED model of SAS.
Values are significantly different from the Con group (*P < 0·05) as analysed with Dunnett's test using PROC MIXED model of SAS.
† For details of animals and procedures, see Materials and methods.
The efficiency of digestive and post-absorptive utilisation of P in response to the consumption of RS, GG and cellulose is presented in Table 5. Pigs consuming RCS had higher (P < 0·05) apparent total tract P digestibility and efficiency of whole-body P retention than the pigs consuming the other two RS preparations. Consumption of the GPS reduced (P < 0·05) total tract P digestibility, increased (P < 0·05) total P excretion and reduced (P < 0·05) the efficiency of P retention in comparison with the control diet. Consumption of GG reduced (P < 0·05) the ileal P digestibility but did not influence (P = 0·85–0·99) total tract P digestibility or whole-body P retention efficiency in comparison with the control pigs.
Table 5 Efficiency of digestive and post-absorptive utilisation of P following the consumption of resistant starch (RS) and conventional fibres in the pig† (Values represent least squares means with their standard errors of the mean)
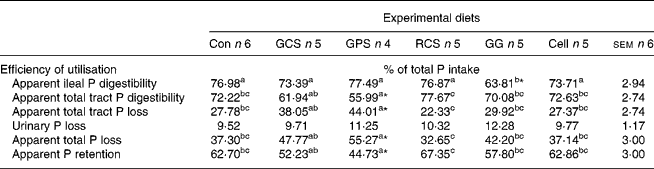
Con, control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % RS content); GPS, granular potato starch (71·51 % RS content); RCS, retrograded high amylose corn starch (41·45 % RS content); GG, guar gum; Cell, cellulose.
a,b,c Values within a row with unlike superscript letters are significantly different (P < 0·05) as analysed with Tukey's multiple comparison test using PROC MIXED model of SAS.
Values are significantly different from the Con group (*P < 0·05) as analysed with Dunnett's test using PROC MIXED model of SAS.
† For details of animals and procedures, see Materials and methods.
Consumption of GG increased (P < 0·05) the caecal production of butyric, hexanoic, isovaleric and valeric acids and resulted in a higher total SCFA production in comparison with the control group (Table 6). Consumption of RCS increased (P < 0·05) the caecal production of butyrate in comparison with the control diet and the other RS diets. Furthermore, consumption of all three RS varieties reduced (P < 0·05) the caecal concentration of indole compared with the control group (Table 6).
Table 6 Individual and total caecal SCFA concentrations following the consumption of resistant starch (RS) and conventional fibres in the pig† (Values represent least squares means with their standard errors of the mean)

Con, control diet with normal corn starch; GCS, granular high amylose corn starch (45·04 % RS content); GPS, granular potato starch (71·51 % RS content); RCS, retrograded high amylose corn starch; GG, guar gum; Cell, cellulose.
a,b,c Values within a row with unlike superscript letters are significantly different (P < 0·05) as analysed with Tukey's multiple comparison test using PROC MIXED model of SAS.
Values are significantly different from the Con group (*P < 0·05) as analysed with Dunnett's test using PROC MIXED model of SAS.
† For details of animals and procedures, see Materials and methods.
Starch thermal properties were measured in order to examine the potential association between these commonly analysed physical–chemical endpoints and the physiological responses observed following consumption of the starch varieties (Table 7). The highest transition temperatures, i.e. onset and peak, were associated with the RCS variety while potato starch had the lowest transition temperatures. Large differences in transition temperatures and enthalpy changes were observed between normal corn starch and GCS (Table 7).
Table 7 Thermal properties of starch varieties heated in the presence of excess water (70 %, w/w)* (Values represent least squares means with their standard errors for three animals)
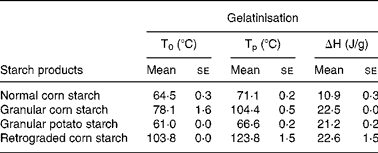
T0, onset temperature; Tp, peak temperature; ΔH, change in enthalpy.
* For details of animals and procedures, see Materials and methods.
Endpoint changes in the efficiency of nutrient utilisation and intestinal fermentation were statistically analysed for their correlations with the thermal physical characteristics measured (Table 8). Apparent CP digestibility values were negatively correlated (P < 0·05) with the enthalpy changes in the three RS preparations. Apparent Ca and P digestibility values were positively correlated (P < 0·001 and 0·02, respectively) with onset and peak temperatures of the starch varieties. Caecal butyrate concentration was positively correlated (P < 0·001, 0·001 and 0·004, respectively) with onset and peak temperatures as well as the enthalpy changes in the RS preparations.
Table 8 Pearson correlations between intestinal endpoints and thermal properties of the starch varieties*
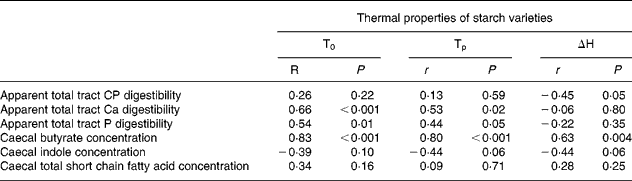
T0, onset temperature; Tp, peak temperature; ΔH, change in enthalpy; CP, crude protein.
r, Pearson partial correlation coefficients; P, probability of significance for six animals.
* For details of animals and procedures, see Materials and methods.
Discussion
Distal ileal starch recovery
As evident from the rather low distal ileal recovery (27–42 %) of the selected RS varieties, a discrepancy exists between the amount of RS measured in vitro and the amount of RS recovered in vivo. In accordance with this observation, previous research with human subjects suggests that current in vitro RS analyses procedures are unreliable predictors of starch that is truly resistant under in vivo conditions(Reference Danjo, Nakaji, Fukuda, Shimoyama, Sakamoto and Sugawara21, Reference Champ, Molis, Flourie, Bornet, Pellier, Colonna, Galmiche and Rambaud22). Alternatively, Silvester et al. (Reference Silvester, Englyst and Cummings23) have concluded that the amount of starch reaching the large intestine can be accurately estimated from in vitro measurements. Thus, the correlation between in vitro and in vivo RS measurements may be dependent on various physiological and methodological factors, including the animal model employed and the in vitro method used to analyse the RS fractions(Reference Bauer, Murphy, Wolf and Fahey24). Additionally, the problem of in vivo RS analyses may be further complicated by the method used to recover intestinal starch. As concerns have been raised regarding the comparison of dietary RS concentrations obtained using ileostomy models and intubation techniques(Reference Danjo, Nakaji, Fukuda, Shimoyama, Sakamoto and Sugawara21), it is possible that the intestinal cannulation procedures in the present study may have altered intestinal physiology and microbial fermentation patterns and ultimately affected the distal ileal starch recovery. Furthermore, a potential microbial contribution to the fermentation of RS in the small intestine is likely, as the distal ileal recovery of GG (38 %) and cellulose (62 %) was lower than expected (Table 2).
Nutrient utilisation
In protein nutrition, fibre supplements are traditionally considered anti-nutritional factors as they have been frequently shown to depress the efficiency of CP utilisation(Reference Eggum25). Previous work has demonstrated that the net efficiency of protein utilisation is reduced in rats fed potato starch compared with rats consuming corn starch(Reference Nageswara and Narasingo26). In the current study, consumption of the two granular starch preparations was associated with increased total tract CP excretion and a reduced urinary:faecal CP ratio. Similarly, previous work confirms that there is a net transfer of plasma urea N to the large intestine with subsequent conversion to NH3 and incorporation into bacterial protein in response to the consumption of RS type 2(Reference van der Meulen, Bakker, Bakker, de Visser, Jongbloed and Everts27). The efficiency of microbial protein synthesis is thought to be partially dependent on the amount and type of carbohydrate that enters the large intestine(Reference Schulze, van Leeuwen, Verstegen and van den Berg28). Under conditions where energy may be derived from a sufficient carbohydrate supply, bacterial growth is maintained through efficient N recycling by ureolytic bacteria(Reference Schulze, van Leeuwen, Verstegen and van den Berg28). Given the reduction in the urinary:faecal CP ratio observed in response to the granular starch preparations (Table 3), it is likely the granular RS varieties were preferentially utilised as bacterial energy sources and resulted in a greater caecal recycling of blood urea N in comparison with the RCS variety and the traditional fibre sources. However, without knowledge of how the RS preparations affected ileal digesta passage rates, it is difficult to make firm conclusions regarding the efficiency of caecal fermentation in response to the different starch varieties.
Literature reports concerned with the effects of RS and conventional fibre supplements on the efficiency of Ca utilisation have been inconsistent(Reference Greger29). The increased apparent total tract Ca digestibility that we observed in response to the consumption of RCS confirms the observations of Morais et al. (Reference Morais, Feste, Miller and Lifschitz30), who reported that feeding 7–10 d old piglets a cooked, cooled high amylose corn starch preparation increased apparent Ca absorption by 30 %. Alternatively, in agreement with previous findings(Reference Heijnen and Beynen31), a reduction in total tract Ca digestibility was observed in response to the consumption of the GPS variety compared with the control group. As shown in Table 4, apparent ileal Ca digestibility was not affected by diets. Furthermore, the much lower apparent total tract Ca digestibility values than the corresponding apparent distal ileal values in GCS- and GPS-based diets would also suggest that the endogenous Ca secretion into the large intestine might have been enhanced by GCS and GPS feeding in the pig. The caecum is considered to be a major site of Ca absorption through both transcellular and paracellular routes(Reference Hoenderop, Nilius and Bindels32). SCFA produced by microbial fermentation enhance Ca absorption by decreasing caecal pH and solubilising luminal Ca complexes(Reference Younes, Coudray, Bellanger, Demigne, Rayssiguier and Remesy33), enlarging the absorptive surface area of the caecum(Reference Remesy, Levrat, Gamet and Demigne34), increasing blood flow to the large intestine(Reference Mortensen, Nielsen, Mulvany and Hessov35) and/or directly affecting the functional properties and the Ca transport pathways associated with the epithelial cell surface(Reference Mineo, Hara and Tomita36). Increased butyrate production following RCS consumption may also help to explain the enhanced total tract Ca digestibility observed with this starch variety as compared with the other RS supplements. Both in vitro (Reference Mineo, Hara and Tomita36) and in vivo (Reference Lutz and Scharrer37) investigations have suggested a relationship between butyrate production and caecal Ca absorption. However, if this is indeed the case, it is surprising that Ca absorption was not enhanced in the pigs consuming GG as caecal butyrate production was also high in this group. However, the effects of GG consumption on intestinal Ca absorption have been reported to be independent of caecal fermentation in rats(Reference Watanabe, Hara, Aoyama and Kasai38).
Dietary P intake and intestinal P utilisation are important factors regulating whole-body Ca and P homeostasis and bone mineralisation(Reference Koshihara, Katsumata, Uehara and Suzuki39). Previous investigations have reported similar total tract absorption and retention of P in rats and pigs fed granular or retrograded corn starch preparations at a level of 6 %(Reference De Schrijver, Vanhoof and Vande Ginste40). However, we observed a significant reduction in the efficiency of P retention in response to the consumption of the GPS variety in comparison with the control group (Table 5). Furthermore, the RCS variety significantly increased the apparent total tract P digestibility and the efficiency of whole-body P retention in comparison with the granular starch preparations (GPS and GCS) (Table 5). Differences in the basal diet formulation and fibre supplementation may be responsible for the apparent discrepancy between the results of the current study and the aforementioned report. Alternatively, the reduction in the apparent ileal P digestibility observed in the current study in response to GG consumption is consistent with previous findings(Reference Elsenhans, Zenker and Caspary41) and is most likely associated with the viscous nature of GG. On the other hand, as shown in Table 5, the much lower apparent total tract P digestibility values than the corresponding apparent distal ileal values in GCS- and GPS-based diets would also suggest that the endogenous P secretion into the large intestine might have been enhanced by GCS and GPS feeding in the pig.
Intestinal fermentation endpoints
Butyrate is important in regulating intestinal function and systemic health and has been shown to act as a signalling molecule in a diverse range of metabolic events including: (i) the absorption and packaging of dietary fat by the intest-inal enterocyte(Reference Nazih, Nazih-Sanderson, Krempf, Michel Huvelin, Mercier and Marie Bard42); (ii) the modulation of pro-inflammatory cytokine expression for the prevention and treatment of inflammatory bowel disease(Reference Miller43); (iii) the expression of intestinal epithelial antimicrobial peptide(Reference Raqib, Sarker, Bergman, Ara, Lindh, Sack, Nasirul Islam, Gudmundsson, Andersson and Agerberth44); (iv) the inhibition of colonic crypt cell proliferation for prevention of colon-rectal cancer(Reference Blottiere, Buecher, Galmiche and Cherbut45); (v) the reduction of cell adhesion molecule expression in endothelial cells(Reference Zapolska-Downar, Siennicka, Kaczmarczyk, Kolodziej and Naruszewicz46); (vi) the modulation of arterial smooth muscle cell proliferation(Reference Ranganna, Yatsu, Hayes, Milton and Jayakumar47). Thus, caecal butyrate production by bacterial fermentation may be a good indicator of intestinal function and systemic health in response to dietary fibre consumption. In the current study, consumption of both the RCS and GG diets significantly increased caecal butyrate concentrations. Therefore, as compared with the granular RS preparations (GCS and GPS), it appears that the retrograded RS variety (RCS) is the most effective RS for the enhancement of microbial butyrate production.
On the other hand, indoles and their derivatives are volatile odour-causing compounds that have been demonstrated to be carcinogenic(Reference Yun, Jung, Son, Ju and Han48). Interestingly, all the RS varieties in the present study were shown to significantly reduce caecal indole concentrations and may therefore be a useful dietary therapy in the prevention and treatment of intestinal cancers.
Relationship between thermal properties of resistant starch and functionality
In the current study, feed intake was similar between the treatment groups and dietary RS supplementation was equalised by adjusting for the starch fraction resistant to in vitro enzymatic hydrolysis. Therefore, the different nutrient utilisation patterns that we observed are most likely related to the unique physiochemical, thermal and molecular structural properties associated with each starch variety. In agreement with previous findings(Reference Singh, Inouchi and Nishinari49), the different starch varieties used in the current study were characterised by unique thermal properties. In the food-processing industry, starch thermal properties are recognised as important parameters influencing the gelling ability and textural quality of food products(Reference Niba, Bokanga, Jackson, Schlimme and Li50). Furthermore, although various starch thermal and structural parameters have been widely examined for their effects on α-amylase function and granular starch release(Reference Yang, Shu, Zhang, Wang, Zhao, Ma and Wu51), the relationship between these starch parameters and physiological responses is not known. To our knowledge, this is the first study to demonstrate significant correlations between starch thermal parameters and nutrient digestibility values and caecal SCFA concentrations. Although a biological explanation for the relationship between the observed physiological responses and the starch thermal parameters is not readily apparent, one can speculate that these thermal properties are a reflection of the starch molecular and structural characteristics that influence the physical interaction between starch granules and pancreatic digestive enzymes, dietary and endogenous nutrients and microbial species within the intestinal lumen. A more comprehensive understanding of these correlations may help predict potential physiological responses based on routine thermal–physical analyses and facilitate the development of future RS-based functional food ingredients.
In conclusion, nutrient utilisation efficiency and intestinal fermentation are differentially affected by the consumption of granular and retrograded starch varieties. Apparent total tract Ca digestibility was increased following consumption of the retrograded starch variety (RCS) but was reduced with the consumption of GPS. Furthermore, consumption of the GPS variety was associated with a reduction in the efficiency of whole body Ca and P utilisation in comparison with the control group. Although all the RS varieties reduced the production of the odour-causing and carcinogenic compound indole, the retrograded starch variety (RCS) was the most effective in enhancing caecal butyrate production. Additionally, the thermal physical properties associated with unique RS varieties were correlated with important nutrient digestion and fermentation endpoints and therefore may be useful in the development of future RS varieties with desired functionality.
Acknowledgements
We thank Elizabeth Donner for help with the RS analyses, Manfred Hansel for N analyses and Ian McMillan for statistical consultation. Each author contributed fully to the design, implementation and interpretation of the results presented in this manuscript. This work was supported by research grants from Ontario Ministry of Agriculture, Food and Rural Affairs – University of Guelph Food Research Program (to M.Z.F.), Agriculture and Agri-Food Canada (to Q.L.) and Ontario Corn Producers' Association (to Q.L.). The authors have no conflict of interest to report.










