Few studies have investigated the effect of water intake on the incidence of CVD. In the Adventist Health Study there was a dose-relationship between water intake and protection against fatal CHD (Chan et al. Reference Chan, Knutsen, Blix, Lee and Fraser2002). One of the suggested mechanisms of this effect is that water ingestion may reduce blood viscosity; however, a recent systematic review found no direct evidence that a decrease in viscosity due to high fluid intake can prevent CVD (Okamura et al. Reference Okamura, Washimi, Endo, Tokuda, Shiga, Miura and Nojiri2005).
The primary determinants of whole-blood viscosity are plasma viscosity, packed cell volume and macromolecules, including fibrinogen. Initial reports indicated that plasma viscosity (Yarnell et al. Reference Yarnell, Baker, Sweetnam, Bainton, O'Brien, Whitehead and Elwood1991) and packed cell volume (Gagnon et al. Reference Gagnon, Zhang, Brand and Kannel1994) were independent predictors of CVD. Subsequently, whole-blood viscosity and packed cell volume were shown to be independent predictors of coronary events (Danesh et al. Reference Danesh, Collins, Peto and Lowe2000). The major CVD risk factors are independently associated with whole-blood or plasma viscosity (Bonithon-Kopp et al. Reference Bonithon-Kopp, Levenson, Scarabin, Guillanneuf, Kirzin, Malmejac and Guize1993; Fossum et al. Reference Fossum, Høieggen, Moan, Nordby, Velund and Kjeldsen1997). Thus, increased viscosity may be one mechanism by which cardiovascular risk factors promote atherosclerosis and plaque formation (Lee et al. Reference Lee, Mowbray, Lowe, Rumley, Rowkes and Allan1998). Whole-blood or plasma viscosity is inversely related to insulin sensitivity (Høieggen et al. Reference Høieggen, Fossum, Moan, Enger and Kjeldsen1998) and strongly related to metabolic risk factors (Fossum et al. Reference Fossum, Høieggen, Moan, Nordby, Velund and Kjeldsen1997).
Given this background, the question of whether increased water intake reduces blood viscosity is of interest, but only limited data exist. We examined the effect of increasing short-term water intake on whole-blood viscosity and its correlates in subjects with cardiovascular risk factors in a randomised, parallel-design, controlled clinical trial that included a control group and a group that ingested 1 litre blueberry juice/d providing a comparison with an energy-containing fluid.
Methods
Subjects
Patients seen at our clinic were informed about the study. Additional recruitment was by newspaper advertisement. Eligibility was men aged 30–70 years and women aged 45–70 years who were at least 12 months postmenopausal. Other inclusion criteria were written informed consent, willingness to drink 1 litre water or blueberry juice per d in addition to usual fluid intake and habitual ingestion of two or fewer cups (0·5 litres) water per d. In addition, the presence of at least one cardiovascular risk factor was required, including cigarette smoking, systolic blood pressure (BP) ≥ 135 but ≤ 160 mmHg or diastolic BP >90 but ≤ 100 mmHg, hyperlipidaemia, or elevated packed cell volume ( ≥ 0·40 for women or ≥ 0·42 for men).
Exclusion criteria were impaired renal function and use of lipid-lowering drugs or other drugs that could affect blood viscosity. Additional exclusions were BMI >31 kg/m2, alcohol or drug abuse or psychiatric illness, more than three units alcohol daily (men) or more than one unit daily (women), endocrine or other disease that affects thirst mechanisms, blood donation in the previous 6 months, CVD, any chronic disease, cancer (within 5 years) or other disease that could affect the results. The study was evaluated by the ethics committee (region 1).
Study design
Subjects completed the study between 2 January and Easter, between Easter and the summer vacation in June or between the end of the summer vacation in August and Christmas to minimise seasonal and holiday effects on physical activity, thirst, and risk factors. A randomised, parallel design with a 1- to 3-week run-in period followed by a 4-week intervention period was used. Following the screening visit 1–3 weeks before randomisation, potential subjects recorded all fluid intake and physical activity for 1 week. Subjects that met all the inclusion criteria were randomised to one of three groups. The control group continued their usual diet, physical activity and fluid ingestion patterns. The intervention groups continued their usual diet, physical activity and fluid ingestion and in addition were asked to increase their water intake by 1 litre/d or to ingest 1 litre blueberry juice/d. Either tap water or bottled still water was allowed; however, less than five subjects drank tap water primarily. Bottled water (Imsdal, Ringnes AS, Oslo, Norway) and blueberry juice produced with no additives or sugar (Coronar Safteri AS, Ranheim, Norway) were provided free of charge. The study coordinator obtained the group assignment for each subject by opening opaque, sealed, consecutively numbered envelopes. Follow-up clinic visits were scheduled weekly for 4 weeks and vital signs and body weight were measured. Subjects were asked to not smoke and avoid caffeine for 30 min before standardised BP measurement.
Lifestyle monitoring
The study coordinator emphasised the importance of maintaining a habitual and stable diet and fluid intake and physical activity. Enough bottled water or blueberry juice was provided to participants in those groups to last for 10 d at each weekly visit. All subjects were asked to record total fluid intake and physical activity during 1 d per week that was predetermined at the start of the study during each of the 4 weeks of the intervention. The recording diary was distributed at each visit, and included questions about the type and amount of fluids consumed and the type and duration of physical activity.
Laboratory methods
Blood collection was done at screening (weeks − 3 to − 1), baseline (week 0) and weeks 2 and 4 (end of study) after a 12 h overnight fast. Samples for haematology, lipids, fibrinogen, Na, K, osmolality and total protein were analysed according to standard laboratory procedures at the Department of Clinical Chemistry, Ullevål University Hospital (Oslo, Norway). Samples for whole-blood viscosity determination (10 ml EDTA-anticoagulated blood) were analysed within 4 h after collection at the Laboratory of Internal Medicine, Medical Department, Ullevål University Hospital using a Bohlin CS 10 rotational double-gap Rheometer (Bohlin Instruments, Lund, Sweden). All analyses were carried out at a temperature of 37°C. The technique has an interassay CV of < 7 % (Fossum et al. Reference Fossum, Høieggen, Moan, Nordby, Velund and Kjeldsen1997; Høieggen et al. Reference Høieggen, Fossum, Moan, Enger and Kjeldsen1998). Because viscosity is high at low shear rate and decreases as shear rate increases we chose a wide range of shear rates of 201, 99, 1·1 and 0·5 per s to evaluate the changes in blood viscosity. Determinations of urine osmolality, Na, K and creatinine concentrations and excretion at weeks 2 and 4 were done using standard procedures.
Statistics
A previous study showed that at high shear rates the mean whole-blood viscosity is 4·23 (sd 0·5) mPa × s. With α set at 0·05 and β at 0·80 and a 10 % decrease in blood viscosity, twenty-five subjects in each group was required. Between-group changes were analysed with repeated-measures ANOVA. Group contrasts were analysed using the Bonferroni test. All tests were two-tailed and P < 0·05 was considered statistically significant. The calculations were done with SPSS (version 13; SPSS Inc., Chicago, IL, USA).
Results
Subjects were randomised to the control group (n 32), increased water intake group (n 34) and blueberry juice group (n 33). One control and two subjects in each of the intervention groups dropped out. The mean age was 53 years, the mean cholesterol level was 6·4 mmol/l, the mean BP was 133/85 mmHg, the mean packed cell volume was 0·41 and about 40 % of subjects were smokers. Total fluid consumption was 1·86 (sd 0·55) and 1·96 (sd 0·87) litre/d at baseline and 4 weeks, respectively, in the control group, while consumption increased from 1·97 (sd 0·69) to 3·01 (sd 0·78) litre/d in the water group and from 1·93 (sd 0·90) to 2·85 (sd 0·75) litre/d in the blueberry juice group.
BP, pulse and body weight remained stable and unchanged between the groups (data not shown). There were no changes in blood haematology and chemistry values including fibrinogen, total protein, lipids, glucose, insulin and C-peptide in the intervention compared with the control group (data not shown). In both intervention groups, urine volume increased and levels of urine osmolality, electrolytes and creatinine decreased (Table 1). The median change in urine volume from baseline to week 4 was 10 (range − 1500 to +1230) ml in the control group v. 872 (range − 920 to +1960) ml in the water group and 725 (range − 700 to +2640) ml in the blueberry juice group.
Table 1 Urine volume and chemistry in the control and intervention (water and blueberry) groups (Mean values and standard deviations)
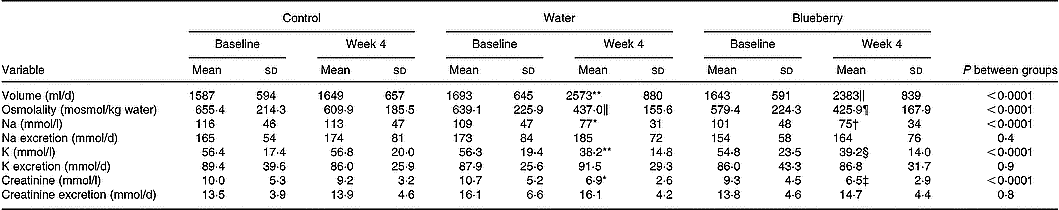
Mean value was significantly different from that of the control group: *P = 0·03, †P = 0·01, ‡P = 0·007, §P = 0·003, ‖P = 0·002, ¶P = 0·001, ** P < 0·0001.
Whole-blood viscosity was unchanged between the groups (Table 2). Baseline levels of blood viscosity (shear rate 99/s) were correlated with BMI (Pearson's correlation coefficient r 0·22; P = 0·03), waist circumference (r 0·39; P < 0·0001), triacylglycerols (r 0·24; P = 0·02) and HDL-cholesterol (r − 0·27; P = 0·008). Blood viscosity was not related to urine volume (r 0·03; P = 0·8), urine osmolality (r 0·18; P = 0·09) or reported fluid intake (r 0·18; P = 0·08) at baseline (shear rate 99/s). All these correlations were similar to those at other shear rates (data not shown).
Table 2 Whole-blood viscosity (mPa×s) at different shear rates in the control and intervention (water and blueberry juice) groups* (Mean values and standard deviations)
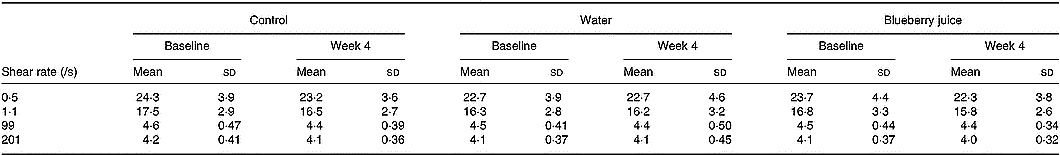
* There were no statistically significant differences between the groups.
Discussion
We observed no change in whole-blood viscosity, fibrinogen, lipids or other chemistry and haematology variables in the present randomised clinical trial of a short-term increase in water or blueberry juice ingestion. Subjects were men and women with one or more risk factors for CVD and thus may have had a high potential for a reduction in blood viscosity. Blood viscosity was correlated with the cardiovascular risk factor levels as expected and as has been demonstrated in previous studies but there was no relationship between blood viscosity and water intake, urine volume or urine osmolality at baseline.
There are several probable explanations for the lack of effect of water ingestion. First, the subjects had no clinical conditions likely to cause mild hypohydration and their intake of total fluids was adequate at baseline (nearly 2 litres/d). Furthermore, the subjects had normal renal function; normal kidneys are very effective in maintaining intravascular volume. Second, the increased fluid ingestion (water or blueberry juice) was distributed throughout the day and not just before the measurement of blood viscosity. Usual homeostatic mechanisms are expected to rapidly compensate for the increase in fluid ingestion leading to an increase in urine volume. In contrast, in a previous study conducted among a small number of elderly subjects, ingestion of a glass of an electrolyte drink at midnight was associated with a drop or a lower rise in blood viscosity at 04.00 and 08.00 hours (Kurabayashi et al. Reference Kurabayashi, Kubota, Tamura and Shirakura1991).
Though we did not evaluate fluid intake from solid foods, the observed increase in urine volume indicates that the primary aim of the study, to attain an increase in fluid intake, was achieved, and that the subjects in the study complied with the instructions to increase fluid intake. A larger increase may have been required to show an effect on viscosity. Urine osmolality was reduced and changes in urinary excretion of Na, K and creatinine were observed. Total fluid intake at the start of the study correlated with urine volume (r 0·56; P < 0·01) and with urine osmolality (r 0·31; P < 0·01), strengthening the validity of the reported fluid intake according to the diary.
In conclusion, increased water intake in the short term did not decrease blood viscosity in subjects with cardiovascular risk factors. Further study is required to determine long-term effects and whether these may occur in other patient groups.
Acknowledgements
We thank Lisa Flakk for study coordination, Roseli Andreassen for technical help with the blood viscosity analyses, Eli Heggen for help with medical evaluations, Ringnes AS for the bottled water and Coronar Safteri AS for the blueberry juice.




