Nitrate (NO3 −) is an ergogenic nutritional supplement widely consumed by exercise practitioners and athletes to improve their health and physical performance( Reference Jones, Haramizu and Ranchordas 1 ). The widespread use of NO3 − likely reflects its abundant availability in many vegetables, and its content ranges from <20 mg/100 g in sweet potato to >250 mg/100 g in beetroot( Reference Jones, Vanhatalo and Bailey 2 ). Although oral bacteria can reduce NO3 − to nitrite (NO2 −), the transit of these foods in the mouth is short, and the resulting increase in NO3 − bioavailability appears to be related to the intrinsic NO3 − content in the vegetable or supplement. Indeed, increased NO3 − bioavailability could favour nitric oxide (NO) synthesis( Reference Jones 3 ). NO is a signalling molecule associated with improved cardiovascular and skeletal muscle functions that may potentially enhance physical performance and even facilitate adaptations to exercise training( Reference Thompson, Wylie and Blackwell 4 ). Nevertheless, the scientific literature provides controversial results regarding the performance-enhancing effects induced by NO3 − supplementation.
Two systematic reviews and meta-analyses on this topic have recently been published, establishing clear practical recommendations and directions for future studies investigating changes in performance induced by NO3 − supplementation( Reference Hoon, Johnson and Chapman 5 , Reference McMahon, Leveritt and Pavey 6 ). Hoon et al.( Reference Hoon, Johnson and Chapman 5 ) and McMahon et al.( Reference McMahon, Leveritt and Pavey 6 ) analysed data according to the exercise protocol used (i.e. time trials, open-ended tests and graded-exercise tests) and observed that dietary NO3 − supplementation improved endurance only when performance was evaluated using open-ended tests. Notably, none of these two meta-analyses divided and analysed separately the studies conducted with athletes or non-athletes, as we are proposing here. McMahon et al. performed a continuous variable meta-regression analysis and reported that the fitness level did not have an influence on the ergogenic effect of dietary NO3 − supplementation( Reference McMahon, Leveritt and Pavey 6 ). However, grouping the data according to the exercise protocol may result in an important bias. In fact, the studies using open-ended tests were mainly performed in non-athletes. In contrast, most studies using time trials were performed in athletes. This disparity might have led to a misinterpretation of the results owing to an unintentional division based on individuals’ physical fitness level. Interestingly, neither of the two recent systematic reviews addressed the following question raised by Jonvik et al.: ‘Can elite athletes benefit from dietary nitrate supplementation?’( Reference Jonvik, Nyakayiru and van Loon 7 – Reference Hultstrom, Amorim de Paula and Antonio Peliky Fontes 9 ). Therefore, information regarding the effectiveness of NO3 − supplementation in individuals with different physical fitness levels is lacking. Moreover, physical performance is modulated by various mechanisms and depends on several factors, including the duration of the test performed (i.e. short or long duration). Thus, the influence of the test duration on the changes in performance induced by NO3 − supplementation in individuals with different fitness levels remains to be investigated.
Increased NO availability resulting from NO3 − supplementation has beneficial effects on health and physical performance and has been largely studied in humans and laboratory animals. In the central nervous system, NO prevented exaggerated increases in the core body temperature in rats subjected to exercise by increasing cutaneous heat loss and decreasing the metabolic cost of running( Reference Lacerda, Marubayashi and Balthazar 10 – Reference Wanner, Leite and Guimaraes 13 ). In these rat studies, the pharmacological blockade of central NO synthesis markedly impaired endurance( Reference Lacerda, Marubayashi and Balthazar 10 , Reference Lima, Santiago and Szawka 12 ), whereas an increased NO availability in the brain did not affect endurance( Reference Wanner, Leite and Guimaraes 13 ). In humans, the physical performance benefits mediated by dietary NO3 − supplementation have been attributed to peripheral effects, including reduced arterial pressure and VO2. The latter effect leads to a reduced oxygen cost during exercise that is most likely due to the reduced cost of ATP for muscle force production, improved mitochondrial efficiency and increased muscle oxygenation( Reference Larsen, Weitzberg and Lundberg 14 , Reference Larsen, Weitzberg and Lundberg 15 ). In contrast, the adverse events related to NO3 − supplementation are minor and restricted to red urine (beeturia) and stool, which usually results from the ingestion of beetroot in juice or meals( Reference Vanhatalo, Bailey and Blackwell 16 , Reference Wylie, Kelly and Bailey 17 ).
Interestingly, both acute and chronic supplementations of NO3 − have been shown to either improve( Reference Bailey, Winyard and Vanhatalo 18 – Reference Masschelein, Van Thienen and Wang 24 ) or have no effect( Reference Larsen, Weitzberg and Lundberg 14 , Reference Bescόs, Ferrer-Roca and Galilea 25 – Reference Wilkerson, Hayward and Stephen 29 ) on endurance performance. The uncertain efficacy of NO3 − supplementation appears to be related to the fitness level of the investigated population as demonstrated by a careful evaluation of the cumulative number of trials reporting the performance benefits or lack thereof in both non-athletes (healthy individuals engaged in regular physical activity but not involved in sports competitions) and athletes (Fig. 1). Notably, nearly 65 % of the publications on this topic did not report the benefits resulting from NO3 − supplementation. However, if only those studies performed in non-athletes are considered, approximately 45 % of the publications show a supplementation-mediated positive effect on physical performance, whereas the percentage of papers showing beneficial effects in athletes is lower than 30 % (Fig. 1). Collectively, these observations reinforce the relevance of the present systematic review and meta-analysis, which help clarify the contradictory reports of the effects of NO3 − supplementation on physical performance.
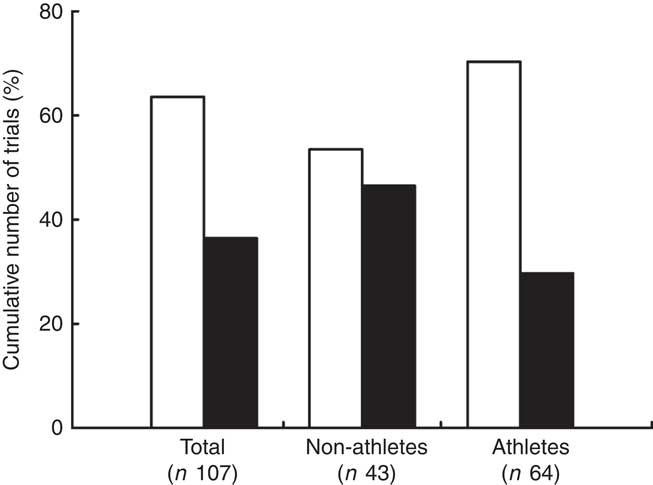
Fig. 1 Number of trials (%) reporting that dietary NO3
− supplementation had no effect (![]() ) and/or a positive effect (
) and/or a positive effect (![]() ) on physical performance in non-athletes and athletes.
) on physical performance in non-athletes and athletes.
Therefore, the present study systematically analysed whether different physical fitness levels (i.e. non-athletes v. athletes) influence the effects of NO3 − supplementation on physical performance. In addition, we also evaluated the influence of the duration of the tests used to measure performance (i.e. short v. long duration) and the test protocol used (i.e. time trials v. open-ended tests v. graded-exercise tests) on the effect of NO3 − supplementation on physical performance in individuals with different physical fitness levels. Thus, the present analyses provide information that is useful to exercise practitioners, athletes, coaches and conditioning professionals who are interested in improving physical performance and achieving health benefits.
Methods
Search strategy
This systematic review and meta-analysis was conducted and reported according to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement( Reference Liberati, Altman and Tetzlaff 30 , Reference Moher, Liberati and Tetzlaff 31 ). A systematic search of electronic databases, including PubMed, Web of Science, SPORTDiscus and ProQuest, was performed in August 2017 without any date restrictions. The search strategy was supplemented by manual cross-matching of each publication reference list and key author searches. Combinations of the following keywords were used: effort, endurance, exercise, fatigue, nitrate, nitrate supplementation, nitrite, nitrite supplementation, performance, power, running, speed, sport and workload.
Study selection
Studies that met the following criteria were included in this systematic review and meta-analysis: (i) the participants were healthy humans (either non-athletes or athletes), (ii) physical performance was measured after the participants were supplemented with NO3 − and (iii) the studies were placebo-controlled trials. Furthermore, all included studies were written in English. Reviews, summaries, case studies and letters were not included, although this bibliography was consulted. Studies involving hypoxic conditions, individuals with diseases, exercise in the heat, children and elderly people, and laboratory animals were excluded. On the basis of the search and inclusion/exclusion criteria, fifty-four studies (106 trials) were selected for inclusion in this systematic review, and fifty-three studies (104 trials) were included in the meta-analysis (Fig. 2). Notably, several studies measured more than one physical performance parameter. The data addressing the effect of NO3 − supplementation on each parameter were included, and therefore the number of trials was greater than the number of studies. Only one study with one trial( Reference Bond, Morton and Braakhuis 20 ) and one trial in a study with several trials( Reference Christensen, Nyberg and Bangsbo 32 ) were excluded from the meta-analysis because they did not include the standard deviation data needed to calculate the effect size.
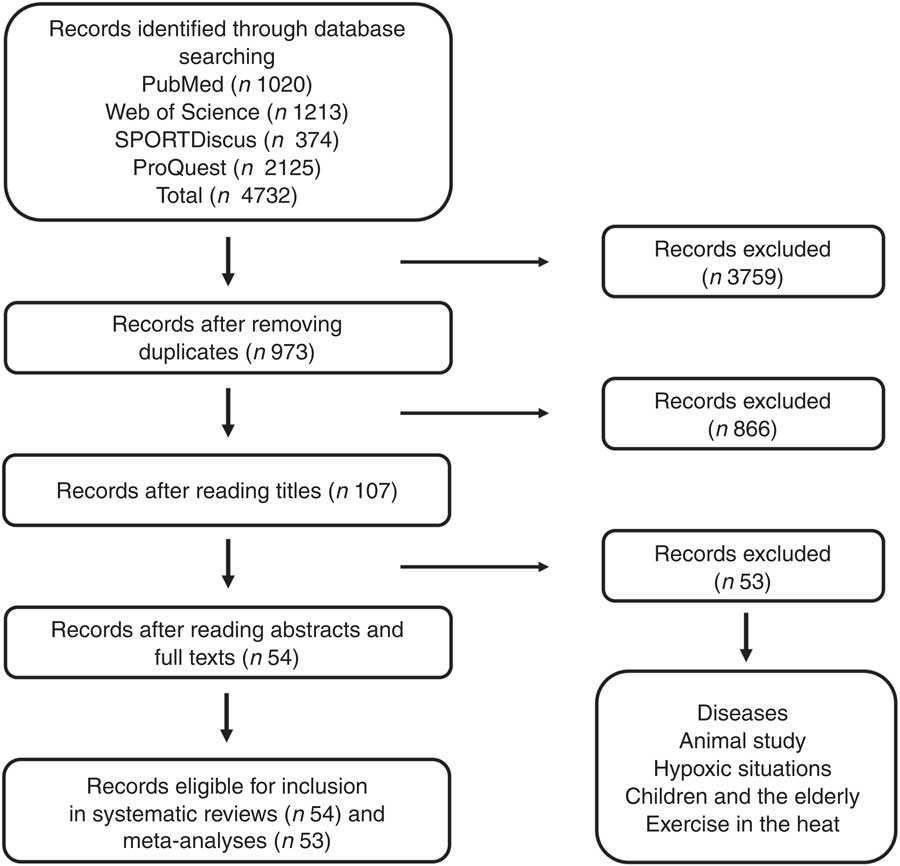
Fig. 2 Summary of the study selection process.
Data grouping
The selected studies were divided into the following two groups according to the physical fitness level of the individuals tested: non-athletes (forty-three trials) and athletes (sixty-three trials). The individuals were allocated into these two groups according to the classification used by the authors of the research papers, which were consulted. This strategy was efficient in dividing the participants into two groups with different functional capacities as demonstrated by the higher VO2max values in the athletes than in the non-athletes (61·1 (sd 1·8) v. 50·5 (sd 1·8) ml/kg per min; t-test, P<0·05). Similarly, the studies selected for inclusion in the meta-analysis were initially divided into the following two groups: non-athletes (forty-three trials) and athletes (sixty-one trials). The two groups were then subdivided according to the duration of the test performed as follows: short duration (non-athletes, eighteen trials; athletes, seventeen trials) or long duration (non-athletes, twenty-five trials; athletes, forty-four trials). Exercises lasting less than 180 s, thereby characterised by a relevant anaerobic contribution to the energy expenditure, were considered short-duration exercises. Alternatively, exercise bouts lasting more than 180 s were considered long-duration exercises( Reference Duffield, Dawson and Goodman 33 ). In addition, because NO3 − supplementation has been shown to have a positive effect on physical performance only in non-athletes during long-duration tests, this group was further subdivided according to the test protocol used (open-ended tests (constant power), fourteen trials; time trials, four trials; and graded-exercise tests (incremental power), five trials). Open-ended tests consist of exercising at a constant power until the participant is volitionally fatigued; the time until fatigue, which may be highly variable among subjects, is considered the main measure of performance in this test. Finally, owing to the large number of studies in cycling athletes, a specific analysis was conducted for this sport (thirty-seven trials).
Analysis of the relationship between the performance level and the response to NO3 − supplementation
Because the authors of the research papers may have been imprecise in the classification of their subjects as athletes, we decided to perform an objective analysis. Thus, the individuals were grouped into different performance levels (PL) according to the classification provided by De Pauw et al.( Reference De Pauw, Roelands and Cheung 34 ). These authors divided the participants in sport science studies into the following five different levels: performance level 1 (PL1) included untrained and sedentary subjects with a VO2max<45·0 ml/kg per min; performance level 2 (PL2) included recreationally trained subjects with a VO2max between 45·0 and 54·9 ml/kg per min; performance level 3 (PL3) included trained subjects with a VO2max between 55·0 and 64·9 ml/kg per min; performance level 4 (PL4) included highly trained subjects with a VO2max between 65·0 and 71·0 ml/kg per min; and performance level 5 (PL5) included professional subjects with a VO2max>71·0 ml/kg per min. On the basis of this study, we grouped the individuals into five levels and then evaluated the relationship between the PL and the changes in performance induced by NO3 − supplementation.
Risk of bias assessment
Two independent reviewers assessed the risk of bias using an adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) instrument( Reference JPT and Green 35 ). Discrepant evaluations were settled via discussion with a third reviewer. Using this approach, it was possible to evaluate the risk of bias in each study included in the present systematic review. Domains reflecting sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other sources of bias were evaluated.
Statistical analysis
The mean and standard deviation values of the performance indexes in both the NO3 − supplementation and control trials were obtained from the data provided in the consulted research papers. Heterogeneity was evaluated using the χ 2 test for homogeneity and the I 2 statistic. The effect size (Cohen’s d or Hedges’ g) was calculated for the performance indexes in each study. Then, a weighted-mean estimate of the effect size was calculated to account for differences in the sample sizes. The mean unweighted effect size and associated 95 % CI were also calculated. We used Cohen’s classification of the effect size magnitude, where d<0·20 for negligible effect; d=0·20–0·49 for small effect; d=0·50–0·79 for moderate effect; and d>0·8 for large effect( Reference Cohen 36 ). The χ 2 test was used to compare the frequency of trials showing improved performance in response to NO3 − supplementation among the different PL. Student’s t test was used to compare the VO2max between the non-athletes and athletes. Pearson’s correlations were performed to evaluate the association between the supplementation parameters (dose, number of days and total amount ingested) and the changes in physical performance. Publication bias was assessed by a visual inspection of funnel plots of the standard error v. effect size( Reference Sterne, Sutton and Ioannidis 37 ).
Results
Systematic review
In total, 4732 studies were identified through the database and reference searches. After removing the duplicates and excluding papers that did not meet the eligibility criteria according to a review of their titles, abstracts and full texts, fifty-four studies (106 trials and 662 individuals) were selected for inclusion in the systematic review (Fig. 2).
The characteristics of the subjects, including information regarding the supplementation regimens and effects of NO3 − supplementation on the physical performance of non-athletes and athletes in each study, are summarised in Tables 1 and 2, respectively. Notably, most studies used beetroot juice as a form of NO3 − supplementation. However, these studies were heterogeneous in several supplementation features, including the ingested volume (70, 140, 250, 280 or 500 ml), dose (between 4·0 and 19·5 mmol), days of supplementation (between 1 and 15 d), timing of supplementation before the trial (between 40 and 1440 min) and the parameter measured to determine physical performance.
Table 1 Study characteristics – non-athletes (Mean values and standard deviations)
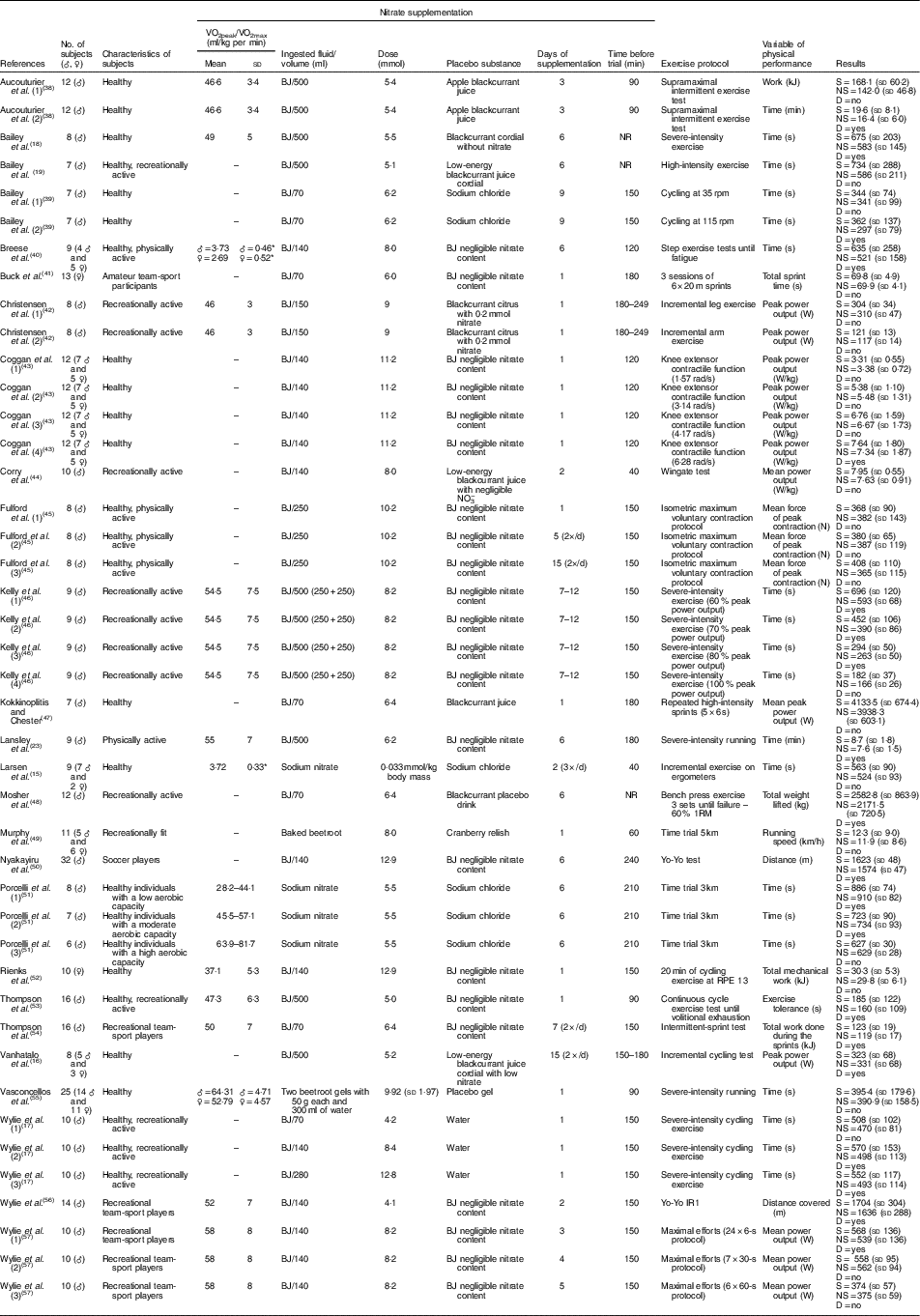
♂, Male; ♀, female; BJ, beetroot juice; NR, not reported; S, supplemented; NS, no supplementation; D, statistical difference.
* Absolute VO2 data in l/min.
Table 2 Study characteristics – athletes (Mean values and standard deviations)
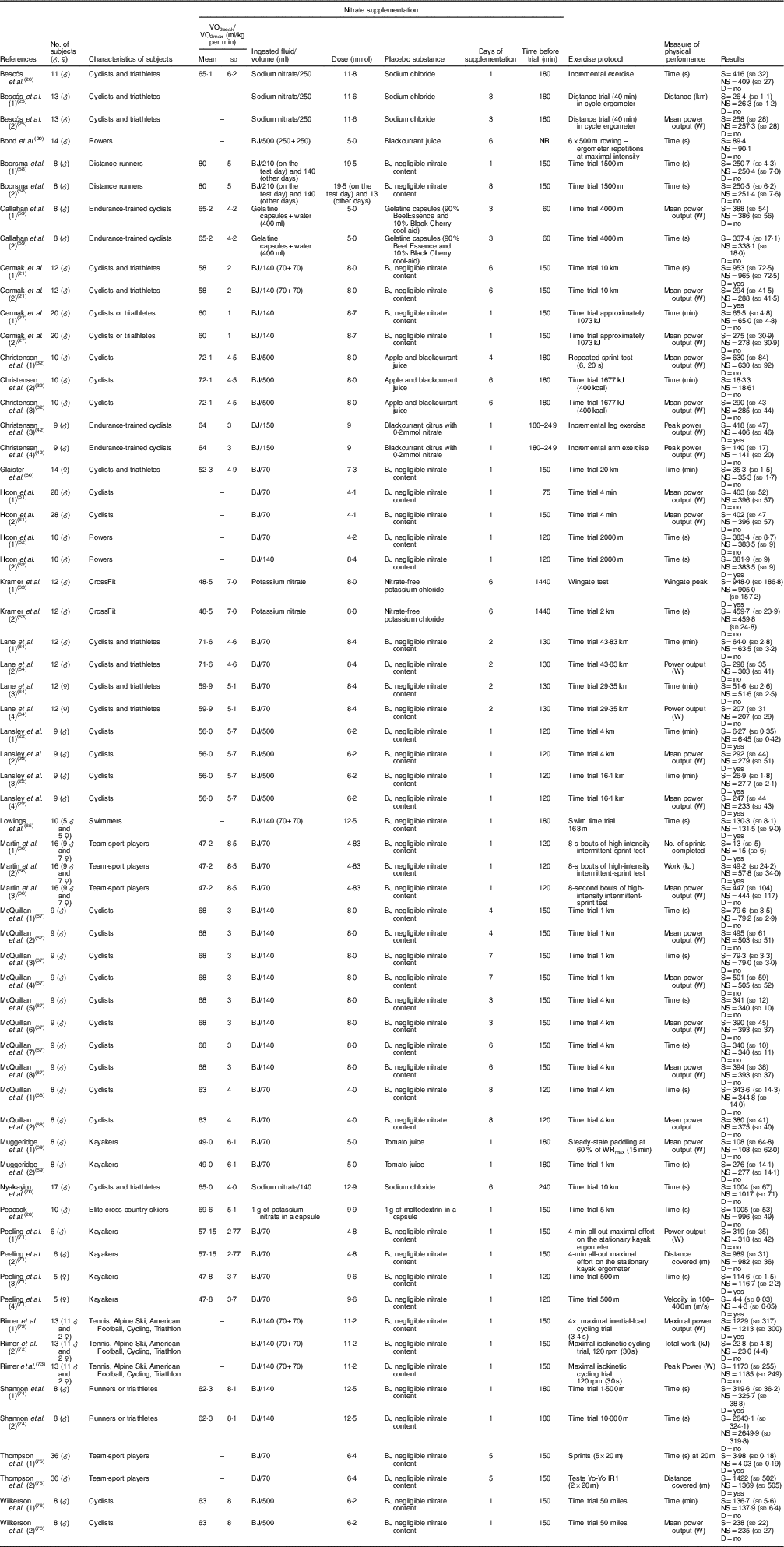
♂, Male; ♀, female; BJ, beetroot juice; NR, not reported; S, supplemented; NS, no supplementation; D, statistical difference.
Meta-analyses
In total, fifty-three studies (104 trials and 648 individuals) were included in the meta-analysis.
Non-athletes
After pooling the data from forty-three trials, the mean effect size was 0·25 (95 % CI 0·11, 0·38), which indicates that the dietary NO3 − supplementation had a small and significant beneficial effect on physical performance (P<0·05; Fig. 3). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=15·26, df=42, P=1·00).
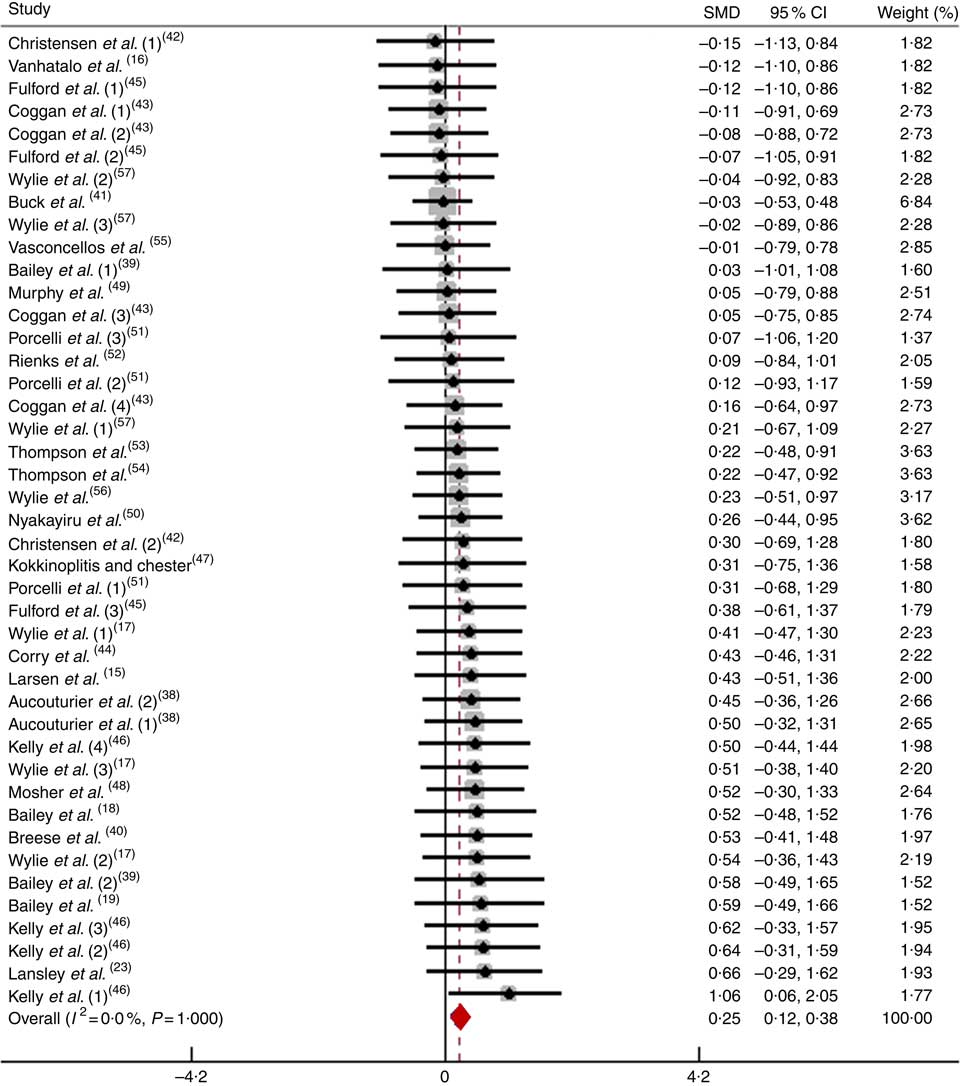
Fig. 3 Forest plot of physical performance following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Athletes
After pooling the data from sixty-one trials, the mean effect size was 0·04 (95 % CI −0·05, 0·15), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on improving physical performance (P>0·05; Fig. 4). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=18·16, df=60, P=1·00). The subsequent analysis consisted of subdividing both the athletes and non-athletes into those performing short- and long-duration tests.
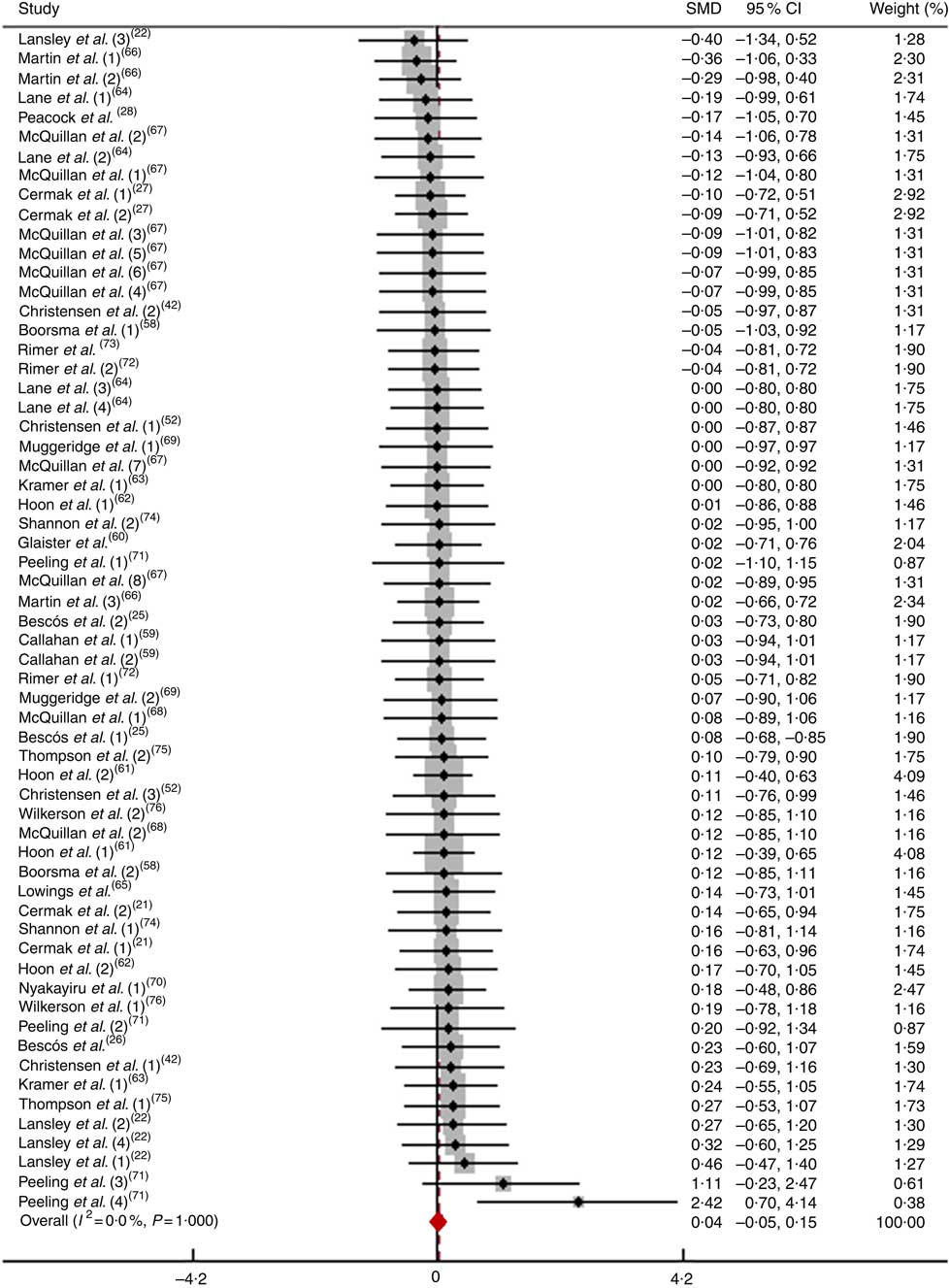
Fig. 4 Forest plot of physical performance following dietary NO3 − supplementation in athletes. SMD, standardised mean difference.
Non-athletes subjected to short-duration tests
After pooling the data from eighteen trials, the mean effect size was 0·12 (95 % CI −0·07, 0·31), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on physical performance (P>0·05; Fig. 5). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=4·43, df=17, P=0·99).

Fig. 5 Forest plot of physical performance during a short-duration test following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Athletes subjected to short-duration tests
After pooling the data from seventeen trials, the mean effect size was 0·03 (95 % CI −0·17, 0·23), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on performance (P>0·05; Fig. 6). According to a random-effects analysis, heterogeneity was observed among these studies (I 2=0 %; Q=13·31, df=16, P=0·65).
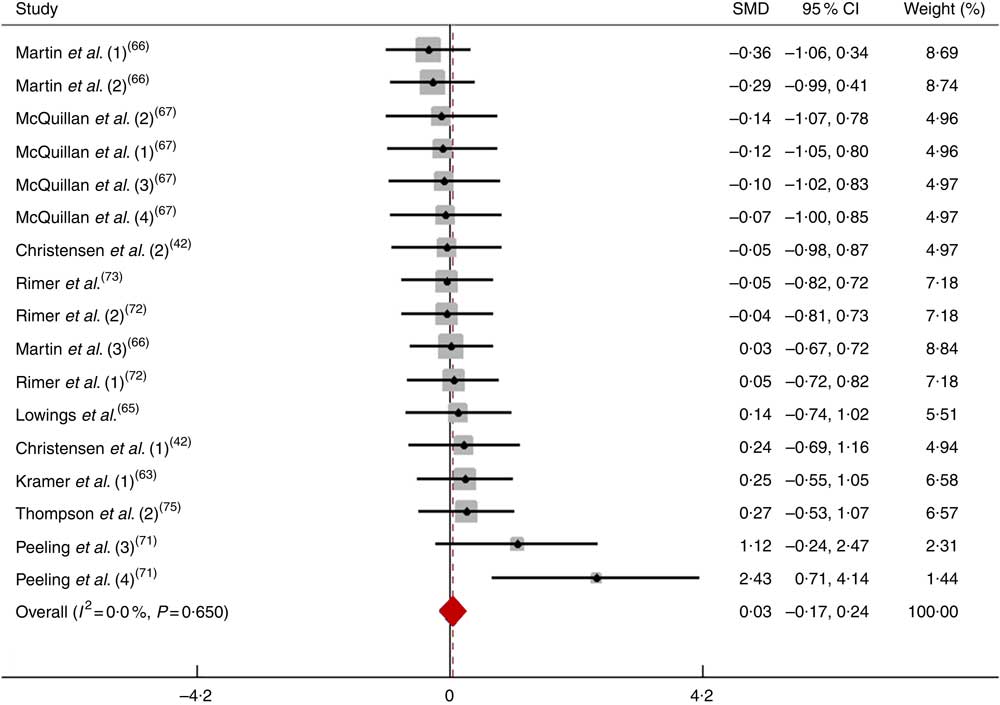
Fig. 6 Forest plot of physical performance during a short-duration test following dietary NO3 − supplementation in athletes. SMD, standardised mean difference.
Non-athletes subjected to long-duration tests
After pooling the data from twenty-five trials, the mean effect size was 0·33 (95 % CI 0·15, 0·51), which indicates that the dietary NO3 − supplementation had a small and significant beneficial effect on physical performance (P<0·05; Fig. 7). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=8·01, df=24, P=0·99).
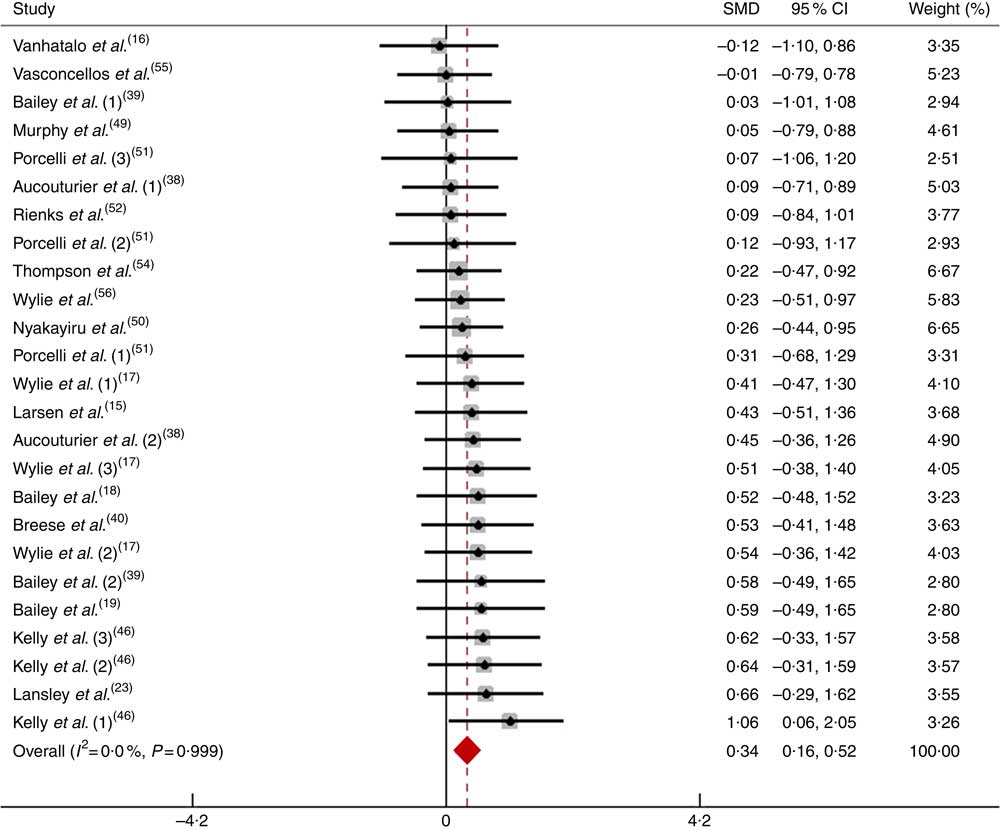
Fig. 7 Forest plot of physical performance during a long-duration test following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Athletes subjected to long-duration tests
After pooling the data from forty-four trials, the mean effect size was 0·05 (95 % CI −0·07, 0·17), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on physical performance (P>0·05; Fig. 8). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=4·82, df=43, P=1·00). The subsequent analysis consisted of subdividing the non-athletes that performed long-duration tests according to the test protocol used.
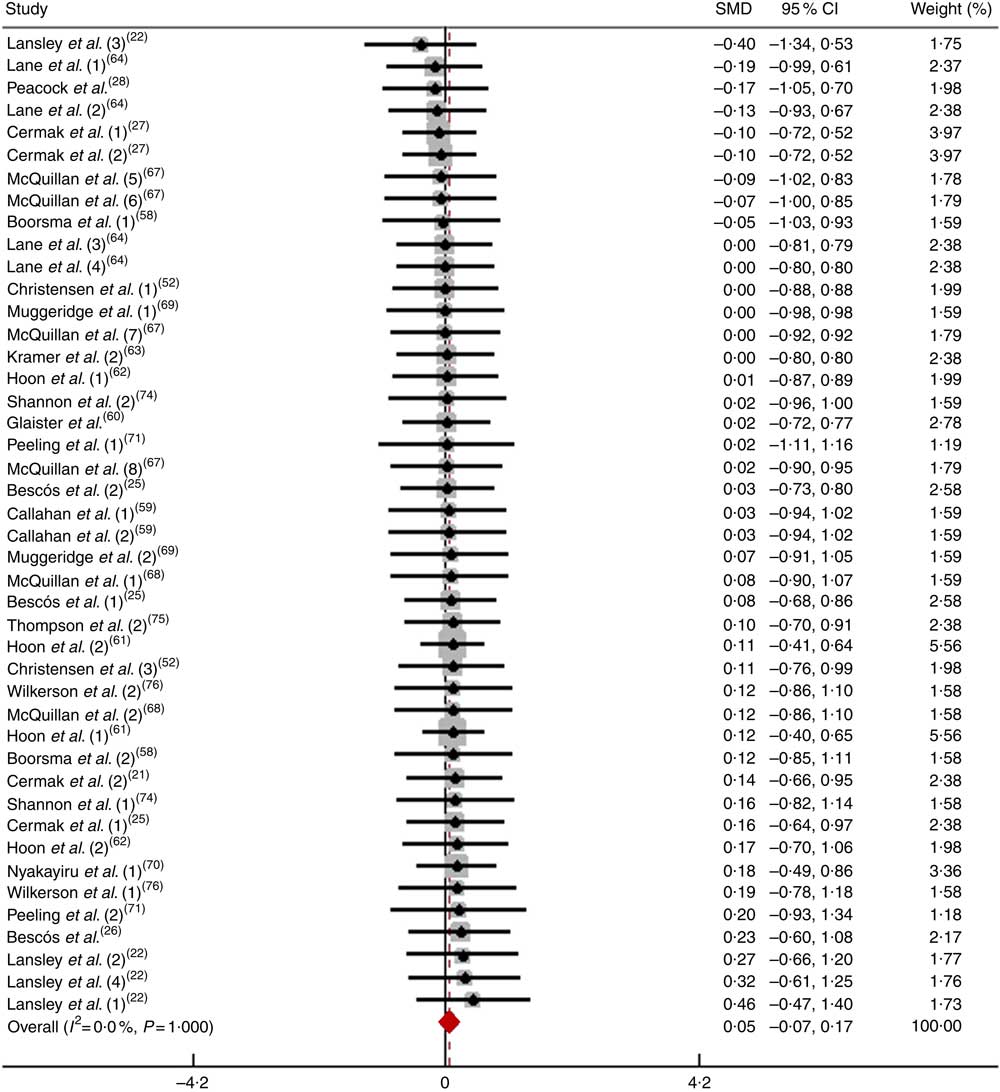
Fig. 8 Forest plot of physical performance during a long-duration test following dietary NO3 − supplementation in athletes. SMD, standardised mean difference.
Non-athletes subjected to long-duration, open-ended tests
After pooling the data from fourteen trials, the mean effect size was 0·47 (95 % CI 0·23, 0·71), which indicates that the dietary NO3 − supplementation had a small and significant beneficial effect on physical performance (P<0·05; Fig. 9). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=3·77, df=13, P=0·99).
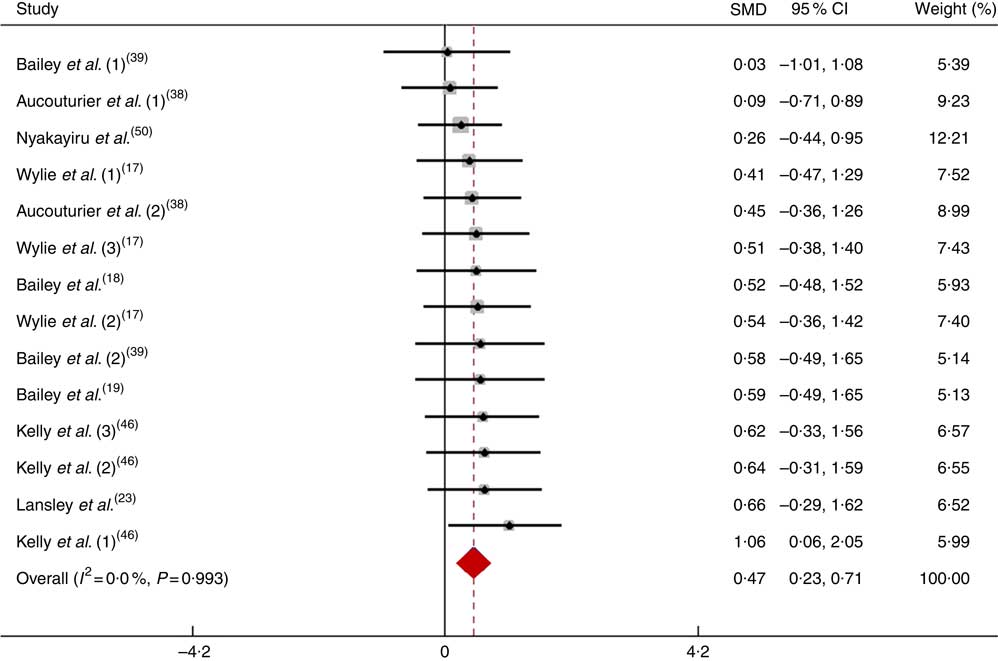
Fig. 9 Forest plot of physical performance during a long-duration open-ended test following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Non-athletes subjected to long-duration time trials
After pooling the data from four trials, the mean effect size was 0·12 (95 % CI −0·37, 0·61), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on physical performance (P>0·05; Fig. 10). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=0·16, df=3, P=0·98).

Fig. 10 Forest plot of physical performance during a long-duration time trial following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Non-athletes subjected to long-duration, graded-exercise tests
After pooling the data from five trials, the mean effect size was 0·20 (95 % CI −0·18, 0·59), which indicates that the dietary NO3 − supplementation had a small but non-significant effect on physical performance (P>0·05; Fig. 11). According to a fixed-effects analysis, no heterogeneity was observed among these studies (I 2=0 %; Q=1·38, df=4, P=0·84).

Fig. 11 Forest plot of physical performance during a long-duration graded-exercise test following dietary NO3 − supplementation in non-athletes. SMD, standardised mean difference.
Cyclists
Most tested athletes were cyclists; therefore, this subgroup was subjected to a special analysis in which they were evaluated alone without the inclusion of athletes engaged in other sports. After pooling the data from thirty-seven trials, the effect size mean was 0·04 (95 % CI −0·09, 0·17), which indicates that the dietary NO3 − supplementation had a negligible and non-significant effect on physical performance (P>0·05; Fig. 12). According to a fixed-effects analysis, heterogeneity was observed among these studies (I 2=0 %; Q=4·90, df=36, P=1·00).
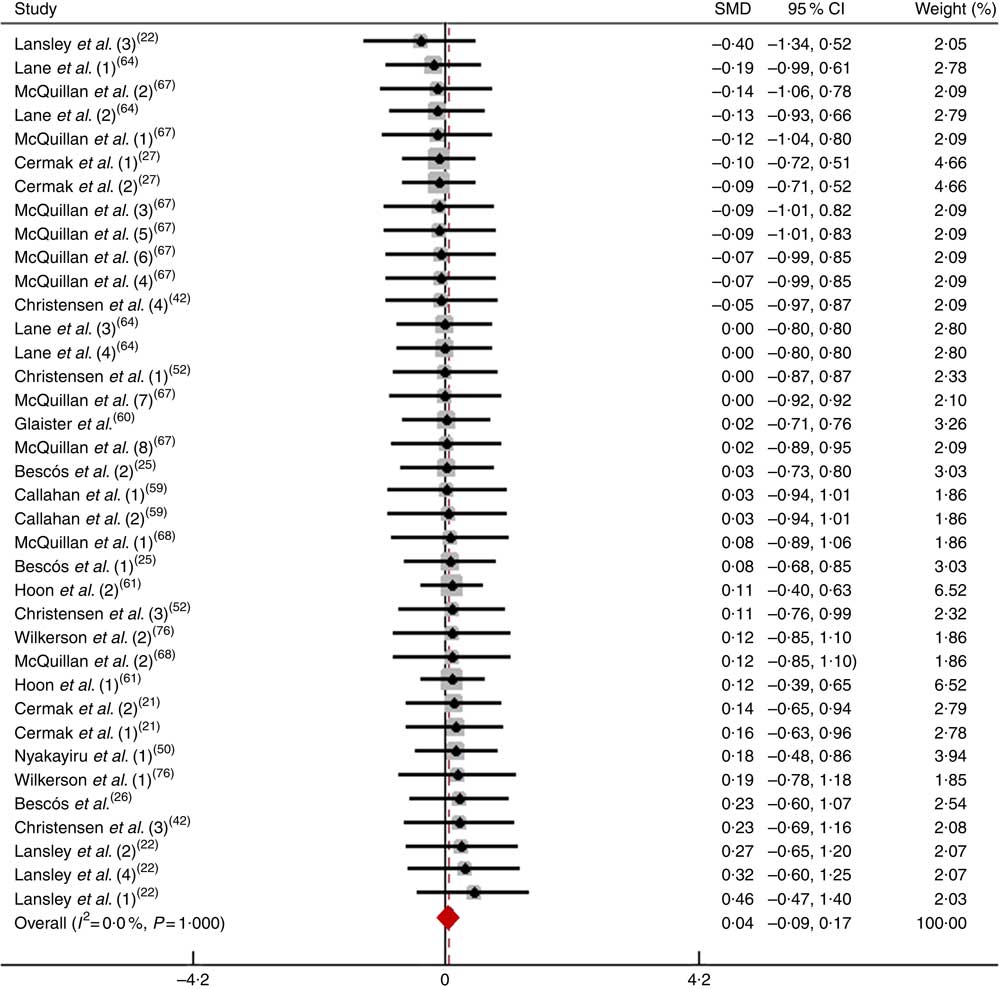
Fig. 12 Forest plot of physical performance in cyclists following dietary NO3 − supplementation. SMD, standardised mean difference.
Analysis of the relationship between the performance level and the ergogenic response to the NO3 − supplementation
By analysing the percentage of trials reporting increased performance in individuals classified into different PL, we observed numerous trials, that is, 50 and 56·5 %, showing increased performance in individuals with PL1 and PL2, respectively. In contrast, approximately 37 % of the trials involving individuals with PL3 showed an increased performance following the NO3 − supplementation, whereas in trials involving individuals with PL4 and PL5 no improvement in performance was observed following the NO3 − supplementation (Fig. 13). The χ 2 test showed a different distribution among the PL (P=0·002).
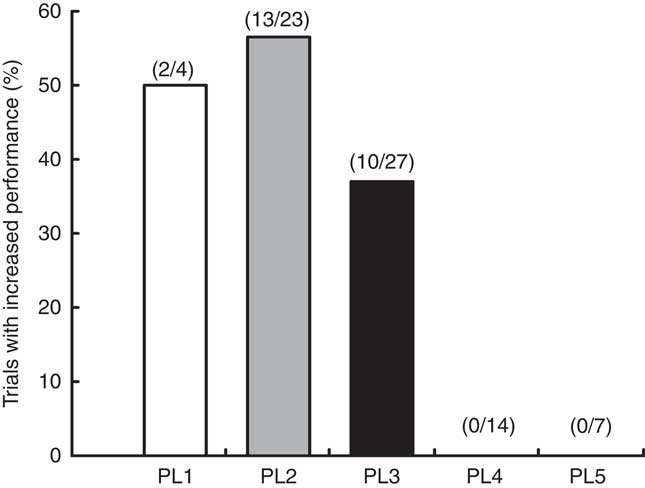
Fig. 13 Number of trials with increased performance (%) in subjects with different performance levels (PL).
Association between supplementation features and changes in physical performance
Pearson’s correlation analyses were performed to verify the association between these variables, including the association between changes in physical performance and the dose of NO3 − (non-athletes: r 0·351, P>0·05; athletes: r 0·099, P>0·05), the number of days of supplementation (non-athletes: r 0·166, P>0·05; athletes: r 0·114, P>0·05) and the total amount ingested (dose multiplied by days under supplementation) (non-athletes: r 0·112, P>0·05; athletes: r 0·088, P>0·05). No significant correlations were observed between the supplementation features evaluated and changes in physical performance.
Publication bias
Publication bias was assessed by a visual inspection of the funnel plot for all subgroups analysed: non-athletes (online Supplementary Fig. S2(a)), athletes (online Supplementary Fig. S1(a)), non-athletes subjected to short-duration tests (online Supplementary Fig. S2(b)), athletes subjected to short-duration tests (online Supplementary Fig. S1(b)), non-athletes subjected to long-duration tests (online Supplementary Fig. S2(c)), athletes subjected to long-duration tests (online Supplementary Fig. S1(c)), non-athletes subjected to long-duration, open-ended tests (online Supplementary Fig. S3(a)), non-athletes subjected to long-duration time trials (online Supplementary Fig. S3(b)) and non-athletes subjected to long-duration, graded-exercise tests (online Supplementary Fig. S3(c)). These analyses revealed minor asymmetrical inverted distributions that were prominent in all plots, suggesting the presence of a small publication bias.
Risk of bias
The risk of bias was assessed in fifty-four studies (twenty-six and twenty-eight conducted with non-athletes and athletes, respectively) in the systematic review. One study( Reference Peeling, Cox and Bullock 71 ) was subjected to two independent evaluations because it presented independent experimental trials. Out of fifty-five evaluations, forty-eight did not present any major risk of bias. Approximately 13 % (non-athletes, two studies; athletes, five studies) of the studies did not blind the participants or researchers. In general, the studies evaluated in the present systematic review showed consistent control of the risk of bias and were deemed to be good-quality studies (online Supplementary Tables S3 and S4).
Discussion
The present systematic review and meta-analysis demonstrated that the level of physical fitness is a determining factor in the performance-enhancing effects associated with NO3 − supplementation. Although athletes are usually less prone to benefit from NO3 − supplementation, non-athletes can experience small but significant advantages in their physical performance, particularly in performance evaluations using long-duration, open-ended tests. Interestingly, this effect is not observed using time trials, which is the most ecologically valid exercise protocol( Reference Goulet 77 ). These findings regarding the beneficial effects induced by NO3 − supplementation in non-athletes are supported by the analysis in which the participants were subdivided according to their PL, and those classified at the lower levels (less conditioned) showed more improvements. This information is very important for exercise practitioners and athletes and provides support in decisions regarding whether to use this potential ergogenic aid to improve physical performance and health.
In the present meta-analysis, we observed that individuals with higher fitness levels benefit less from NO3 − supplementation (Fig. 13). Consistently, the effect size of NO3 − supplementation-mediated changes on performance in athletes was mostly irrelevant (Fig. 4). In contrast, non-athletes can benefit from NO3 − supplementation (Fig. 3). This was the first study to systematically show the importance of characterising the fitness levels of individuals before adopting a nutritional NO3 − supplementation ergogenic strategy. Similarly, Porcelli et al.( Reference Porcelli, Ramaglia and Bellistri 51 ) assessed athletic performance in subjects with three aerobic fitness levels after 6 d of supplementation with 5·5 mmol per d of NO3 −. The authors observed that individuals with lower and moderate aerobic capacities performed better during the time trial after the NO3 − supplementation. However, the performance during the time trial was not improved in individuals with a higher aerobic capacity.
Several mechanisms may act collectively to improve performance following NO3 − supplementation in non-athletes, including beneficial effects of an increased NO bioavailability in the skeletal muscles, blood vessels and even in the brain (Fig. 14). In contrast, the mechanisms underlying the limited ergogenic effects of NO3 − supplementation in high-performance athletes have not been well elucidated. The ergogenic effects of NO3 − supplementation are related to enhanced NO bioavailability, and athletes probably already have optimal levels of NO( Reference Porcelli, Ramaglia and Bellistri 51 ). Highly trained subjects are likely to have high NOS activity( Reference McConell, Bradley and Stephens 83 ), which might render the NO3 −–NO2 −–NO pathway less important for NO production. Therefore, the resulting increase in NO bioavailability due to supplementation does not appear to be relevant in athletes. In addition to these factors, Porcelli et al.( Reference Porcelli, Ramaglia and Bellistri 51 ) suggested that high-performance athletes have a high daily energy expenditure and possibly an enriched diet. Therefore, a diet consisting of a higher intake of NO3 − in these subjects should be considered. Furthermore, recent evidence that NO3 − supplementation may preferentially alter contractile function in type II fibres( Reference Hernández, Schiffer and Ivarsson 79 ) suggests that endurance athletes, who typically have a low proportion of such fibres in their musculature( Reference Tesch and Karlsson 84 ), might experience a blunted physiological response to NO3 − supplementation.
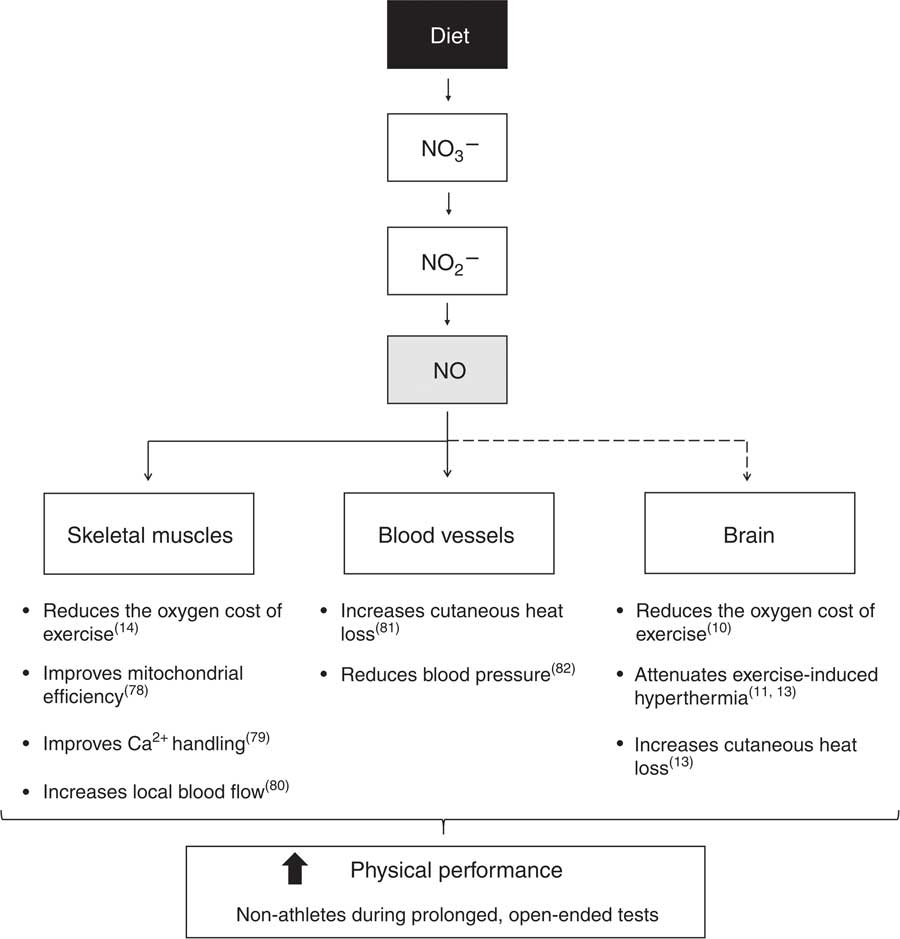
Fig. 14 Mechanisms underlying improved physical performance induced by nitrate (NO3 −) supplementation in non-athletes subjected to prolonged, open-ended tests. Through a series of reduction reactions along the gastrointestinal tract and at target tissues, NO3 − acts as the main nitric oxide (NO) donor. Increased NO bioavailability promotes beneficial effects on performance through effects in skeletal muscles, blood vessels and likely in the brain. To date, no study has provided direct evidence showing that NO3 − supplementation increases brain NO levels (this is the reason why a dashed line is connecting NO to the brain in the schematic). In the skeletal muscles, NO reduces the oxygen cost of exercise( Reference Larsen, Weitzberg and Lundberg 14 ), improves mitochondrial efficiency( Reference Larsen, Schiffer and Borniquel 78 ) and Ca2+ handling( Reference Hernández, Schiffer and Ivarsson 79 ) and increases local blood flow( Reference Ferguson, Hirai and Copp 80 ). In the blood vessels, NO increases cutaneous heat loss( Reference McNamara, Keen and Simmons 81 ) and reduces blood pressure( Reference Larsen, Ekblom and Sahlin 82 ). Experiments conducted in rats showed that NO in the brain reduces the oxygen cost of exercise( Reference Lacerda, Marubayashi and Balthazar 10 ), attenuates exercise-induced hyperthermia( Reference Lacerda, Marubayashi and Coimbra 11 , Reference Wanner, Leite and Guimaraes 13 ) and increases cutaneous heat loss( Reference Wanner, Leite and Guimaraes 13 ). Collectively, these physiological responses induced by NO3 − supplementation improve performance in the conditions mentioned above.
The effects of NO3 − supplementation on exercise performance in non-athletes appear to be more robust in evaluations using long-duration, open-ended tests rather than time trials. Time-trial tests are the most ecologically valid options to assess performance( Reference McMahon, Leveritt and Pavey 6 , Reference Currell and Jeukendrup 85 ). Compared with time trials, constant-power (open-ended) tests are more influenced by psychological factors, such as boredom and motivation( Reference Amann, Hopkins and Marcora 86 , Reference Jeukendrup, Saris and Brouns 87 ). In addition, open-ended tests are more efficient in measuring endurance capacity rather than exercise performance, which is best measured by time-trial protocols( Reference McMahon, Leveritt and Pavey 6 , Reference Jeukendrup and Currell 88 ).
Although the dietary NO3 − supplementation did not exert positive effects on the performance of athletes as previously described, the use of this supplement in sports competitions may still be applicable. During competitions, the winner is often determined by narrow differences between athletes, thus creating opportunities for the implementation of practices that may have subtle improvements in performance. Therefore, the distinct sensitivity of different athletes to supplementation should not be disregarded( Reference Jonvik, Nyakayiru and van Loon 7 , Reference Wilkerson, Hayward and Bailey 76 ) and further research on this topic is warranted.
It is important to understand the physiological meaning of the doses that were supplemented in the included studies. These doses ranged from 4·0 to 19·5 mmol (Tables 1 and 2). Considering that the daily ingestion of NO3 − corresponds on average to 91 mg (1·5 mmol) in people from the UK( Reference Alexander, Diane and Cockburn 89 ), the supplementation would increase the daily ingestion of nitrate by 3- to 13-fold in this population. However, the dose of the NO3 − supplementation, the number of days of supplementation and the total amount ingested do not appear to influence the effects of NO3 − supplementation on physical performance in non-athletes and athletes as shown by the lack of significant associations between these parameters. Studies using a single dose showed that NO3 − supplementation had either no effects( Reference Buck, Henry and Guelfi 41 , Reference Coggan, Leibowitz and Kadkhodayan 43 , Reference Fulford, Winyard and Vanhatalo 45 , Reference Kokkinoplitis and Chester 47 ) or positive effects( Reference Wylie, Kelly and Bailey 17 , Reference Thompson, Turner and Prichard 53 , Reference Wylie, Mohr and Krustrup 56 ) on exercise performance. Likewise, studies using several days (≥5 d) of supplementation showed that NO3 − supplementation had either no effects( Reference Bailey, Fulford and Vanhatalo 19 , Reference Bailey, Varnham and DiMenna 39 , Reference Fulford, Winyard and Vanhatalo 45 , Reference Wylie, Bailey and Kelly 57 ) or positive effects( Reference Bailey, Winyard and Vanhatalo 18 , Reference Lansley, Winyard and Bailey 22 , Reference Breese, McNarry and Marwood 40 , Reference Kelly, Vanhatalo and Wilkerson 46 ) on exercise performance. A similar rationale can be applied to the supplementation dose, which does not appear to influence physical performance. For example, studies using low doses (4–5·5 mmol) showed that NO3 − supplementation had either no effects( Reference Wylie, Kelly and Bailey 17 , Reference Bailey, Fulford and Vanhatalo 19 , Reference Aucouturier, Boissiere and Pawlak-Chaouch 38 , Reference Porcelli, Ramaglia and Bellistri 51 ) or positive effects( Reference Vanhatalo, Bailey and Blackwell 16 , Reference Porcelli, Ramaglia and Bellistri 51 , Reference Thompson, Turner and Prichard 53 , Reference Wylie, Mohr and Krustrup 56 ) on exercise performance. Finally, studies using high doses (>10 mmol) also showed that NO3 − supplementation had no effects( Reference Coggan, Leibowitz and Kadkhodayan 43 , Reference Fulford, Winyard and Vanhatalo 45 , Reference Rienks, Vanderwoude and Maas 52 ) or positive effects( Reference Wylie, Kelly and Bailey 17 , Reference Coggan, Leibowitz and Kadkhodayan 43 ) on exercise performance.
Despite the high variability in the experimental protocols used in the studies analysed in the present review, the analysed subgroups did not include heterogeneous samples. Therefore, the data homogeneity, the quality of the studies assessed by the risk of bias and the absence of publication bias in the studies used in this systematic review and meta-analysis are sufficient to draw conclusions.
A major limitation of this review is related to the wide variation in the methods (differences in the dose of NO3 −, number of days of supplementation, total amount ingested and mode of NO3 − delivery) used in the analysed studies. This methodological diversity complicates the interpretation of the results and precludes clear conclusions regarding certain features of supplementation, such as those listed above. In addition, most studied individuals were men, and whether a sex-related sensitivity to the enhancing effects of nitrate exists in non-athletes is unclear. Thus, future studies should include women as participants.
Practical applications
The present results may encourage coaches, athletes and exercise practitioners to consider the following: (1) NO3 − supplementation appears to be more effective in non-athletes than in athletes, particularly in performance evaluations using long-duration, open-ended tests; (2) the ergogenic effects mediated by NO3 − supplementation do not affect physical performance in athletes, including cyclists, which are the most studied athletic population; and (3) subjects classified at a lower PL (i.e. less conditioned) are more responsive to the effects of NO3 − supplementation than are subjects classified at a higher PL.
Conclusion
The present systematic review and meta-analysis indicates that dietary NO3 − supplementation improves physical performance in non-athletes, particularly in performance evaluations using long-duration, open-ended tests. In contrast, dietary NO3 − supplementation does not appear to benefit the performance of athletes.
Acknowledgements
The authors thank Dr Luc Van Loon for his helpful comments to the first draft of this manuscript.
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The funding institutions had no role in the study design, data analysis, decision to publish or preparation of the article.
All authors contributed to the development of the research question and study design. H. O. C., L. R. D., Q. T. R. and W. P. performed the literature search. H. O. C., L. R. D., Q. T. R. and F. S. M. M. performed the study selection. H. O. C., L. R. D. and Q. T. R. analysed the data. All authors interpreted the results and wrote the manuscript. All authors reviewed and approved the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114518000132



















