There has been a well-documented increase in the incidence of acute pancreatitis (AP)( Reference Yadav and Lowenfels 1 ), which now accounts for more than 210 000 hospital admissions per year in the USA and represents 0·7 hospitalisations per 1000( Reference Frossard, Steer and Pastor 2 , Reference Fagenholz, Castillo and Harris 3 ). The estimated annual cost amounts to 2·2 billion dollars( Reference Fagenholz, Fernandez-del Castillo and Harris 4 ).
The severity of pancreatitis is highly variable, such that stratification is required to support clinical decision-making. Previously, severity had been classified into mild and severe forms according to the 1992 Atlanta classification( Reference Bradley 5 ). More recently, however, a new classification system of mild, moderate, severe and critical pancreatitis has been developed to accurately predict outcome in the various patient subgroups( Reference Dellinger, Forsmark and Layer 6 ).
In the most severe forms, pancreatic inflammation triggers a systemic inflammatory response syndrome, increases metabolic rates and generates a catabolic state. Nutritional requirements increase at a time when intake is reduced, leading to an overall deficit. Thus, nutritional support is a crucial component of disease management. Historically, total parenteral nutrition (TPN) was provided to all patients with severe pancreatitis to meet their increased requirements and to provide for pancreatic ‘rest’. More recently, research has demonstrated maladaptive changes in intestinal morphology and function, where TPN is the sole source of nutrition( Reference Buchman, Moukarzel and Bhuta 7 ), and shown the protective role of enteral feeding in preserving intestinal mucosal integrity and limiting bacterial translocation( Reference Kotani, Usami and Nomura 8 ). This has clinical implications such that a number of trials have reported that enteral nutrition reduces mortality, multiple-organ failure, systemic infections and the need for surgery when compared with parenteral nutrition( Reference Kalfarentzos, Kehagias and Mead 9 – Reference Louie, Noseworthy and Hailey 11 ). These findings were confirmed in a Cochrane Review in 2010( Reference Al-Omran, Albalawi and Tashkandi 12 ). Accordingly, professional guidelines now recommend enteral nutrition over parenteral nutrition where tolerated( Reference Banks and Freeman 13 , 14 ).
Short-term enteral feeding is administered to critically ill patients via nasogastric (NG) or nasojejunal (NJ) tubes. A 2006 meta-analysis from critical care literature failed to demonstrate a clinically significant benefit of postpyloric feeding via NJ tubes when compared with gastric feeding in patients with no evidence of delayed gastric emptying( Reference Ho, Dobb and Webb 15 ). Similarly, a recent randomised controlled trial of ventilated patients with elevated levels of gastric residuals has found no improvement in energy delivery by NJ feeding or attenuation of the severity of aspiration pneumonia( Reference Davies, Morrison and Bailey 16 ). The pathophysiology of pancreatitis leads to extra considerations when deciding on nutritional strategies for this population of critically ill patients. The rationale behind jejunal feeding is to ‘rest’ the pancreas: reduce stimulation and limit exocrine function to modulate this pro-inflammatory cascade. Animal models of AP, however, have shown that pancreatic secretion in response to cholecystokinin is reduced soon after the onset of AP( Reference Niederau, Niederau and Luthen 17 , Reference Czako, Yamamoto and Otsuki 18 ). This being the case, there may be no clinical benefit of delivering enteral nutrition beyond the pylorus.
Petrov et al. ( Reference Petrov, Correia and Windsor 19 ) conducted a systematic review of trials of NG feeding in severe AP published up to December 2007. This review included ninety-three patients. Only three controlled studies were available at that time and just two trials were included in the meta-analysis. NG nutrition was considered safe and well tolerated and, when compared with NJ nutrition, there was no statistically significant difference with respect to mortality, diarrhoea, exacerbation of pain following feeding, or intolerance to feeding. More recent trials, with greater numbers of participants, suggest that NG feeding is reasonable in both mild-to-moderate( Reference Petrov, McIlroy and Grayson 20 ) and severe pancreatitis( Reference Singh, Sharma and Sharma 21 ) cases. Despite this, NG feeding has not been widely adopted and NJ feeding is still much more commonly employed. An updated review is warranted such that the role of NG feeding is assessed in the context of the current literature. Given that this review includes more trials and hence a larger number of patients than previous papers, it provides for a more valuable evaluation of the efficacy of NG nutrition.
The primary objective of this systematic review was to evaluate the efficacy of NG feeding in patients with AP (the ability to deliver adequate nutrition exclusively by the NG route). Secondary objectives were to compare NG and NJ feeding to establish equipoise with respect to the constraints of enteral feeding and to assess the possible limitations and side effect profile of the NG route.
Materials and methods
A systematic review was performed, and the results are reported according to PRISMA guidelines( Reference Moher, Liberati and Tetzlaff 22 ). All English-language, clinical studies (case series, case–control studies, cohort studies, non-randomised pragmatic trials and randomised clinical trials) published since the inception of indexed databases to June 2013 were eligible for inclusion in this systematic review. Controlled clinical trials comparing NG and NJ feeding were eligible for inclusion in the meta-analysis.
Adult patients with a diagnosis of AP as defined by the primary authors were considered for inclusion, regardless of underlying aetiology. Predicted severe pancreatitis was defined according to previous classification systems; newer classification systems now differentiate between mild, moderate, severe and critical forms. Enteral nutrition delivered by NG tube (the intervention) was compared with NJ nutrition.
The primary objective of this review was to determine the feasibility and efficacy of NG nutrition in patients with AP. The former refers to exclusive NG delivery of nutrition without the need to withdraw feeding or change to another modality of nutrition. Efficacy is determined by delivery of more than 75 % of the nutritional target.
Secondary objectives were to compare the NG and NJ routes with respect to the constraints of enteral nutrition in patients with pancreatitis: ability to deliver adequate nutrition; the frequency of change to parenteral nutrition; exacerbation of pain or disease severity; symptoms of gastric stasis (elevated levels of aspirates or abdominal distension). The side effects of NG nutrition examined were vomiting, diarrhoea, tube displacement and a need to reduce the rate of delivery.
Progression was quantified by the deterioration of APACHE II (Acute Physiology and Chronic Health Evaluation) scores or elevation of serum C-reactive protein measurements. Exacerbation of abdominal pain was defined as increased pain scores or increased analgesia requirements.
The following bibliographic databases were used to search for and identify relevant primary studies: Cochrane Controlled Trials Register (CCTR issues 5–12, May 2013) and MEDLINE (Ovid MEDLINE (R) 1946 to May Week 4 2013).
The registry of ongoing clinical trials at www.clinicaltrials.gov and the bibliographies of review articles were examined to identify additional studies. The MeSH terms ‘Pancreatitis’, ‘Pancreatitis, Acute Necrotizing’, ‘Intubation, Gastrointestinal’ and ‘Enteral Nutrition’ in combination with a keyword search of variations of ‘nasogastric’, ‘naso-jejunal’ and ‘post-pyloric’ were employed in the search strategy.
Studies were selected and retrieved by two authors independently. The titles and abstracts of all the retrieved studies were scanned to determine potential eligibility for inclusion. Full articles were retrieved for those found to be relevant. Data were collected according to a pre-piloted standardised format by the lead author with a second author involved in the verification of 5 % of the studies. Decisions regarding eligibility for inclusion or on issues surrounding data collection were made by consensus or by the senior author if agreement was not reached.
Data on the details of each study, demographics of enrolled patients and their disease particulars, specifics regarding the intervention (e.g. type of tube and type of formula), and the primary and secondary outcomes outlined above were collected.
For controlled trials, the risk of bias was assessed using a tool recommended by the Cochrane Collaboration( Reference Higgins, Altman, Higgins and Altman 23 ). Judgements on allocation concealment, blinding, incomplete outcome data reporting and selective reporting were categorised as low risk of bias, high risk of bias and unclear risk of bias.
Where outcomes could be sensibly combined (outcomes measured in comparable ways and low levels of heterogeneity), pooled risk ratios with 95 % CI were used as the measure of effect for each dichotomous outcome. The I 2 statistic was used to determine heterogeneity between the studies. A fixed-effects model was employed in all analyses due to very low levels of heterogeneity across the studies. For variables where meta-analytic techniques were not suitable, descriptive analyses were used. All analyses were carried out using the STATA statistical software package (version 12.0; StataCorp).
Results
Search results
A total of 915 articles were identified in the initial search. After exclusion of duplicates, 450 records were screened. Full-text articles were retrieved for sixteen studies, of which ten were excluded. Fig. 1 shows a PRISMA flow diagram of the search results and the reasons for study exclusion. After study exclusion, six studies( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 – Reference Piciucchi, Merola and Marignani 28 ) were found to be eligible for the qualitative review and four for the meta-analysis. Of note, the most recent randomised controlled trial pertaining to NG nutrition in AP( Reference Petrov, McIlroy and Grayson 20 ) was excluded on the basis that it focused on mild and moderate disease states.
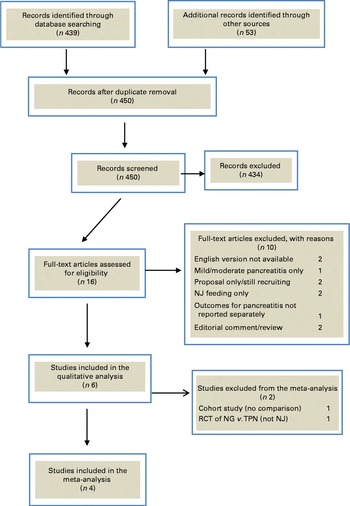
Fig. 1 Flow diagram depicting the process of study identification and selection for systematic review and meta-analysis. NJ, nasojejunal; RCT, randomised controlled trial; NG, nasogastric; TPN, total parenteral nutrition. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Studies included
A number of different study designs were identified: four randomised controlled trials( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 – Reference Kumar, Singh and Prakash 26 ); one cohort study( Reference Eatock, Brombacher and Steven 27 ); one non-randomised pragmatic study( Reference Piciucchi, Merola and Marignani 28 ). NG nutrition was compared with TPN in one randomised controlled trial( Reference Eckerwall, Axelsson and Andersson 25 ) and NG nutrition was compared with NJ nutrition in the remaining three trials( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 , Reference Kumar, Singh and Prakash 26 ). The included studies spanned the period from December 1996( Reference Eatock, Brombacher and Steven 27 ) to November 2009( Reference Piciucchi, Merola and Marignani 28 ). Of the studies, two were published in India( Reference Singh, Sharma and Sharma 21 , Reference Kumar, Singh and Prakash 26 ), two in Scotland( Reference Eatock, Chong and Menezes 24 , Reference Eatock, Brombacher and Steven 27 ), and one study each in Italy( Reference Piciucchi, Merola and Marignani 28 ) and Sweden( Reference Eckerwall, Axelsson and Andersson 25 ). The cohort study carried out by Eatock et al. ( Reference Eatock, Brombacher and Steven 27 ) in 2000 was a pilot, feasibility study that prompted the researchers to conduct a larger controlled trial comparing NG and NJ feeding; this was also included in this review( Reference Eatock, Chong and Menezes 24 ). Similarly, the studies carried out by Kumar et al. ( Reference Kumar, Singh and Prakash 26 ) and Singh et al. ( Reference Singh, Sharma and Sharma 21 ) involved the same investigators. In both instances, there was no overlap between the preliminary and follow-up studies and separate, distinct patient groups were recruited.
Intervention as described
Patients randomised to the NG feeding arm had tubes placed at the bedside by nursing and medical staff in five studies( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 – Reference Eatock, Brombacher and Steven 27 ) and the position was checked by aspiration, pH measurement and radiography. In the study carried out by Piciucchi et al. ( Reference Piciucchi, Merola and Marignani 28 ), an enteral feeding tube was placed at the bedside, tip progression was monitored by serial X-rays over 3 d and, depending on the final position, the patient was allocated to the NG or NJ feeding arm. In the other studies comparing NG and NJ feeding( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 , Reference Kumar, Singh and Prakash 26 ), patients receiving NJ nutrition underwent endoscopic tube placement. The position of NJ tubes was verified radiologically by Singh et al. ( Reference Singh, Sharma and Sharma 21 ), but neither Eatock( Reference Eatock, Chong and Menezes 24 ) nor Kumar( Reference Kumar, Singh and Prakash 26 ) reported confirmation of tube position. The control group in the study carried out by Eckerwall et al. ( Reference Eckerwall, Axelsson and Andersson 25 ) received parenteral nutrition.
The study design and intervention provided in each study included in this review are summarised in Table 1. The type and rate of formula delivered are also outlined.
Table 1 Summary of study design and intervention provided in each study included in this systematic review

RCT, randomised controlled trial; NJ, nasojejunal; TPN, total parenteral nutrition.
Patients included in this review
Of a total of 258 patients, 147 were allocated to the NG feeding arm across the six studies( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 – Reference Piciucchi, Merola and Marignani 28 ). Men accounted for 59·2 % of the patients. Biliary pancreatitis was most common, occurring in 49·7 % of the patients. A median age of 59·5 years was reported by four studies( Reference Eatock, Chong and Menezes 24 , Reference Eckerwall, Axelsson and Andersson 25 , Reference Eatock, Brombacher and Steven 27 , Reference Piciucchi, Merola and Marignani 28 ); a mean age of 41·2 years was reported by two studies( Reference Singh, Sharma and Sharma 21 , Reference Kumar, Singh and Prakash 26 ). Patient demographics, underlying aetiology and disease severity are outlined in Table 2.
Table 2 Demographics of patients receiving nasogastric nutrition
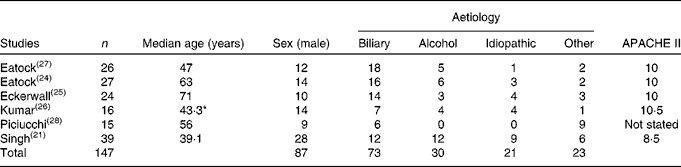
APACHE II, Acute Physiology and Chronic Health Evaluation.
* Mean age.
Assessment of the quality of studies included in this review
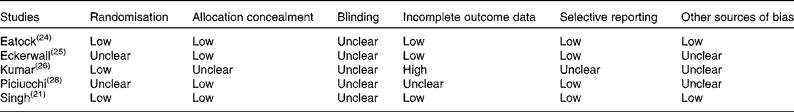
The quality of the controlled trials included in this review was assessed using the Cochrane Collaboration Tool for Assessing Risk of Bias. The findings are summarised in Table 3.
Table 3 Summary of risk of bias assessment for controlled clinical trials included in this systematic review
Primary outcomes
The primary outcome of this systematic review was exclusive NG feeding without any other modality of nutrition. This was achieved in 90·5 % (133/147) of the patients across the six studies( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 – Reference Piciucchi, Merola and Marignani 28 ). Of the other fourteen patients, eleven changed to TPN, one changed to NJ feeding, one required a feeding jejunostomy, and NG feeding was abandoned in the last patient due to repeated displacement of the tube. Of the 147 patients assigned to the NG feeding arm, 129 (87·8 %) achieved ≥ 75 % of the nutritional targets set by the investigators. In studies where all subjects received exclusive NG nutrition( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 , Reference Eckerwall, Axelsson and Andersson 25 ), 82·2 % (seventy-four of the ninety patients) received at least 75 % of the intended energy. In the remaining studies( Reference Kumar, Singh and Prakash 26 – Reference Piciucchi, Merola and Marignani 28 ), there were no differences in nutritional target outcomes between those who received exclusive NG nutrition and those who changed to another modality, and so this data point is not available. These primary outcomes are outlined in Table 4.
Table 4 Primary outcomes
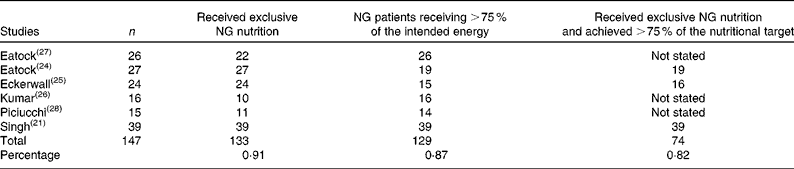
NG, nasogastric.
Secondary outcomes
Comparison with nasojejunal feeding
In the eligible trials, ninety-seven patients received nutrition via the NG route and eighty-five patients received nutrition via the NJ route. Of the eighty-five patients assigned to the NJ feeding arm, seven (8·2 %) changed to TPN and, including those who changed, 91 % received >75 % of the target energy.
The meta-analysis showed that there was no significant difference between the two routes in the delivery of nutrition: the pooled risk ratio for the delivery of 75 % of the nutritional target for NG v. NJ feeding was 1·02 (95 % CI 0·75, 1·38). This is shown in the forest plot in Fig. 2. There was no difference in the risk of change to TPN between the NG and NJ groups (pooled risk ratio 1·05; 95 % CI 0·45, 2·48; Fig. 3). A pooled risk ratio of 1·28 (95 % CI 0·6, 2·6) for diarrhoea for the NG v. NJ route was not statistically significant (forest plot shown in Fig. 4). Similarly, a pooled risk ratio of 1·10 (95 % CI 0·4, 2·6) was not significant for exacerbation of pain in NG v. NJ feeding (forest plot shown in Fig. 5). A pooled risk ratio of 0·44 (95 % CI 0·11, 1·73) indicates that NG feeding is less likely to lead to tube dislodgement, but this difference was not statistically significant (Fig. 6).

Fig. 2 Forest plot comparing the nasogastric and nasojejunal routes with respect to the delivery of more than 75 % of the target energy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 3 Forest plot comparing the nasogastric and nasojejunal routes with respect to the risk of change to total parenteral nutrition. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 4 Forest plot comparing the nasogastric and nasojejunal routes with respect to the risk of diarrhoea. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 5 Forest plot comparing the nasogastric and nasojejunal routes with respect to the risk of exacerbation of pain. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 6 Forest plot comparing the nasogastric and nasojejunal routes with respect to the risk of tube displacement. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Only two studies( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 ) included in this meta-analysis reported reduced rate of delivery and abdominal distension. The pooled risk ratios for these outcomes suggest that there was no significant difference between the NG and NJ groups (reduced rate of delivery: pooled risk ratio 1·57; 95 % CI 0·4, 5·1; abdominal distension: pooled risk ratio 0·56; 95 % CI 0·08, 4·03.)
During quality assessment using the Cochrane Collaboration Tool, the study carried out by Kumar et al. ( Reference Kumar, Singh and Prakash 26 ) was judged to be at the greatest risk of bias. Given this judgement, sensitivity analysis was performed to determine whether the exclusion of this study affected any of the above results, but no significant effect was found.
Side effects of nasogastric feeding
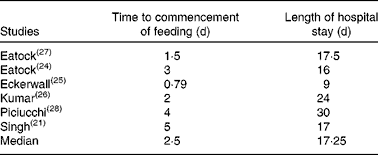
Tables 5 and 6 address the practicalities of NG feeding. The median time to commencement of NG nutrition was 2·5 (range 0·79–5) d; the median length of hospital stay was 17·25 (range 9–30) d. With respect to the delivery of nutrition, 3·4 % of the patients dislodged their tube or required replacement, while thirteen patients (11·2 %) required a reduction in the rate of delivery. Vomiting and diarrhoea were the most common side effects, affecting 13·3 and 12·9 % of the patients, respectively. Elevated levels of aspirates were observed in 9·1 % of the patients and abdominal distension affected 1·5 %. Pain scores or analgesia requirements increased in 7·5 % of the patients, and 1·6 % had an exacerbation of disease severity.
Table 5 Secondary outcomes: adverse effects of nasogastric nutrition

Table 6 Secondary outcomes: time intervals
Discussion
The benefits of enteral nutrition in patients with AP are well documented and accepted( Reference Al-Omran, Albalawi and Tashkandi 12 ). The primary outcome of this systematic review shows that NG delivery of enteral nutrition to patients with severe AP is efficacious: 90 % of the patients received exclusive NG nutrition and 82 % of the NG-fed patients achieved the nutritional target. Indeed, these values are comparable to the historical failure rate values of NJ feeding established in the literature: a retrospective review of postpyloric nutrition in intensive care unit (ICU) patients by Boulton-Jones et al. ( Reference Boulton-Jones, Lewis and Jobling 29 ) revealed successful tube placement in 92 % of the patients and nutritional requirements to be met by this route alone in 83 %. Furthermore, in this review, we found that there was no statistically significant difference between the NG and NJ routes with respect to the delivery of nutrition or change to TPN.
Patients with AP were previously kept fasting to minimise pancreatic stimulation and hence reduce the secretion of proteolytic enzymes, which might otherwise promote inflammation within the gland. When it was first introduced, enteral nutrition was delivered distally to avoid the exacerbation of disease severity in this manner. However, the dogma of ‘pancreatic rest’ is currently under challenge, although conflicting outcomes have been reported. Results from animal models have shown reduced exocrine activity in the pancreas of patients with AP( Reference Keller and Layer 30 ), and Boreham & Ammori( Reference Boreham and Ammori 31 ) found a similar exocrine insufficiency in humans recovering from their first episode of pancreatitis. However, O'Keefe et al. ( Reference O'Keefe, Broderick and Turner 32 ) found reduced exocrine activity manifesting as a consequence of impaired enzyme secretion and not reduced synthesis. These authors assert that this distinction is clinically relevant and provides an argument against oro-enteral nutrition. Pancreatitic stimulation is, therefore, a highly topical issue as ‘gut-rousing’ nutritional strategies are being promoted( Reference Petrov 33 , Reference Petrov and Windsor 34 ). These studies suggest that restoration or maintenance of gastrointestinal function is the highest priority in the nutritional management of AP and that avoidance of pancreatic stimulation and delivery of adequate nutrition are secondary goals. In this review, NG nutrition was found to satisfy each of these requirements: 82 % of the patients received their target energy and there was no difference between the NG and NJ groups with respect to increased pain intensity as might be expected if proximal (NG) feeding was stimulating the pancreas excessively and promoting disease progression.
Further secondary outcomes of this review address possible limitations of NG delivery of nutrition in patients with AP. Diarrhoea is a recognised side effect of enteral nutrition occurring in up to 29 % of the patients with pancreatitis receiving NJ nutrition but in only 7 % of those receiving TPN (risk ratio 0·20; 95 % CI 0·09, 0·43)( Reference Petrov and Whelan 35 ). In this review, however, diarrhoea was found to be less problematic, occurring in 12·9 % of the patients who received NG nutrition. NG delivery of nutrition did not increase the severity of diarrhoea when compared with NJ delivery of nutrition.
Pancreatic inflammation predisposes to gastric stasis such that abdominal distension or elevated levels of aspirates might limit gastric feeding more than postpyloric delivery of nutrition. Where reported in this review, these symptoms were found to affect 1·5 and 9·1 % of the NG-fed patients, respectively. When compared with NJ nutrition, however, there was no increased risk of abdominal distension, although it must be noted that this is based on only two studies reporting this outcome.
Advantages of nasogastric nutrition
NJ tubes are more difficult to insert, requiring at least specialist staff, if not intra-hospital transfer of a critically unwell patient for endoscopic or fluoroscopic placement. In addition, as specialist involvement is required, providing NJ nutrition is more expensive: Hauschild et al. ( Reference Hauschild, Fu and Hipwell 36 ) calculated the cost of fluoroscopic and endoscopic NJ tube insertion to be US $226 and US $328, respectively. In increasingly resource-conscious health care, instituting more economical interventions without compromising clinical care is a priority. This is particularly relevant in the management of severe AP, given the increasing prevalence of the condition.
Introducing enteral nutrition early in critically ill patients has been shown to be beneficial( Reference Marik and Zaloga 37 , Reference Heyland, Dhaliwal and Drover 38 ). This has also been shown to be the case in patients with severe AP( Reference Li, Yu and Chen 39 , Reference Petrov, Pylypchuk and Uchugina 40 ): a systematic review of eleven trials found that enteral nutrition within 48 h reduced mortality, multiple-organ failure and pancreatic compilations( Reference Petrov, Pylypchuk and Uchugina 40 ). Similarly, a recent study carried out by Sun et al. ( Reference Sun, Li and Ke 41 ) has found that early enteral nutrition reduces intra-abdominal hypertension and disease severity. One of the innate advantages of NG feeding is that in normal clinical practice, insertion of a NG tube should be achieved with greater ease and speed than NJ intubation, allowing initiation of a feeding regimen earlier. In the setting of clinical trials similar to those included in this review, however, NG nutrition could not be commenced until after randomisation. This prevents the anticipated more prompt commencement of feeding via the NG route, which is more likely in clinical practice rather than in a trial setting.
Existing evidence and potential for further research in this area
A systematic review of NG feeding was published by Petrov et al. ( Reference Petrov, Correia and Windsor 19 ) in 2008; ninety-three patients were included( Reference Eatock, Chong and Menezes 24 – Reference Kumar, Singh and Prakash 26 ) and two studies( Reference Eatock, Chong and Menezes 24 , Reference Kumar, Singh and Prakash 26 ) were pooled for meta-analysis. The present systematic review incorporates the most current research in this area. Furthermore, with 147 patients and four trials being eligible for meta-analysis( Reference Singh, Sharma and Sharma 21 , Reference Eatock, Chong and Menezes 24 , Reference Kumar, Singh and Prakash 26 , Reference Piciucchi, Merola and Marignani 28 ), this review includes more patients for qualitative review and more trials for pooled analysis, increasing the confidence with which conclusions can be drawn, particularly those pertaining to efficacy. The results of this review and the 2008 reviews, however, are consistent: Petrov et al. ( Reference Petrov, Correia and Windsor 19 ) defined full tolerance as delivery of NG nutrition without the need to withdraw, stop or reduce the rate of delivery. This criterion was met by 79·3 % of the patients. Our primary outcome, exclusive NG feeding, was achieved in 91 % of the patients. Overall, 11 % of the patients whom we included required a reduction in the rate of delivery.
The quality of any systematic review or meta-analysis depends on the quality of included studies. The greatest limitation of this review is the lack of high-quality level one trials pertaining to this subject. Furthermore, not all the secondary endpoints of this systematic review are reported in all studies. This restricted meta-analysis of variables pertaining to vomiting and gastric residuals. Given the number of studies available, it was impossible to construct a funnel plot to detect possible publication bias.
A high-quality randomised controlled trial comparing NG and NJ feeding could provide further clarity on the equipoise of these two routes and give further insights into ‘gut-rousing’ nutritional strategies. Further research in this area should address ways to optimise the delivery of NG nutrition to those for whom it is the first-line approach. Equally, it is important to identify the 10–20 % of patients who might benefit from alternative delivery routes such that their outcomes are not moderated by novel nutritional approaches.
Conclusion
NG feeding is efficacious in patients with severe AP. Overall, a small number of trials are available, but nonetheless, on meta-analysis, the NG route represents a viable alternative to NJ feeding.
Acknowledgements
The authors’ contributions are as follows: D. M. N. was the first investigator and prepared the manuscript; E. G. K. was the second investigator; M. C. was the methodological supervisor and carried out the statistical analysis; P. R. was the methodological and clinical supervisor.
None of the authors has any conflicts of interest to declare.














