A Mediterranean diet (MD) has been associated with reduced risk for major chronic diseases( Reference Sofi, Abbate and Gensini 1 , Reference Bonaccio, Iacoviello and de Gaetano 2 ) such as CVD, cancer, diabetes and neurodegenerative diseases; its beneficial effects have been partially ascribed to its high content of PUFA, antioxidants and fibres that exhibit an anti-inflammatory action( Reference Estruch 3 ). Nuts represent an important component of the MD pattern and are rich in unsaturated fatty acids, minerals (Mg, K and Ca) and other bioactive compounds( Reference Ros, Tapsell and Sabaté 4 ). Epidemiological evidence has linked nut intake to reduced inflammatory status( Reference Jiang, Jacobs and Mayer-Davis 5 ) or lower risk of CVD( Reference Zhou, Yu and He 6 – Reference Estruch, Ros and Salas-Salvadó 8 ). Limited evidence also suggests an inverse association with cancer risk attributable to the presence of anti-carcinogenic compounds( Reference Sabaté and Ang 9 ). Benefits of nut intake on cardiovascular health have been ascribed to the direct effect of nuts on oxidation, inflammation and vascular reactivity( Reference Kris-Etherton, Hu and Ros 10 ). In addition, possible mechanisms that are able to explain the CVD advantages of nuts may be found in the documented positive effects on blood pressure( Reference Steffen, Kroenke and Yu 11 , Reference Doménech, Roman and Lapetra 12 ), blood lipids( Reference Mukuddem-Petersen, Oosthuizen and Jerling 13 , Reference Sabaté, Oda and Ros 14 ), diabetes( Reference Jiang, Manson and Stampfer 15 , Reference Afshin, Micha and Khatibzadeh 16 ) and the metabolic syndrome( Reference Salas-Salvadó, Guasch-Ferré and Bulló 17 ). Yet the possible role of inflammation as a mediator of the association between nut consumption and total mortality has been poorly explored so far( Reference Bao, Han and Hu 18 – Reference Fernández-Montero, Bes-Rastrollo and Barrio-López 21 ).
The purpose of this study was to address the relationship between nut intake and total and cause-specific mortality in a large Mediterranean cohort. Furthermore, our study investigated a possible role of inflammation( Reference Bonaccio, Di Castelnuovo and De Curtis 22 ) in explaining the association and likely advantages of nut intake in population groups sharing common risk factors for both CVD and cancer, with particular focus on subjects with obesity, diabetes, the metabolic syndrome, smokers or those with poor adherence to an MD.
Methods
Study population
Data presented here are from the Moli-sani study, which is a large population-based cohort study that recruited 24 325 subjects from the general population of the Molise region, a central-southern area of Italy( Reference Iacoviello, Bonanni and Costanzo 23 ). Individuals were enrolled from March 2005 to April 2010 and were followed up for mortality for a median of 4·3 years (interquartile range: 3·5–5·3 years, 84 302 person/years).
After excluding individuals with prevalent CVD (n 1320), cancer (n 781) or missing data for both diseases (n 543), unreliable medical (n 235) or dietary questionnaires (n 955), subjects lost to follow-up (n 44) or with incomplete personal data (n 408), those reporting extremely low or high values for total energy intake (<3347·2 kJ/d (<800 kcal/d) in men and 2092 kJ/d (500 kcal/d) in women or >16 736 kJ/d (>4000 kcal/d) in men and 14 644 kJ/d (3500 kcal/d) in women) and individuals with missing information on blood pressure (n 186), lipid profile (n 373) or diabetes (n 229), a total of 19 386 subjects were included in the analysis. Mortality was recorded until December 2011. Overall mortality and cause-specific mortality were assessed by the Italian mortality registry (ReNCaM registry), validated by Italian death certificates (ISTAT form) and coded according to the International Classification of Diseases (ICD-9). Cardiovascular deaths were defined when the underlying cause of death had an ICD-9 code of 390-459 or 745-747, and for cancer deaths an ICD-9 code of 140-208. A critical evaluation of the diagnosis was performed, by analysing hospital medical records for hospital deaths and for other deceased patients if previously hospitalised during the follow-up. The process of ascertainment of death causes was conducted by qualified personnel blinded to the present analyses.
The Moli-sani study was approved by the Ethics Committee of the Catholic University of Rome, Italy. All participants signed an informed consent.
Dietary information
Food intake during the year before enrolment was ascertained by the validated Italian version of the ‘European Project Investigation into Cancer and Nutrition’ (EPIC) FFQ( Reference Pala, Sieri and Palli 24 , Reference Pisani, Faggiano and Krogh 25 ), which includes 188 food items, classified into forty-five predefined food groups on the basis of similar nutrient characteristics or culinary usage.
The EPIC questionnaire has a specific question about nut intake, including the frequency of consumption of walnuts, hazelnuts, almonds and peanuts. Frequency was categorised as never, rare (≤2 times/month), 3–7 or ≥8 times/month.
Adherence to the MD was evaluated by the Mediterranean diet score (MDS) developed by Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 26 ). The scoring was based on the intake of the following nine items: vegetables, legumes, fruit and nuts, dairy products, cereals, meat and meat products, fish, alcohol and monounsaturated/saturated fats. For most items, consumption above the study population median received 1 point; all other intakes received 0 points. Consumption below the median of dairy products, meat and meat products received 1 point. Medians were sex-specific. For ethanol, men who consumed 10–50 g/d and women who consumed 5–25 g/d received 1 point; otherwise, the score was 0. The possible scores thus ranged between 0 and 9, the latter reflecting the maximal adherence to MD. In the present study, nut intake was not included in the MDS in order to control for dietary habits. Adherence to the MD was also categorised as poor (0–3), average (4–5) and good (>5). Daily energy intake (kcal/d) was divided into quintiles.
Laboratory analyses
All blood samples were obtained from participants who had fasted overnight and had refrained from smoking for at least 6 h. Serum lipids and blood glucose were assayed by enzymatic reaction methods using an automatic analyser (ILab 350; Instrumentation Laboratory). LDL-cholesterol was calculated according to Friedewald( Reference Cleeman 27 ).
High-sensitivity (hs) C-reactive protein (CRP) was measured in fresh serum samples within 3 h of collection by a particle-enhanced immunoturbidimetric assay (IL-Coagulation-Systems ACL9000; Instrumentation Laboratory). Quality control for hs-CRP was maintained using in-house serum pool and internal laboratory standard at 1·5 mg/l; inter-day CV of hs-CRP were 5·5 and 4·2 %, respectively.
Haemocromocytometric analysis was performed by cell count (Coulter HMX, Beckman Coulter; Instrumentation Laboratory). Neutrophil (granulocyte):lymphocyte ratio (NLR) was calculated as a marker of low-grade inflammation( Reference Bonaccio, Di Castelnuovo and De Curtis 22 , Reference Bhat, Teli and Rijal 28 ).
Assessment of risk factors
BMI was calculated as kg/m2 and then categorised into three levels as normal (≤25 kg/m2), overweight (>25 and <30 kg/m2) or obese (≥30 kg/m2). Hypertension was defined as systolic blood pressure≥140 mm Hg or diastolic blood pressure≥90 mm Hg or treatment for hypertension. Hypercholesterolaemia was defined as total cholesterol ≥240 mg/dl (6.2 mmol/l) or the use of specific medication. Diabetes was defined as blood glucose≥126 mg/dl (7 mmol/l) or the use of specific pharmacological treatment. The metabolic syndrome was defined according to Adult Treatment Panel III criteria( Reference Grundy, Cleeman and Daniels 29 ), based on at least three of these criteria: elevated waist circumference (>102 cm in men, >88 cm in women); elevated TAG (>150 mg/dl; 1.7 mmol/l) or drug treatment for elevated TAG; reduced HDL-cholesterol<40 mg/dl (1·03 mmol/l) in men and <50 mg/dl (1·3 mmol/l) in women or drug treatment for reduced HDL-cholesterol, elevated blood pressure (>130 mmHg systolic blood pressure or >85 mmHg diastolic blood pressure) or anti-hypertensive drug treatment in a patient with a history of hypertension; and elevated fasting glucose (>100 mg/dl; 5.55 mmol/l) or drug treatment for elevated glucose. Subjects were classified as never-smokers, current smokers or ex-smokers (quitting from at least 1 year).
Leisure-time physical activity was assessed by a structured questionnaire (questions on walking, gardening and sport participation)( Reference Pereira, Fitzgerald and Gregg 30 ), and expressed as daily energy expenditure in metabolic equivalent task-hours (MET/d) and then categorised as below (≤2·27 MET/d) or above the median (>2·27 MET/d).
Educational level as an indicator of socio-economic status was considered as secondary school or lower and high school or higher.
Statistical analysis
ANOVA for continuous or categorical variables was used to identify variables associated with the frequency of nut consumption and included socio-demographic variables (age, sex, smoking habit, educational level and leisure-time physical activity), BMI, hypertension, systolic and diastolic blood pressure, hypercholesterolaemia, diabetes, blood glucose, adherence to the MD and total energy intake. In addition, associations with biomarkers of low-grade inflammation (CRP, platelet and leucocyte counts or NLR) and lipid profile (HDL-cholesterol, LDL-cholesterol, total cholesterol and TAG) were tested. Associations with P value<0·10 were used in the multivariable model. However, hypercholesterolaemia and diabetes were not included in the multivariable model, as these factors are likely to be in the causal pathway of mortality protection by nuts.
Crude, age/sex-adjusted or multivariable hazard ratios (HR) with corresponding 95 % CI were calculated using the Cox proportional hazard model considering subjects in the lowest category of nut consumption as the reference group. The multivariable model was controlled for energy intake, leisure-time physical activity, smoking, educational level, BMI and the MDS not including nut consumption. An additional multivariable model was further controlled for inflammatory biomarkers to test their role as possible mediators of the effect. Sensitivity analyses were undertaken to estimate the impact of nut intake within subgroups at different CVD risk. Appropriate interaction terms were added to the models to test for differences of the effect. In cause-specific and sensitivity analyses, nut intake was compared with the no-intake group.
The data analysis was generated using the SAS/STAT software, version 9.1.3 of the SAS System for Windows©2009. SAS Institute Inc. and SAS are registered trademarks of SAS Institute Inc.
Results
Baseline characteristics of the population according to the frequency of nut intake are reported in Table 1. Compared with subjects with no nut consumption, those with the highest nut consumption had lower prevalence of diabetes and lower blood glucose levels, higher total cholesterol, LDL-cholesterol and HDL-cholesterol levels and higher energy intake.
Table 1 Main characteristics of the study population according to the frequency of nut intakeFootnote * (Mean values and standard deviations for continuous variables; numbers and percentages for categorical variables)

PA, physical activity; MET, metabolic equivalent task-hours; BP, blood pressure; CRP, C-reactive protein.
* Means for biomarkers of inflammation, biochemical parameters and systolic or diastolic BP are adjusted for age and sex. All P values are adjusted for age and sex.
† Number of subjects and percentage.
‡ Numbers do not add up to 100 % because of missing values.
§ To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
|| P values obtained from the model adjusted for age, sex, educational level (low/high), smoking (never, smoker, former), leisure-time PA (continuous), BMI (continuous), energy intake (continuous), Mediterranean diet score without nuts (continuous).
¶ Values for CRP are reported as geometric means with corresponding 95 % confidence intervals.
During a median follow-up of 4·3 years, 334 overall deaths occurred, among which 104 CVD and 124 cancer deaths could be identified. Compared with those reporting no nut intake, a reduction in mortality was observed at increased consumption of nuts (P for trend=0·0010), with a protection of 47 % (10–68 %) at highest intake (Table 2, model 1); these values likely reflect a plateau-like effect.
Table 2 Risk of death associated with the frequency of nut intake (Hazard ratios and 95 % confidence intervals)
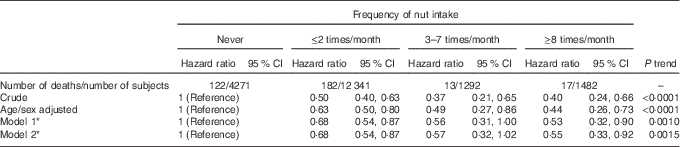
* Model 1: multivariable hazard ratios with corresponding 95 % confidence intervals obtained from the model adjusted for age, sex, educational level (low/high), smoking (never, smoker, former), leisure-time physical activity (continuous), BMI (continuous), energy intake (continuous), Mediterranean diet score without nuts (continuous). Model 2: same as model 1, but further controlled for biomarkers of inflammation (C-reactive protein, platelet count and the neutrophil:lymphocyte ratio).
Cause-specific risks of death are reported in Table 3. As compared with those who never ate nuts, subjects consuming nuts showed a lower risk of overall mortality of 34 (95 % CI 17, 47 ) % and reduced cancer death of 3 6 (95 % CI 6, 56) %; CVD mortality exhibited a similar, but not significant, inverse trend (HR=0·87; 95 % CI 0·57, 1·32). When CVD deaths were limited to CHD, a non-significant trend of protection of 26 % was found (Table 3). Stroke deaths were not associated with any nut intake (Table 3).
Table 3 Cause-specific risks of death by nut intake v. no intakeFootnote * (Hazard ratios and 95 % confidence intervals)

* Model 1 and P (1): hazard ratios with 95 % confidence intervals and P values obtained from the model adjusted for age, sex, educational level (low/high), smoking (never, smoker, former), leisure-time physical activity (continuous), BMI (continuous), energy intake (continuous) and Mediterranean diet score without nuts (continuous). Model 2 and P (2): same as in model 1, but further controlled for C-reactive protein platelet count and the neutrophil:lymphocyte ratio.
Subgroup analyses are reported in Table 4. The protection against mortality of nut intake was more evident in subjects with poor adherence to MD (HR=0·47; 95 % CI 0·31, 0·71) as compared with those with greater adherence (P for interaction=0·022; Table 4). Among all other subgroups, the effects of nuts on mortality were similar across different categories of consumption (Table 4).
Table 4 Subgroup analyses by nut intake v. no intake for total mortalityFootnote * (Hazard ratios (HR) and 95 % confidence intervals)

* HR with 95 % CI and P values obtained from the model adjusted for age, sex, educational level (low/high), smoking (never, smoker, former), leisure-time physical activity (continuous), BMI (continuous), energy intake (continuous), Mediterranean diet score without nuts (continuous).
† Numbers do not add up to 100 % because of missing values.
The relationship between nut intake and biomarkers of low-grade inflammation is reported in Table 1. More frequent nut consumption was associated with lower levels of CRP, platelet count and the NLR. No association was found with leucocyte count.
The association between mortality and nut intake was not modified by the inclusion of CRP, platelet count and NLR (Table 2, model 2; Table 3, model 2), or by the inclusion of lipid profile (HDL- and LDL-cholesterol; data not shown).
Discussion
Results from this large prospective study show that nut intake is associated with a reduced risk of all-cause death in a general Italian population that is apparently free, at enrolment, from CVD and cancer.
As compared with subjects who did not eat nuts, participants consuming nuts ≥8 times/month showed a reduced risk (47 %) of dying from any cause after controlling for potential confounders, including other dietary components of the MD. In addition, those eating nuts more rarely (≤2 times/month) exhibited a lower risk of mortality of 32 %. When analyses were restricted to the two main groups – that is, nut consumers v. no consumers – we found a protection of 34 % for those consuming nuts.
Cause-specific risks of death
An effort was made to address the association of nut intake with specific causes of death. Our results show that nut intake was inversely and significantly associated with reduced risk of cancer death, in agreement with a recent investigation suggesting a beneficial effect of nuts, even at modest intakes, in lowering cancer deaths in two large, independent cohorts of nurses and other male health care professionals, from the USA( Reference Bao, Han and Hu 18 ). Further studies will be needed to identify possible biological mechanisms explaining the observed inverse relationship; so far, limited evidence supports benefits of nut consumption on some types of cancer, such as colorectal cancer( Reference Jenab, Ferrari and Slimani 31 ) or endometrial cancer in women( Reference Petridou, Kedikoglou and Koukoulomatis 32 ), but the consumption of nuts has often been reported in association with seeds and legumes( Reference Gonzalez and Salas-Salvado 33 ). In addition, in previous studies, the anti-carcinogenic advantages of nuts appeared only in women, whereas poor effects have been documented in men( Reference Jenab, Ferrari and Slimani 31 , Reference Gonzalez and Salas-Salvado 33 ). Unfortunately, we were not able to address cancer or sex-specific analyses because of lack of a sufficient number of cases. It is noteworthy that our findings are also in agreement with results of an analysis conducted within the framework of the PREDIMED study, the very first large intervention trial on MD( Reference Estruch, Ros and Salas-Salvadó 8 , Reference Guasch-Ferré, Bulló and Martínez-González 19 ): that analysis( Reference Guasch-Ferré, Bulló and Martínez-González 19 ) revealed that increased frequency of nut consumption was associated with a 40 % reduced risk of cancer death, comparable with a 36 % reduction in cancer mortality observed in our study. As the PREDIMED study was conducted within high CVD risk subjects older than 55 years, our study somehow extends the positive results documented in the PREDIMED trial to a more general, younger population. The actual role of nut intake in relation to cancer risk surely deserves further investigation( Reference Sabaté and Ang 9 ).
When specific CVD mortality was considered, a nonstatistically significant trend for protection was found. However, when only CHD death was considered, the reduction in death risk was similar to that observed for cancer mortality, but, most probably, the small number of CHD cases did not allow to reach any statistical significance. Yet, a recent meta-analysis found that cardiovascular advantages from nut consumption were primarily driven by decreased coronary artery disease deaths( Reference Grosso, Yang and Marventano 34 ).
It is noteworthy that the association of nut consumption with stroke deaths was weaker than that observed for other causes, and no statistical significance was reached. Our overall results on CVD events also appear in agreement with data from a meta-analysis that suggested similar protection for CVD, ischaemic heart disease and total mortality, but no protection for type 2 diabetes or stroke( Reference Luo, Zhang and Ding 7 ).
However, benefits from nut intake against diabetes were observed in the PREDIMED-Reus trial( Reference Salas-Salvadó, Bulló and Babio 35 ).
Subgroup analyses
Our study has also examined the effects of nut intake within subgroups at different risk for both CVD or cancer. The protection against mortality offered by nut intake was more evident in subjects with poor adherence to the MD as compared with those showing greater adherence. Nut intake appeared to add no additional protection against mortality within individuals who already followed an MD, probably because they already benefit from other fundamental components of this healthy dietary pattern, such as olive oil, moderate alcohol intake or antioxidant-rich foods. Conversely, nut intake alone seems to convey some health advantage to those who stick less to this dietary pattern. These data are in agreement with those observed in non-Mediterranean population settings, such as in the USA, in which health advantages from nut consumption have been reported( Reference Rohrmann and Faeh 36 ).
Role of inflammation
Finally, we addressed the possible role of low-grade inflammation on the causal pathway between nuts and the risk of death. Results showed that subjects consuming higher amounts of nuts had lower values of biomarkers of inflammation (CRP, platelet count or NLR), probably indicating a reduced subclinical inflammatory status in nut consumers, in agreement with previous studies( Reference Jiang, Jacobs and Mayer-Davis 5 ). However, we observed that inflammation did not account for the inverse associations between nut intake and overall or cancer deaths. Similarly, adjustment for lipid status did not modify the association.
Strengths and limitations of this study
The strengths of this large epidemiological study in a Mediterranean population are represented by its prospective nature. In addition, further control of all analyses by a wide panel of possible confounding factors and diet-related behaviours should assure consistency to the observed association.
A major limitation of the present study in an apparently healthy population is the relatively small number of deaths, but this did not prevent statistically significant differences to be consistently observed, at least for both total and cancer mortality. The relatively low number of CVD, CHD or stroke events did not allow to provide conclusive and consistent results or to explore more deeply the plateau effect, which may be linked to specific death causes (i.e. stroke, for which a J-shape was already observed( Reference Djoussé, Gaziano and Kase 37 )) rather than others. As far as total and cancer deaths are concerned, our study did reach the same major conclusions of a much larger population study( Reference Bao, Han and Hu 18 ).
Conclusions
The present results obtained in people living in a Mediterranean country provide evidence of an inverse association between nut consumption and risk of death in a general population that is apparently free from CVD and cancer at enrolment. To our knowledge, this study is one of the first observational investigations to address the health advantages of nut intake for subjects at risk for CVD or cancer, such as subjects who are obese, affected by diabetes, metabolic syndrome or are less adherent to the MD. In addition, this study adds knowledge on the relationship between nut consumption and cancer, an issue on which evidence is still scarce and controversial.
Finally, our findings suggest the opportunity to consider nuts as an important part of a healthy dietary pattern for the prevention of chronic diseases, as already suggested by the PREDIMED study( Reference Estruch, Ros and Salas-Salvadó 8 , Reference Guasch-Ferré, Bulló and Martínez-González 19 , Reference Babio, Toledo and Estruch 38 , Reference Salas-Salvadó, Bulló and Estruch 39 ). Possibly, nut consumption should be mainly encouraged in countries in which nut intake is not regular and MD is not followed as a regular eating pattern.
The health advantages of nuts observed at low to moderate doses are in agreement with the beneficial effects of specific foods such as salt( Reference O’Donnell, Mente and Rangarajan 40 ), dark chocolate( Reference di Giuseppe, Di Castelnuovo and Centritto 41 ) or alcohol( Reference Di Castelnuovo, Costanzo and Bagnardi 42 ), whose protective role has only been detected at low to moderate intakes.
Acknowledgements
The Moli-sani research group thanks Dr Vittorio Krogh and Dr Sabina Sieri (Istituto Nazionale dei Tumori, Milan, Italy) for their contribution to dietary questionnaire analysis and interpretation, the Associazione Cuore Sano Onlus (Campobasso) for its financial support and the Azienda Sanitaria Regionale del Molise (ASReM, Campobasso, Italy), the Offices of vital statistics of the Molise region and the Molise Dati Spa (Campobasso, Italy) for their collaboration and support provided during the follow-up activities.
The enrolment phase of the Moli-sani Project was conducted at the Research Laboratories of the Catholic University in Campobasso (Italy) and supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy) – Programma Triennale di Ricerca, Decreto no. 1588 and Instrumentation Laboratory, Milan, Italy. These funders had no role in study design, collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors were and are independent from funders. Marialaura Bonaccio was supported by a Fondazione Umberto Veronesi Fellowship.
M. B., L. I. and A. Di. C. designed the present research; A. De. C., S. C., M. P. and F. B. managed data collection; M. B. and A. Di. C. analysed the data; M. B. wrote the paper; and M. B. D., G. d. G. and L. I. originally inspired the research and critically reviewed the manuscript.
There are no conflicts of interest.
Appendix: Moli-sani study investigators
Steering committee
Licia Iacoviello (Neuromed, Pozzilli, Italy), Chairperson, Maria Benedetta Donati and Giovanni de Gaetano (Neuromed, Pozzilli, Italy) and Simona Giampaoli (Istituto Superiore di Sanità, Roma, Italy).
Safety and data monitoring committee
Jos Vermylen (Catholic Univesity, Leuven, Belgio), Chairman, Ignacio De Paula Carrasco (Accademia Pontificia Pro Vita, Roma, Italy), Fabrizio Oleari (Istituto Superiore di Sanità, Roma, Italy) and Antonio Spagnolo (Catholic University, Roma, Italy).
Event adjudicating committee
Deodato Assanelli (Brescia, Italy), Vincenzo Centritto (Campobasso, Italy), Paola Muti (Hamilton, Ontario, Canada), Holger Schünemann (Hamilton, Ontario, Canada), Pasquale Spagnuolo and Dante Staniscia (Termoli, Italy).
Scientific and organizing secretariat
Francesco Zito (Coordinator), Americo Bonanni, Chiara Cerletti, Amalia De Curtis, Augusto Di Castelnuovo, Licia Iacoviello, Roberto Lorenzet, Antonio Mascioli, Marco Olivieri and Domenico Rotilio.
Data management and analysis
Augusto Di Castelnuovo (Coordinator), Marialaura Bonaccio, Simona Costanzo and Francesco Gianfagna.
Informatics
Marco Olivieri (Coordinator), Maurizio Giacci, Antonella Padulo and Dario Petraroia.
Biobank and biochemical analyses
Amalia De Curtis (Coordinator), Federico Marracino, Maria Spinelli and Christian Silvestri.
Communication and press office
Americo Bonanni (Coordinator), Marialaura Bonaccio and Francesca De Lucia.
Moli-family project
Francesco Gianfagna and Branislav Vohnout.
Recruitment staff
Franco Zito (General Coordinator); Secretariat: Mariarosaria Persichillo (Coordinator), Angelita Verna, Maura Di Lillo, Irene Di Stefano; Blood sample: Agostino Pannichella, Antonio Rinaldo Vizzarri, Branislav Vohnout, Agnieszka Pampuch; Spirometry: Antonella Arcari (Coordinator), Daniela Barbato, Francesca Bracone, Simona Costanzo, Carmine Di Giorgio, Sara Magnacca, Simona Panebianco, Antonello Chiovitti, Federico Marracino, Sergio Caccamo, Vanesa Caruso; Electrocardiograms: Livia Rago (Coordinator), Daniela Cugino, Francesco Zito, Francesco Gianfagna, Alessandra Ferri, Concetta Castaldi, Marcella Mignogna; Tomasz Guszcz; Questionnaires: Romina di Giuseppe (Coordinator), Paola Barisciano, Lorena Buonaccorsi, Floriana Centritto, Antonella Cutrone, Francesca De Lucia, Francesca Fanelli, Iolanda Santimone, Anna Sciarretta, Maura Di Lillo, Isabella Sorella, Irene Di Stefano, Emanuela Plescia, Alessandra Molinaro and Christiana Cavone.
Call center
Giovanna Galuppo, Maura Di Lillo, Concetta Castaldi, Dolores D’Angelo and Rosanna Ramacciato.






