Nutritional influences on the development of CVD may operate throughout life(Reference Harding1–Reference McMillen and Robinson3). During fetal life, marine n-3 PUFA, mainly found in fatty fish, are transferred to the fetus from the mother(Reference Uauy, Peirano and Hoffman4). The fact that marine n-3 PUFA are incorporated into cell membranes(Reference Martinez5) and are involved in hormonal pathways(Reference Poudyal, Panchal and Diwan6, Reference Russo7) and have effects on the expression of genes involved in inflammation and lipid metabolism(Reference Sun, Wei and Li8–Reference Jump10) and on the proliferation and differentiation of adipose tissue(Reference Parrish, Pathy and Angel11–Reference Raclot, Groscolas and Langin13) suggests that dietary long-chain n-3 PUFA could have an impact on cardiometabolic risk factors through several mechanisms.
Previous human trials that have investigated the effect of supplementation with long-chain n-3 PUFA during pregnancy on factors associated with cardiometabolic risk in offspring vary in terms of both the intervention period and the amount of n-3 PUFA given(Reference Helland, Smith and Blomen14–Reference Rytter, Christensen and Bech19). Also, the follow-up period in most of the studies is relatively short. We have recently followed up the offspring of women who had participated in a randomised controlled trial with fish oil supplementation during the third trimester of pregnancy(Reference Rytter, Bech and Christensen17–Reference Rytter, Christensen and Bech19). The offspring were followed up after 19 years, and we found no effect of fish oil supplementation on any of the measured factors associated with cardiometabolic risk. However, particularly the development of adipose tissue already starts in the second trimester of pregnancy(Reference Ailhaud, Massiera and Weill20), and therefore it is possible that the intake of marine n-3 PUFA during this period could influence later adiposity.
Previous studies that have investigated the intake of marine n-3 PUFA as early as the second trimester of pregnancy have reported inconsistent results(Reference Helland, Smith and Blomen14–Reference Stein, Wang and Martorell16, Reference Donahue, Rifas-Shiman and Gold21). Hence, the evidence for a possible programming effect of marine n-3 PUFA intake during mid-pregnancy on later cardiometabolic risk in humans is still sparse and inconclusive.
In the present study, we investigated the hypothesis that the intake of long-chain n-3 PUFA during the second trimester of pregnancy has an impact on factors associated with cardiometabolic risk in the offspring at the age of 19–20 years. Thus, the offspring from a birth cohort study conducted in 1988–9 were followed up after 20 years.
Methods
Original study
Details about the recruitment and dietary assessments have been described in detail previously(Reference Olsen, Hansen and Sandstrom22).
In brief, the original study population included 965 pregnant women recruited for a birth cohort study in Aarhus, Denmark, from April 1988 until January 1989. These women represented 80 % of a consecutive sample of 1212 women making visits for routine antenatal care to a specific midwife centre in the city. The women filled out a self-administered dietary questionnaire 1 week before the scheduled antenatal visit in week 30 of gestation, and provided that consent was given, a supplementary face-to-face interview was conducted after the visit. A blood sample was obtained from the women after the interview. The mean age of the participating women was 29 years; approximately 58 % were nulliparous and 40 % had been smoking during pregnancy. Approximately 9 % ate no fish during pregnancy.
Intake of marine n-3 PUFA
The intake of marine n-3 PUFA was quantified by a combination of the answers given by the women in response to the self-administered questionnaire and the face-to-face interview. The questionnaire mainly covered breakfast and lunch. The interview focused on quantifying the main ingredients of cooked meals and on completing the information collected from the questionnaire in order to make a quantitative estimation of the selected food items. Photographs modelling portion sizes were used in the quantification procedure. Emphasis was mainly placed on the intake of marine n-3 PUFA and energy-containing ingredients. Both in the mailed questionnaire and in the structured interview, the women were asked to ensure that the reported intakes represented those taken by them in the recent 3 months, approximately corresponding to the second trimester of pregnancy. In the structured interview, a trained person checked and corrected the questionnaire for any possible misunderstandings.
The estimated contents of marine n-3 PUFA in various food items used to quantify the total intake of marine n-3 PUFA have been described previously(Reference Olsen, Hansen and Sandstrom22).
A random sample (14 %) of the participating women had their blood samples analysed with regard to the fatty acid profile of erythrocytes. The reported daily intake of marine n-3 PUFA was found to be correlated with the sum of marine n-3 PUFA in erythrocytes with a correlation coefficient of 0·45(Reference Olsen, Hansen and Sandstrom22).
Follow-up
The offspring of the observational birth cohort constituted the study population in the present study. At the time of the study, the offspring were aged between 19 and 20 years. A total of 915 (95 %) mother and child pairs could be identified in the central registration registry and were alive and living in Denmark at the time of the follow-up.
The mothers were contacted by mail and informed about the follow-up. Thereafter, the offspring were contacted and asked to complete a self-administered Web-based questionnaire concerning their anthropometric measures, health and lifestyle. They were also asked whether they were interested in participating in a clinical examination. Those who agreed and those who did not answer the questionnaire were all invited to participate in a physical examination. A total of 688 (71 %) filled out the questionnaire and 443 (46 %) participated in the clinical examination.
The physical examination was performed by a trained nurse and a trained medical laboratory technician. Height, weight and waist circumference were measured. Following this, the participants rested for 5 min and their short-term (2 min) heart rate (HR) variability (HRV) was measured in a horizontal position using a validated handheld device (HealthMate; Medicus Engineering)(Reference Ejskjaer, Fleischer and Fleischer23, Reference Fleischer, Nielsen and Laugesen24). HRV was included as a measure of the balance between sympathetic and parasympathetic activities on the sinus node. The standard deviation of the time intervals between consecutive heart beats was determined. While still in the horizontal position, resting blood pressure (BP) and HR were measured three times (at an interval of 2 min) with an automatic BP device (OMRON M6 Comfort (HEM-7000-E)). The mean of the last two measurements was used in the analyses. Finally, as a measure of the parasympathetic nervous function, the mean ratio between the highest and the lowest HR within a breathing cycle (HRVE/I) was measured with the participant sitting at a desk, holding the HealthMate in both hands. The participant was instructed to breathe slowly for 1 min according to an illustration on the HealthMate display.
Blood analyses
A fasting (10 h) blood sample was drawn, centrifuged and frozen at − 80°C. Blood glucose was measured using bedside equipment (Accu-chek; Roche Diagnostics) immediately after blood sampling.
High-sensitivity C-reactive protein was measured by an immunoturbidimetric assay (Randox Laboratories Limited) on an Advia 1650 (Bayer Diagnostics). Detection limit was 0·20 mg/l.
HbA1c was measured by an HPLC assay (Bio-Rad Laboratories) on a Variant II Turbo.
Serum adiponectin, leptin and IgF-1 were determined at the Medical Research Laboratories in Aarhus, Denmark, by in-house validated assays. Adiponectin was measured by a time-resolved immunofluorometric assay based on two antibodies and recombinant human adiponectin (R&D Systems) as described previously(Reference Frystyk, Tarnow and Hansen25). Serum leptin was determined by a time-resolved immunofluorometric assay based on commercially available reagents (R&D Systems) and recombinant human leptin as the standard, and carried out essentially as had been done for adiponectin(Reference Frystyk, Tarnow and Hansen25). Serum total IgF-I was measured by RIA(Reference Frystyk, Dinesen and Orskov26). Plasma insulin concentrations were determined using a commercial ELISA kit (DAKO).
Insulin resistance was estimated using homeostatic model assessment (HOMA-IR). HOMA-IR was calculated as follows:
Serum TAG and cholesterol (total, LDL and HDL) were measured according to standard methods on a Modular P (Roche Diagnostics). ApoA1 and apoB were measured using antibodies obtained from DAKO, on an Advia 1650 from Bayer Diagnostics.
The study was conducted according to the guidelines given in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethics committee (journal no. 20070157) and the Danish Data Protection Agency (journal no. 2006-41–6257). Written informed consent was obtained from all participants.
Statistical analyses
All statistical tests were performed using STATA software package 11.0 (Stata Corporation).
A two-sided P value below 0·05 was considered as statistically significant.
Exposure
As maternal energy intake is a potential confounding factor when examining the attributes of metabolic disorders in the offspring, the intake of marine n-3 PUFA was adjusted for total energy intake using the residual method(Reference Willett, Howe and Kushi27). Following this, exposure was divided into quintiles. This was done for the entire study population, regardless of their participation in the follow-up.
Covariates
χ2 Tests and Student's t tests were used to compare categorical and normally distributed continuous variables, respectively, between the participants and non-participants. It was a priori decided to adjust for major risk factors for the outcome. Hence, maternal pre-pregnancy BMI (continuous, 5 % missing), maternal education (elementary school, high-school or technical school, university, higher academic or other, 7 % missing) and smoking during pregnancy (never, < 10 and ≥ 10 cigarettes/d, 5 % missing) were included in the model to adjust for potential genetic and/or environmental disposition to the outcomes. Also, maternal age (continuous, no missing) and parity (0, 1 or ≥ 2, 1 % missing) were included to adjust for factors associated with the diet and other factors that could indirectly affect growth and adiposity. Energy intake was included in the model (quintiles, 1 % missing), since it may have an important association with the outcomes independent of marine n-3 PUFA. Differential programming effects of marine n-3 PUFA had been described previously in males and females, and therefore all analyses were initially stratified by sex. However, since no sex-specific effects were found, sex was included in the model as a potential confounder (no missing). Only participants with complete information on covariates were included in the main analyses.
Analyses
Multiple linear regression modelling, using the lowest quintile of marine n-3 PUFA intake as the reference, was used to estimate the association with all outcomes.
A total of 16 % of the participants had an high-sensitivity C-reactive protein value below the detection limit of 0·20 mg/l. Assuming that the data below the detection limit were missing at random, multiple imputations were used to impute the values below the detection limit on the basis of sex, BMI, smoking habit and parental overweight.
The distributions of the biochemical and HRV variables were generally skewed and therefore log-transformed for normalisation. Following this, all regressions were found to fulfil the assumptions of normally distributed residuals and approximately equal variance of residuals.
Due to the relatively high loss to follow-up and an association between participation in the clinical examination and self-reported BMI, a sensitivity analysis was performed in order to investigate whether the results obtained for BMI were biased. The correlation between self-reported and clinically measured BMI for the two sexes was used to calculate and impute BMI values for those who filled out the questionnaire but did not participate in the clinical examination. For those who did not fill out the questionnaire or participate in the clinical examination, a value of 25 kg/m2 was imputed. A BMI of 25 kg/m2 was chosen, since this is a relatively high and still not totally an unrealistic guess.
Results
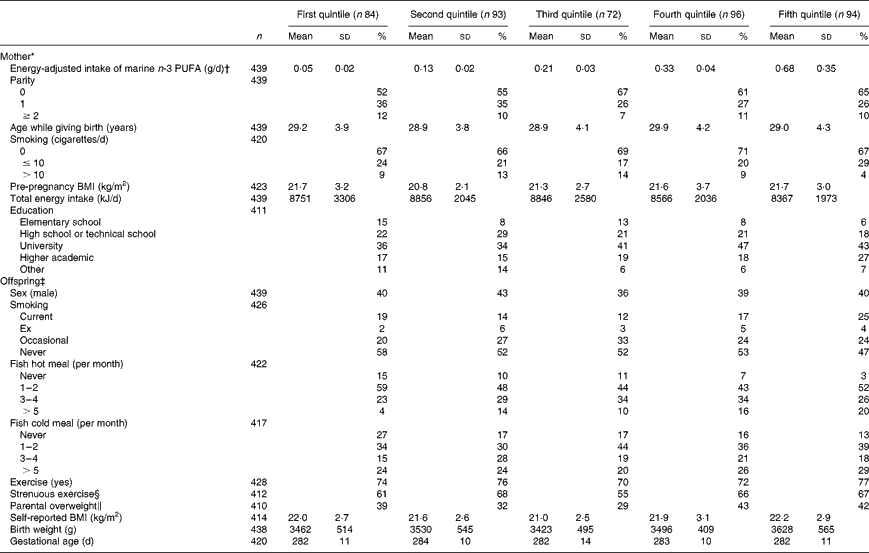
The participants and non-participants of the clinical examination were similar with respect to most of the known characteristics, such as parity, pre-pregnancy BMI, maternal age, offspring smoking and exercise habits and parental overweight. However, the participants were more likely to be females (60 v. 36 % among non-participants), have a slightly lower self-reported BMI (21·8 v. 22·5 kg/m2), eat more fish (42 v. 37 % ate fish as a warm meal at least three times per month) and to be born to mothers with a higher education (59·7 v. 47·1 % had a university or higher academic education) who were smoking less during pregnancy compared with the non-participants (37 v. 43 %). The participants and non-participants did not differ with regard to maternal intake of marine n-3 PUFA during the second trimester of pregnancy. Information about maternal intake of marine n-3 PUFA during pregnancy was lacking for four participants, leaving 439 participants available for analysis.
When comparing participant characteristics across quintiles of energy-adjusted n-3 PUFA intake, the groups only differed significantly with regard to offspring fish intake (Table 1). Thus, offspring intake of fish increased with maternal intake of marine n-3 PUFA during pregnancy. The proportion with a university education or an academic education tended to be higher in the highest fish intake quintile compared with the lowest one (43 plus 27 % v. 36 plus 17 %), as was observed for primiparous women compared with multiparous one (65 v. 52 %). Otherwise, the groups were similar with regard to the known characteristics.
Table 1 Characteristics of the participants according to the intake of marine n-3 PUFA during the second trimester of pregnancy (Mean values, standard deviations and percentages)

* Information collected from the self-administered questionnaire and structured interview of pregnant women in week 30 of gestation. Additional data collected from birth records.
† The intake of marine n-3 PUFA during the second trimester of pregnancy adjusted for daily energy intake by the residual method.
‡ Information collected from a self-administered Web-based questionnaire filled up by the offspring at the age of 19 years. Sex, birth weight and gestational age collected from birth records.
§ Defined as exercise of at least 20 min duration, resulting in breathlessness.
∥ The participants were asked as to whether they would consider any of their parents to be overweight.
BMI, waist circumference and related biochemical characteristics
The mean BMI was 22·8 and 22·1 kg/m2 in males and females, respectively, and the prevalence of overweight (BMI ≥ 25 kg/m2) was 20 % among males and 16 % among females. The mean waist circumference was 83 cm in males and 80 cm in females.
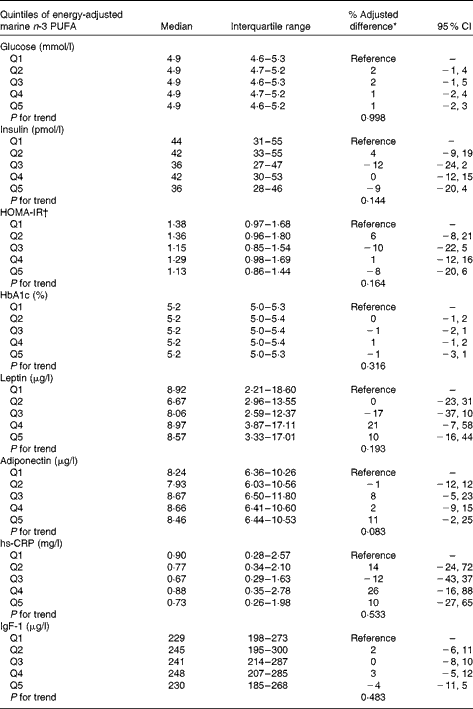
There was no association between the intake of marine n-3 PUFA during pregnancy and BMI or waist circumference in the offspring (Table 2). Sensitivity analysis for BMI did not change the estimated differences substantially. Also, overall no statistically significant association was found between the intake of marine n-3 PUFA and glucose metabolism, evaluated by fasting glucose, insulin, HOMA and HbA1c in the offspring (Table 3). Furthermore, the adipose tissue-derived hormones leptin and adiponectin as well as high-sensitivity C-reactive protein and IgF-1 were not associated with the intake of marine n-3 PUFA during pregnancy.
Table 2 Association between quintiles (Q) of energy-adjusted intake of marine n-3 PUFA during the second trimester of pregnancy and offspring adiposity (Mean values and standard deviations; adjusted differences and 95 % confidence intervals)

* Adjusted for maternal pre-pregnancy BMI, maternal education, smoking during pregnancy, maternal age, parity, energy intake and sex.
Table 3 Association between quintiles (Q) of energy-adjusted intake of marine n-3 PUFA during the second trimester of pregnancy and markers of cardiometabolic risk in the offspring (Medians and interquartile ranges; % adjusted differences and 95 % confidence intervals)

HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein.
* Adjusted difference in % relative to the lowest quintile of marine n-3 PUFA intake. Adjusted for maternal pre-pregnancy BMI, maternal education, smoking during pregnancy, maternal age, parity, energy intake and sex.
† HOMA index calculated as follows: fasting insulin (μU/ml) × fasting glucose (mmol/l)/22·5.
Blood pressure, heart rate and heart rate variability
The mean systolic BP and diastolic BP in males were 118 and 64 mmHg, respectively. A total of 35 % had a systolic BP above 120 mmHg, whereas only 2 % had a diastolic BP above 80 mmHg.
In females, the mean systolic BP was 105 mmHg and diastolic BP was 66 mmHg. The prevalence of women with a systolic BP above 120 mmHg and a diastolic BP above 80 mmHg was 3 and 1 %, respectively.
Maternal intake of marine n-3 PUFA during pregnancy was non-significantly associated with offspring BP, HR and HRV (Table 4).
Table 4 Association between quintiles (Q) of energy-adjusted intake of marine n-3 PUFA during the second trimester of pregnancy and offspring blood pressure, heart rate (HR) and short-term HR variability indices (Mean values and standard deviations; adjusted differences and 95 % confidence intervals)

SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats/min; SDNN, standard deviation of the time intervals between consecutive heart beats; HRE/I, median ratio between the highest and the lowest heart rate within every breathing cycle.
* Difference in % relative to the lowest quintile of marine n-3 PUFA intake during the second trimester of pregnancy.
† SDNN in a 2 min heart rate recording.
‡ HRE/I in a 1 min recording.
Lipid profile
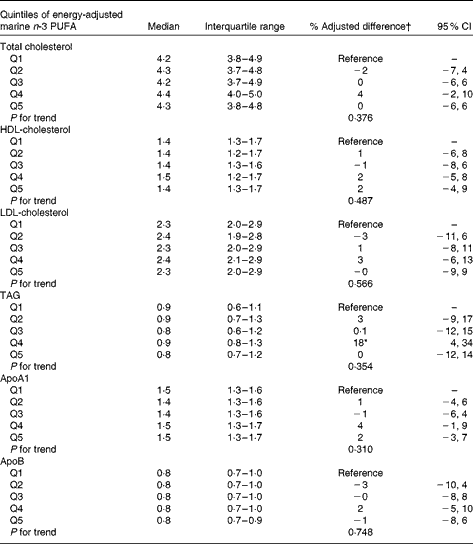
Of the entire study population, 19 % had total cholesterol above 5 mmol/l, 8 % had an HDL-cholesterol concentration below 1·03 mmol/L, 19 % had an LDL-cholesterol concentration above 3 mmol/l and 3 % had a TAG concentration above 2 mmol/l.
The overall intake of marine n-3 PUFA during the second trimester of pregnancy was not associated with cholesterol (HDL, LDL and total), apoA1 and apoB or TAG concentration in the offspring (Table 5). However, the concentration of TAG was statistically significantly higher in the fourth quintile compared with that in the lowest quintile of marine n-3 PUFA intake.
Table 5 Association between quintiles (Q) of energy-adjusted intake of marine n-3 PUFA during the second trimester of pregnancy and offspring lipid parameters (Medians and interquartile ranges; % adjusted differences and 95 % confidence intervals)

* Statistically significant difference relative to the lowest quintile.
† Difference in % relative to the lowest quintile of marine n-3 PUFA intake during the second trimester of pregnancy.
Discussion
We found no association between the intake of marine n-3 PUFA in the second trimester of pregnancy and several cardiometabolic risk factors in the 19–20-year-old offspring. Thus, no association was found with BMI and waist circumference, glucose metabolism, adipose tissue-derived hormones, BP, HR, HRV or lipid profile. The only difference reaching statistical significance was the higher TAG concentration in the fourth quintile compared with the lowest quintile of marine n-3 PUFA intake. However, no general trend towards an association between the intake of marine n-3 PUFA and TAG was found, and since a lot of comparisons have been made, this may be a chance finding.
The relatively high loss to follow-up could potentially have led to a bias of the estimates. A description of the participants and non-participants has been published previously(Reference Halldorsson, Rytter and Haug28). Self-reported BMI was higher among the non-participating females compared with the participating ones in the clinical examination. Also, we did not have any information about BMI for 221 subjects. The prevalence of overweight in the study was somewhat lower than the estimated one among young people in Denmark in 2010 (20·5 v. 25·8 % in males and 16·1 v. 20·2 % in females)(29). These data put together could indicate a lower participation among subjects with a high BMI. Nevertheless, participation was not associated with exposure in the study, indicating that the possible larger attrition among subjects with a high BMI would not lead to a bias of the estimates. To further investigate a possible selection bias, a sensitivity analysis was performed. Imputing a BMI of 25 kg/m2 for those offspring who were completely lost to follow-up, and using self-reported BMI to calculate and impute expected values for those who only filled out the questionnaire, did not change the estimated difference in BMI between the exposure groups materially and, if anything, made the estimated differences even smaller. Participation per se would not be expected to be directly associated with BP or lipid profile, since most of the participants were probably unaware of these values.
Originally, 80 % of eligible pregnant women agreed to participate in the study. No information is available for the remaining 20 %, and it cannot be ruled out that these women were different from those who agreed to participate. There is, however, no reason to believe that they should be different with respect to the association between the intake of marine n-3 PUFA during the second trimester of pregnancy and later cardiometabolic risk in the offspring.
The lack of associations in the present study may be due to a statistical type 2 error. Hence, the study may not have been large enough to detect small differences in the outcomes. Also, the exposure contrast may not have been large enough to cause differences between the groups. However, most of the estimated differences were small, and the present study is one of the largest and most comprehensive studies to date that has examined the association between early intake of marine n-3 PUFA intake and later cardiometabolic risk. The intake of marine n-3 PUFA was calculated based on the self-reported dietary intake of different food items. This information could contain some degree of imprecision, making it difficult to detect differences between the groups. However, the self-reported dietary intake was shown to be positively correlated with the sum of marine n-3 PUFA in erythrocytes(22). Also, both in the original questionnaire and in the following interview, emphasis was mainly placed on making it possible to quantify the intake of marine n-3 PUFA and more effort was put to get as correct and precise answers as possible. Hence, we believe that the dietary assessment of fish intake corresponds relatively well to the actual intakes during the second trimester of pregnancy. Any potential misclassification of the exposure is most probably not associated with the outcome, but could be the reason for us being unable to detect differences between the groups.
The observational design of the study also increases the risk of confounding. We did adjust for a number of potential confounders, and most of the important characteristics were found to be not associated with the exposure. The intake of fish is generally associated with higher social status and a healthier lifestyle, but assuming the positive effects of marine n-3 PUFA on cardiovascular health, confounding from such factors does not explain the null findings of the present study.
The evidence from previous studies on the association between fish intake during pregnancy and later adiposity is relatively sparse(Reference Helland, Smith and Blomen14–Reference Rytter, Bech and Christensen17, Reference Donahue, Rifas-Shiman and Gold21). We have recently followed up a randomised controlled trial with fish oil supplementation from the 30th week of gestation, undertaken in the same population of Aarhus, and found no effects on adiposity in the 19-year-old offspring(Reference Rytter, Bech and Christensen17). In a randomised controlled trial carried out in Norway also, no association has been reported between supplementation with marine n-3 PUFA from gestational week 18 until 3 months after delivery and BMI at the age of 7 years(Reference Helland, Smith and Blomen14). Likewise, no overall association could be documented between supplementation with the marine n-3 PUFA DHA from gestational week 20 until delivery and anthropometry at 18 weeks of age in a large trial with marine n-3 PUFA supplementation carried out in pregnant women(Reference Stein, Wang and Martorell16). In contrast, Lucia et al. (Reference Lucia, Bergmann and Haschke-Becher15) reported a lower BMI among 21-month-old children whose mothers had been randomised to marine n-3 PUFA together with a vitamin and mineral supplement compared with vitamins and minerals taken alone. This finding has recently been supported by results from an observational study reporting an inverse association between the intake of marine n-3 PUFA during mid/late pregnancy and the risk of obesity at 3 years of age(Reference Donahue, Rifas-Shiman and Gold21).
Only two studies have previously investigated the association between the intake of marine n-3 PUFA during pregnancy and later offspring BP(Reference Rytter, Christensen and Bech19, Reference Leary, Ness and Emmett30). In our 19-year follow-up of a randomised trial, no effect of supplementation with marine n-3 PUFA during the third trimester of pregnancy on offspring BP was found(Reference Rytter, Christensen and Bech19). Similarly, the observational study by Leary et al. (Reference Leary, Ness and Emmett30) reported no association between the intake of marine n-3 PUFA during late pregnancy and offspring systolic BP at the age of 7·5 years.
The present study supports the findings on lipid profile from our previous randomised controlled trial with marine n-3 PUFA supplementation, where no effect of supplementation with marine n-3 PUFA could be documented(Reference Rytter, Schmidt and Bech18).
In conclusion, we found no association between the intake of marine n-3 PUFA during the second trimester of pregnancy and cardiometabolic risk in the 19–20-year-old offspring.
Acknowledgements
The present study was supported by the Danish Council for Strategic Research (grant no.: 09-067124, 2101-07-0025 and 2101-06-0005). The study sponsors were not involved in the study design; in the collection, analysis or interpretation of the data; in the writing of the report, or in the decision to submit the article for publication.
The authors' contributions were as follows: S. F. O. was responsible for the original pregnancy study and initiated the follow-up of the offspring; D. R., J. H. C., B. H. B., S. F. O., E. B. S. and T. B. H. designed the research; D. R. and B. H. B. conducted the research; D. R. analysed the data and wrote the first draft; D. R., B. H. B., T. H., J. H. C., E. B. S., I. D., T. B. H. and S. F. O. wrote the article; D. R. had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors had a conflict of interest.