Volatile fatty acids (VFA), carbon dioxide (CO2) and molecular H (H2) are produced during carbohydrate fermentation by bacteria, protozoa and fungi in the rumen. VFA serve as a major energy source for the host animal( Reference Vyas, McGinn and Duval 1 ), with acetate and propionate being the main precursors of fat and glucose, respectively. The main consumers of H2 are methanogenic Archaea, producing methane (CH4) as an end product of fermentation, which keeps a low H2 partial pressure in the rumen( Reference Janssen 2 ). It is understood that the accumulation of H2, which typically occurs when methanogenesis is inhibited, could hinder the re-oxidation of reduced electron carriers and adversely affect fermentation and fibre digestion( Reference Joblin 3 ).
Besides being used as a substrate for methanogenesis, H2 is also involved in VFA production( Reference Janssen 2 , Reference Ungerfeld and Kohn 4 ). This is because different numbers of moles of H2 (or of reducing equivalent pairs in reduced co-factors), are released or incorporated in the production pathway of each VFA. For example, fermentation of 1 mol of glucose to acetate releases 4 mol of electron pairs, most of which are transferred to protons to form H2. In contrast, fermentation of glucose to propionate involves H2 or metabolic H incorporation( Reference Janssen 2 ). When animals are switched from fibrous to starchy diets, or rumen methanogenesis is inhibited, dissolved H2 (dH2) in the rumen elevates, and, as expected from the stoichiometry of acetate and propionate production, fermentation shifts from acetate to propionate( Reference Wang, Wang and Xie 5 – Reference Mitsumori, Shinkai and Takenaka 7 ).
However, when the effect of direct addition of H2 as gas (gaseous H2, gH2) has been studied in in vitro batch cultures( Reference Patra and Yu 8 – Reference Qiao, Tan and Guan 10 ) and in vivo ( Reference Olijhoek, Hellwing and Weisbjerg 11 ), the results observed in acetate and propionate do not fully agree with that rationale. In batch cultures, Broudiscou et al. ( Reference Broudiscou, Offner and Sauvant 12 ) found that the effect of an initial 0·5 or 1 kPa gH2 headspace on acetate and propionate production depended on the inoculum used, and when using an inoculum adapted to a fibrous substrate, gH2 addition unexpectedly increased acetate and decreased propionate. Similarly, Patra & Yu( Reference Patra and Yu 8 ) found that including gH2 in the initial culture headspace unexpectedly resulted in lower propionate molar percentage. Similarly, Qiao et al. ( Reference Qiao, Tan and Guan 10 ) found that increasing gH2 in the headspace of batch cultures actually increased acetate molar percentage and the acetate:propionate ratio, and decreased propionate molar percentage. Infusion of gH2 in the rumen of dairy cows did not result in changes in total VFA concentration or VFA profile( Reference Olijhoek, Hellwing and Weisbjerg 11 ). The effect of gH2 on dH2 concentration was not reported in those studies. Given that dH2 and gH2 have been shown not to be at equilibrium in vitro ( Reference Wang, Sun and Janssen 13 ) and in vivo ( Reference Wang, Ungerfeld and Wang 14 ), and that the form of H2 available to microbes is dH2 rather than gH2, we propose that the role of H2 on fermentation needs further examination by studying the effect of dH2 addition.
Our first hypothesis for this study using goats as the experimental ruminant model was that dH2 could be increased through supplementing elemental Mg to the diet, as elemental Mg would react with water in rumen fluid releasing H2:Mg + 2 H2O→Mg(OH)2+H2 Reference Ng, Kittelmann and Patchett (15 ). Our second hypothesis was that increased dH2 would alter rumen fermentation and the microbial community composition.
Methods
All animal procedures were approved by the Animal Care Committee, Institute of Subtropical Agriculture, the Chinese Academy of Sciences (CAS), Changsha, China.
Goats and diets
A randomised block design with two treatments was used to investigate the effects of H2 generated by the reaction of elemental Mg with water in the rumen fluid of goats. In all, twenty growing Xiangdong black male goats (mean initial body weight=20·3 (sd 3·42) kg) were allocated to ten blocks according to body weight and CH4 emission (g/kg DM intake) measured in a preliminary experiment (unpublished results). Two treatments were formulated with equal dietary Mg content: basal diet plus 1·45 % (DM basis) Mg(OH)2 powder (99 % purity; Beijing Taizejiaye Technology Development Co., Ltd) for the control group and basal diet with 0·60 % (DM basis) elemental Mg powder (99 % purity; Beijing Taizejiaye Technology Development Co., Ltd) for the hypothesised elevated dH2 group. In a preliminary experiment (unpublished results), these treatments had been shown to be harmless to the health of goats. Each block of animals contained two goats and each goat within a block was randomly assigned to one of the two dietary treatments.
Goats were kept in individual pens and had free access to fresh water. The diet was formulated to meet 1·4 times maintenance requirements of goats. The composition of the basal diet is shown in Table 1. Dietary forage and concentrate were not mixed, and both forage and concentrate were offered separately, each divided into two equal portions at 08.00 and 17.00 hours. Diets were offered for a 28-d adaptation period before conducting measurements. The elemental Mg and Mg(OH)2 supplements were mixed with the concentrate fraction immediately before feeding to avoid the reaction with water in the environment, and the concentrate fraction containing the elemental Mg and Mg(OH)2 supplements was eaten completely within approximately 1 h. During the initial 10 d of adaptation to diets, feed was offered ad libitum targeting 5 % refusals. The amount of feed allocated daily during the next 18 d of adaptation was adjusted to 100 % of the DM intake previously measured in order to minimise feed selection. The refusals, when present, were collected and analysed to determine the actual nutrient intakes.
Table 1 Ingredients and chemical composition of the basal diet offered to goats (g/kg DM)
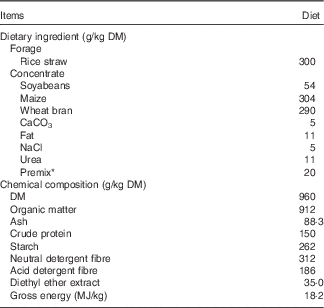
* Premix was formulated to provide the following (per kg of premix): 400 g of NaHCO3, 2 g of Fe, 1 g of Cu, 0·01 g of Co, 0·05 g of I, 6·6 g of Mn, 4·4 g of Zn, 0·003 g of Se, 333 mg of retinol, 5 mg of cholecalciferol, 838 mg of α-tocopherol.
Nutrient digestibility
Nutrient digestibility was determined over a 5-d period from days 29 to 33. Total faeces and urine were collected twice daily, weighed and mixed daily. A subsample (approximately 1 %) was frozen immediately at −20°C, and another subsample (approximately 1 %) was acidified using 10 % (w/w) H2SO4 to prevent N loss and then frozen immediately at −20°C. The faeces samples were later dried at 60°C for 48 h in a forced-air oven, and ground through a 1-mm screen. The acidified oven-dried samples were used for total N analysis, whereas non-acidified, oven-dried samples were used for other chemical analyses.
Rumen sampling
Collection of rumen contents was performed at 0, +2·5 and +6 h relative to the commencement of the morning feeding on days 34 and 35. Rumen contents (300 ml) were collected by oral stomach tubing as described by Wang et al. ( Reference Wang, Wang and Janssen 16 ), with the initial 100 ml discarded to avoid saliva contamination. The pH of rumen contents was measured immediately after sampling using a portable pH meter (Starter 300; Ohaus Instruments Co. Ltd). Two subsamples of 35 ml each were immediately transferred into 50-ml plastic syringes for measuring dH2 and dissolved CH4 (dCH4) concentration, as described by Wang et al. ( Reference Wang, Sun and Janssen 13 ). Two other 35-ml subsamples were immediately frozen at −80°C in liquid N2 for DNA extraction and subsequent microbial analyses. In addition, 2-ml samples of rumen contents were collected and centrifuged at 15 000 g for 10 min at 4°C, and 1·5 ml of supernatant was acidified using 0·15 ml of 25 % (w/v) metaphosphoric acid, and stored at −20°C for subsequent measurement of VFA concentration. The remaining sample of rumen contents was stored at −20°C for the measurements of ammonium (NH4 +), Mg2+ and glucose concentration.
Methane and carbon dioxide emissions
CH4 and CO2 emissions were measured in three plexiglass respiration chambers that permitted the goats to see each other, thereby minimising stress. CH4 and CO2 emissions were measured individually for each goat for 48 h using the protocol of Wang et al.( Reference Wang, Wang and Sun 17 ) slightly modified. In brief, during seven periods of 2 d each, each block of animals containing two goats assigned to different treatments was randomly assigned to a chamber. One goat from each block was then randomly assigned to a measurement period, and the second goat from that block was placed in the same chamber in the subsequent period. Within the chamber, the goats were restrained with free access to a feed bin and drinking water. Airflow was maintained under negative pressure (flow rate=35 m3/h). Outlet gas from the chamber and ambient gas were connected to a multiport inlet unit of a gas analyser (GGA-30p; Los Gatos Research) for measuring CH4 and CO2 concentration. The cycling time to measure CH4 and CO2 concentration produced in the chamber was 30 min, with 8 min for analysing gases from each of the three chambers and 6 min for analysing background environmental concentrations of CH4 and CO2 in incoming air. Daily CH4 and CO2 emissions were calculated using net CH4 and CO2 concentrations and flow rate of air through each chamber at 30-min intervals, and differences between chambers were corrected using the methodology described by McGinn et al.( Reference McGinn, Beauchemin and Coates 18 ). The chambers were opened twice a day at 08.00 and 18.00 hours to deliver the diets. The chamber cleaning, such as swapping faeces and urine trays, took place during the morning before feeding.
Sample analysis
All samples of feeds, refusals and faeces were dried and ground to pass through a 1-mm sieve. Contents of DM (method 945.15), organic matter (OM) (method 942.05), crude protein (method 954.01) and diethyl ether extract (method 920.39) were determined according to published methodologies( 19 ). Gross energy was determined using an isothermal automatic calorimeter (5E-AC8018; Changsha Kaiyuan Instruments Co. Ltd). Contents of neutral detergent fibre (NDF) and acid detergent fibre were determined and expressed inclusive of residual ash( Reference Van Soest, Robertson and Lewis 20 ), and NDF was assayed with the addition of a heat-stable amylase, but without sodium sulphite. The starch content was determined after pre-extraction with ethanol (80 %), and glucose released from starch by enzyme hydrolysis was measured using amyloglucosidase( Reference Kartchner and Theurer 21 ).
Frozen acidified rumen samples were thawed and centrifuged at 15 000 g for 10 min at 4°C, and individual VFA concentrations in the supernatant were measured using GC (Agilent 7890A; Agilent Inc.), according to the method described by Wang et al. ( Reference Wang, Sun and Janssen 13 ). The estimated net H2 production relative to the amount of total VFA produced (RNH2) was estimated according to the stoichiometric equation developed by Wang et al. ( Reference Wang, Wang and Janssen 16 ), under the assumption of equal fractional rates of individual VFA absorption. Ammonia and glucose in the supernatant were determined colorimetrically according to the methods of Weatherburn( Reference Weatherburn 22 ) and Nelson( Reference Nelson 23 ), respectively. The concentration of Mg2+ in the supernatant was determined by Inductively Coupled Plasma-Optical Emission Spectrometers using Varian 720-ES series (Agilent Inc.).
Dissolved gases were also measured immediately after sampling using the procedure described by Wang et al.( Reference Wang, Wang and Janssen 16 ) with a slight modification. In brief, a 20-ml syringe containing 10 ml of N2 gas was connected to a 50-ml plastic syringe containing 35-ml rumen content samples via polyurethane tubing. The N2 gas was then injected into the 50-ml syringe, and the gases dissolved in the rumen fluid were extracted into the N2 gas phase by vigorously hand shaking for 5 min. Gaseous H2 and CH4 (gCH4) concentrations in the gas phase were measured by GC (Agilent 7890A). The dH2 and dCH4 concentrations in the original rumen fluid were calculated using equations described by Wang et al. ( Reference Wang, Sun and Janssen 13 ). Gaseous CO2 (gCO2) was calculated as the total dissolved gas extracted minus dCH4 extracted, assuming that rumen gases would be composed of CO2 and CH4. Total dissolved CO2 (dCO2) concentration in the original rumen fluid (CTdCO2 , mol/l) was calculated by combining equations from Wang et al.( Reference Wang, Wang and Janssen 16 ) and Hille et al.( Reference Hille, Hetz and Rosendahl 24 ), and expressed as follows:
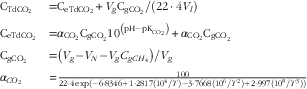 $$\matrix{ {{\rm C}_{{{\rm TdCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}C}_{{{\rm eTdCO}_{{\rm 2}} }} {\rm {\plus}}V_{g} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} {\rm /}\left( {{\rm 22}\cdot{\rm 4}V_{l} } \right)} \hfill \cr {{\rm C}_{{{\rm eTdCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}}\alpha _{{{\rm CO}_{{\rm 2}} }} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} {\rm 10}^{{\left( {{\rm pH}{\minus}{\rm pK}_{{{\rm CO}_{{\rm 2}} }} } \right)}} {\rm {\plus}}\alpha _{{{\rm CO}_{{\rm 2}} }} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} } \hfill \cr {{\rm C}_{{{\rm gCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}}\left( {V_{g} {\minus}V_{N} {\minus}V_{g} C_{g}_\rm_{{CH}_{\rm 4}}} \right){\rm /}V_{g} } \hfill \cr {\alpha _{{CO_{2} }} } \hfill & {{\equals}{{100} \over {22\cdot 4\,{\rm exp}\left( {{\minus}6\cdot8346{\plus}1\cdot2817\left( {10^{4} /T} \right){\minus}3\cdot7668\left( {10^{6} /T^{2} } \right){\plus}2\cdot997\left( {10^{8} /T^{3} } \right)} \right)}}} \hfill \cr } \,$$
$$\matrix{ {{\rm C}_{{{\rm TdCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}C}_{{{\rm eTdCO}_{{\rm 2}} }} {\rm {\plus}}V_{g} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} {\rm /}\left( {{\rm 22}\cdot{\rm 4}V_{l} } \right)} \hfill \cr {{\rm C}_{{{\rm eTdCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}}\alpha _{{{\rm CO}_{{\rm 2}} }} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} {\rm 10}^{{\left( {{\rm pH}{\minus}{\rm pK}_{{{\rm CO}_{{\rm 2}} }} } \right)}} {\rm {\plus}}\alpha _{{{\rm CO}_{{\rm 2}} }} {\rm C}_{{{\rm gCO}_{{\rm 2}} }} } \hfill \cr {{\rm C}_{{{\rm gCO}_{{\rm 2}} }} } \hfill & {{\rm {\equals}}\left( {V_{g} {\minus}V_{N} {\minus}V_{g} C_{g}_\rm_{{CH}_{\rm 4}}} \right){\rm /}V_{g} } \hfill \cr {\alpha _{{CO_{2} }} } \hfill & {{\equals}{{100} \over {22\cdot 4\,{\rm exp}\left( {{\minus}6\cdot8346{\plus}1\cdot2817\left( {10^{4} /T} \right){\minus}3\cdot7668\left( {10^{6} /T^{2} } \right){\plus}2\cdot997\left( {10^{8} /T^{3} } \right)} \right)}}} \hfill \cr } \,$$
where
![]() $${\rm C}_{{{\rm eTdCO}_{{\rm 2}} }} $$
is the total dCO2 concentration (mol/l) in the rumen fluid at equilibrium after extraction;
$${\rm C}_{{{\rm eTdCO}_{{\rm 2}} }} $$
is the total dCO2 concentration (mol/l) in the rumen fluid at equilibrium after extraction;
![]() $${\rm C}_{{{\rm gCO}_{{\rm 2}} }} $$
the gCO2 concentration (litres/l) measured in the gas phase of the 20-ml syringe at equilibrium after extraction of dissolved gases;
$${\rm C}_{{{\rm gCO}_{{\rm 2}} }} $$
the gCO2 concentration (litres/l) measured in the gas phase of the 20-ml syringe at equilibrium after extraction of dissolved gases;
![]() $${\rm C}_{{{\rm gCH}_{{\rm 4}} }} $$
the gCH4 concentration (litres/l) measured in the gas phase of the 20-ml syringe at equilibrium after extraction of dissolved gases; V
g
the gas volume (ml) at equilibrium after extraction of dissolved gases; V
l
the volume of liquid (ml); V
N
the injected N2 gas volume (10 ml);
$${\rm C}_{{{\rm gCH}_{{\rm 4}} }} $$
the gCH4 concentration (litres/l) measured in the gas phase of the 20-ml syringe at equilibrium after extraction of dissolved gases; V
g
the gas volume (ml) at equilibrium after extraction of dissolved gases; V
l
the volume of liquid (ml); V
N
the injected N2 gas volume (10 ml);
![]() $${\rm pK}_{{{\rm CO}_{{\rm 2}} }} $$
the dissociation constant of bicarbonate and set to be 6·11(
Reference Hille, Hetz and Rosendahl
24
);
$${\rm pK}_{{{\rm CO}_{{\rm 2}} }} $$
the dissociation constant of bicarbonate and set to be 6·11(
Reference Hille, Hetz and Rosendahl
24
);
![]() $$\alpha _{{{\rm CO}_{{\rm 2}} }} $$
the Bunsen absorption coefficient (mol/l·atm) for CO2
(
Reference Carroll, Slupsky and Mather
25
); and T the temperature in K (273+temperature in °C).
$$\alpha _{{{\rm CO}_{{\rm 2}} }} $$
the Bunsen absorption coefficient (mol/l·atm) for CO2
(
Reference Carroll, Slupsky and Mather
25
); and T the temperature in K (273+temperature in °C).
Estimation of Gibbs free-energy changes
Gibbs energy changes (ΔG) of fermentation reactions (online Supplementary Table S1) were estimated using measured concentrations of metabolites at 0, +2·5 and +6 h relative to the commencement of the morning feeding. Gibbs energy changes of reactions at standard conditions of 298 K were calculated from standard Gibbs energy of formation of reactants and products, and then adjusted to a rumen temperature of 312 K (39°C) using the van’t Hoff equation( Reference Guyader, Eugène and Doreau 26 ). Gibbs energy changes estimated for actual rumen conditions were subsequently adjusted using measured concentrations of soluble metabolites (10−pH, glucose, acetate, propionate and butyrate) and dissolved gases (dH2, dCO2 and dCH4)( Reference Wang, Ungerfeld and Wang 14 ).
Quantitative real-time PCR analyses
Rumen samples (2 ml) at each of the three sampling time points were pooled and freeze-dried, and then physically disrupted using a bead beater for 1 min. Genomic DNA was extracted using the QIAamp DNA Stool Mini kit (Qiagen) according to the manufacturer’s instructions. The quantity of DNA was measured based on absorbance at 260 and 280 nm using a NanoDrop ND 100 (Nano Drop Technologies). The absolute quantification of total bacteria, protozoa, fungi, methanogens and select bacterial species was measured by quantitative real-time PCR (qPCR) using primers validated in our laboratory (online Supplementary Table S2)( Reference Jiao, Lu and Tan 27 ). Quantitative PCR was performed according to the procedures described by Jiao et al.( Reference Jiao, Lu and Tan 27 ). Final absolute amounts of target groups or species were estimated by relating the C T value to the standard curves and expressed as log10 copies/DM rumen contents. The abundances of six select rumen bacterial species (Ruminococcus albus, R. flavefaciens, Fibrobacter succinogenes, Selenomonas ruminantium, Ruminobacter amylophilus and Prevotella ruminicola) and of genus Prevotella spp. were measured using qPCR and species-specific 16S rRNA gene-targeted primers (online Supplementary Table S2) and expressed relative to the total bacterial DNA. The abundances of each bacterial species and of genus Prevotella spp. were determined using the ∆C T method( Reference Stevenson and Weimer 28 ).
High-throughput sequencing and analysis
Extracted purified DNA (50 ng) from each rumen sample was subjected to PCR amplification of the V3–V4 region of 16S rRNA gene using universal bacterial primers 338 F (5'-ACTCCTACGGGAGGCAGCA-3') and 806 R (5'-GGACTACHVGGGTWTCTAAT-3')( Reference Hackmann and Firkins 29 ). PCR was performed using a GeneAmp® 9700 thermal cycler (Applied Biosystems). The PCR products were purified using the AxyPrep™ DNA Gel Extraction Kit (Axygen Biosciences) according to the manufacturer’s instructions and quantified using the QuantiFluor™-ST system (Promega). Purified PCR products were high-throughput-sequenced using an IlluminaMiSeq PE300 instrument at Majorbio Bio-Pharm Technology Co., Ltd, using protocols recommended by procedures of Miseq reagent kits v3 (Illumina). Sequences were quality filtered and demultiplexed using exact matches to the supplied DNA barcodes.
Bacterial phylotypes were identified using uclust( Reference Edgar 30 ) and assigned to operational taxonomic units (OTU) at 97 % sequence identity. Taxonomic identity of each phylotype was determined using the Greengenes database( Reference DeSantis, Hugenholtz and Larsen 31 ). The resulting OTU were combined into an OTU table that represented abundance of each OTU in each microbial sample. Alpha diversity of bacterial communities was obtained using Mothur version 1.30.1. Similarity between bacterial communities was assessed using the Bray–Curtis distance metric and visualised using principal coordinates (PCoA) analysis.
Statistical analyses
The statistical model used included dietary treatment and block as fixed effects and sampling day as random effect. When sampling time was included, the model included dietary treatment and block as fixed effects, sampling time as a repeated-measure variable, and the interaction between dietary treatment and sampling time and sampling day as random effects. The best linear or log linear regression was derived between dH2 and concentration of other rumen metabolites using ordinary least squares. Associations between response variables were studied through calculating their Pearson’s correlation coefficient (r) and statistical significance. P≤0·05 was considered significant, and 0·05<P≤0·1 was accepted as a tendency to significance. The SPSS 12.0 software was used for the statistical analyses.
Results
Although no differences were observed for feed intake and digestibility between both treatments, elemental Mg supplementation increased CH4 emissions expressed as g/d (+11·7 %, P=0·03) and as g/kg OM intake (+9·87 %, P=0·03) (Table 2). No differences were observed in ruminal Mg2+ concentration and pH between the two treatments (Table 3). The pH was negatively correlated (r−0·75, P<0·001) with ruminal Mg2+ concentration (online Supplementary Fig. S1).
Table 2 Feed intake and digestibility, methane (CH4) and carbon dioxide (CO2) emissions in growing goats fed diets with 1·45 % Mg(OH)2 or 0·6 % elemental magnesium after 28 d of adaption (n 10) (Mean values with their standard errors)
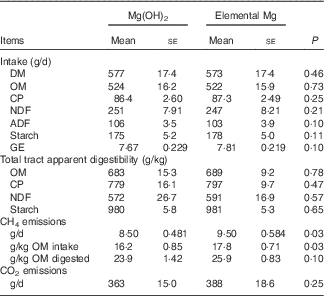
CP, crude protein; NDF, neutral detergent fibre; ADF, acid detergent fibre; GE, gross energy.
Table 3 Concentration of rumen metabolites in growing goats fed diets with 1·45 % Mg(OH)2 or 0·6 % elemental magnesium after 28 d of adaption (n 10) (Mean values with their standard errors)

dH2, dissolved H2; dCH4, dissolved methane; dCO2, dissolved carbon dioxide; VFA, volatile fatty acids.
Elemental Mg supplementation increased the average dH2 (+95·1 %, P=0·02) and dCH4 concentrations (+39·0 %, P<0·001) (Table 3). Interactions between treatment and time were observed for dH2 (P=0·02) and dCH4 (P<0·001). Elemental Mg supplementation increased dH2 only at +2·5 h (+180 %, P<0·001) but not at 0 or +6 h relative to the commencement of the morning feeding, and increased dCH4 at +2·5 (+63·4 %, P<0·001) and +6 h (+50·5 %, P<0·001) but not at 0 h relative to the commencement of the morning feeding (Fig. 1). At +2·5 h, ruminal dH2 was positively correlated with dCH4 (r 0·50, P =0·02) (online Supplementary Fig. S2). Furthermore, ruminal dH2 concentration varied widely among the 10 goats treated with elemental Mg supplementation, the highest dH2 concentration being 17 mmol/l. When this highest dH2 point was removed, the Pearson’s correlation coefficient between dH2 and dCH4 was 0·486 (P=0·03, data not shown).

Fig. 1 Dissolved hydrogen (dH2, a) and methane (dCH4, b) in rumen contents at 0, 2·5 and 6 h after the commencement of the morning feeding in goats fed diets with 1·45 % Mg(OH)2 (![]() ) or 0·6 % elemental magnesium (
) or 0·6 % elemental magnesium (![]() ) after 28 d of adaption. Values are means (n 10), with their standard errors represented by vertical bars. *** P<0·001.
) after 28 d of adaption. Values are means (n 10), with their standard errors represented by vertical bars. *** P<0·001.
Elemental Mg supplementation decreased total VFA molar concentration (−11·6 %, P<0·001), acetate molar percentage (−3·80 %, P<0·001), the acetate to propionate ratio (−11·8 %, P<0·03) and RNH2 (−42·1 %, P<0·03), and increased propionate molar percentage (+11·6 %, P<0·001) (Table 3). Elemental Mg supplementation slightly increased ∆G of glucose fermentation to acetate (P=0·03) and to butyrate (P=0·04), did not alter ∆G of glucose to 2/3 acetate + 4/3 propionate (P=0·36) and tended (P=0·009) to decrease ∆G of glucose + 2H2 to propionate at +2·5 h relative to the commencement of the morning feeding (Table 4).
Table 4 Estimated Gibbs energy changes (kJ/reaction) of seven reaction pathways in the rumen of goats fed diets 1·45 % Mg(OH)2 or 0·6 % elemental magnesium after 28 d of adaption (n 10)Footnote * (Mean values with their standard errors)

* Water is omitted for simplicity.
Because the hypothesised effect on dH2 was observed only at +2·5 h relative to the commencement of the morning feeding, we focused on this time point for studying the effects of elemental Mg supplementation on the associations between concentration of dH2 and other rumen metabolites. Ruminal dH2 was negatively correlated with total VFA concentration (r −0·60, P=0·005), acetate molar percentage (r −0·76, P<0·001), acetate:propionate ratio (r −0·60, P=0·005) and RNH2 (r −0·40, P=0·08), and positively correlated with propionate molar percentage (r 0·47, P=0·03) at +2·5 h relative to the commencement of the morning feeding (online Supplementary Fig. S3). When the greatest dH2 data point was removed, only a trend (r −0·39, P=0·09, data not shown) to a correlation between dH2 and acetate molar percentage at +2·5 h was observed.
Elemental Mg supplementation decreased the copy numbers of fungi (−63·6 %, P=0·006) and the relative abundance of S. ruminantium (−39·7 %, P=0·008), and increased the copy numbers of methanogens (+47·9 %, P<0·001) and relative abundance of R. flavefaciens (+71 %, P=0·02) (online Supplementary Table S3). Ruminal +2·5 h dH2 was negatively correlated with copy numbers of fungi (r −0·53, P=0·02) and the relative abundance of S. Ruminantium (r −0·43, P=0·05), and positively correlated with copy numbers of methanogens (r 0·54, P =0·01) (Fig. 2). When the greatest dH2 data point was removed, a significant correlation (r −0·54, P=0·01, data not shown) between +2·5 h dH2 and copy numbers of fungi was still observed.

Fig. 2 Relationships between 2·5 h post-feeding dissolved hydrogen (dH2) and select microorganisms (a, copy number of fungi; b, copy number of methanogens; c, relative abundance of Selenomonas ruminantium), as determined by RT-PCR, in goats (n 10) fed diets with 1·45 %Mg(OH)2 or 0·6 % elemental Mg after 28 d of adaption. Each point represents one goat, with a total of 20 data points. ![]() , Mg (OH)2 treatment;
, Mg (OH)2 treatment; ![]() , elemental magnesium treatment.
, elemental magnesium treatment.
Pyrosequencing of the 16S rRNA gene resulted in 38 730 (sd 3761) sequences per sample (range of 30 525–44 554 sequences per sample). Supplementation with elemental Mg increased community Ace (P=0·04) and bias-corrected Chao 1 (P=0·04) richness, but did not alter bacterial diversity of Shannon and Simpson index (online Supplementary Table S4). The PC1 and PC2 explained 15·2 and 10·1 % of variation in bacterial OTU, respectively, and the score plot did not show a distinct clustering of goats supplemented with Mg(OH)2 or elemental Mg (online Supplementary Fig. S4). Bacteroidetes and Firmicutes were the most abundant ruminal bacterial phyla, and did not differ between treatments (Table 5). Few differences were observed in the abundance of genera with >1 % abundance, which are shown in Table 5. Elemental Mg supplementation decreased the abundance of Bacteroidaceae (P=0·03) and increased the abundance of Veillonellaceae (P=0·06) and Succiniclasticum (P=0·009) (Table 5).
Table 5 Select bacterial phyla and genera, as determined by 16S rRNA pyrosequencing, in the rumen contents of growing goats fed diets with 1·45 % Mg(OH)2 or 0·6 % elemental magnesium after 28 d of adaption (n 10)Footnote * (Mean values with their standard errors)
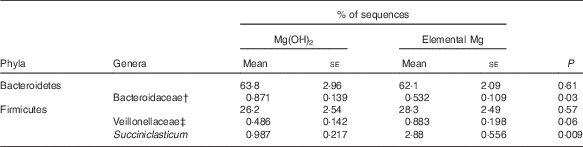
* Only the most abundant phyla and the genera that differed (P<0·10) between treatments are shown.
† Unknown genera within family Bacteroidaceae.
‡ Unknown genera within family Veillonellaceae.
Discussion
H2 is produced during the reaction of elemental Mg with water: Mg + 2H2O→Mg(OH)2+H2. The Mg(OH)2 so produced would be further hydrolysed in the rumen environment, in a reaction favoured by low pH: Mg(OH)2+2 H+→Mg2++2 H2O. Ruminal Mg2+ concentration was thus expected to be pH dependent, which agreed with the negative association observed between Mg2+ concentration and pH. At all time points, ruminal Mg2+ concentration was similar for both treatments of elemental Mg and Mg(OH)2 supplementation, which suggests that rumen fluid passage rates to the lower tract were similar for both treatments. The reaction of elemental Mg with water resulting in H2 formation might have been short-lived, or perhaps incomplete because of rumen outflow of elemental Mg, because dH2 increased at +2·5 h, but not at +6 h, relative to the commencement of the morning feeding.
It is understood that ruminal H2 accumulation hampers the oxidation of reduced electron carriers, such as NADH, to form H2, leading to impaired glycolysis, decreased microbial ATP generation and fibre degradation in the rumen( Reference Ungerfeld 32 ). However, the rumen microbial ecosystem might exhibit the capability to cope with elevated H2. Previous in vivo studies indicated that H2 accumulation under methanogenesis inhibition did not affect feed intake, total tract digestibility and meat or milk production in ruminants( Reference Mitsumori, Shinkai and Takenaka 7 , Reference Hristov, Oh and Giallongo 33 , Reference Romero-Perez, Okine and McGinn 34 ). In the current study, we also did not find differences in feed intake and digestibility in goats fed diets supplemented with elemental Mg and Mg(OH)2, but elevated dH2 resulting from elemental Mg supplementation decreased total VFA concentration in the rumen, which suggests that fermentation might have been impaired by elevated rumen dH2. At first sight, it is difficult to reconcile the lack of effect of elevated dH2 on digestibility with its negative effects on total VFA concentration. However, both measurements are proxies of digestion and fermentation events. We measured apparent overall tract digestibility, but any changes in microbial biomass in faeces could have masked differences in true overall digestibility. Similarly, we measured VFA concentration in the rumen, but we did not measure actual fluxes of VFA production. A more complete understanding of the effects of elevated dH2 would require a more in-depth study of the dynamics of digestion, microbial biomass production and fluxes of VFA production in the rumen.
The rumen VFA profile varies widely depending on the types of carbohydrates fermented. Diets rich in readily degraded starch generally stimulate propionate production, whereas on the other hand cellulose is fermented preferentially to acetate( Reference Bannink, Kogut and Dijkstra 35 ). These shifts of fermentation pathways are associated with the concentration of ruminal dH2 ( Reference Wang, Wang and Xie 5 ). Lower dH2 facilitates H2 generation through acetate production, whereas greater dH2 facilitates the disposition of electrons derived from fermentation into propionate production( Reference Janssen 2 ). In this study, we confirmed rumen dH2 as a direct effector of the shift of glucose fermentation pathways, as elevation of dH2 caused by elemental Mg supplementation decreased acetate molar percentage and increased propionate molar percentage. This study allowed isolating the effects of elevated dH2 on fermentation from other effects caused by changes in the diet.
Elevated dH2 facilitated electron-incorporating glucose fermentation pathways, which agreed with the negative correlation between dH2 and RNH2. The shifts in fermentation pathways caused by elevated dH2 resulting from elemental Mg supplementation also agreed with the effects of elemental Mg supplementation on ∆G of the main fermentation pathways. The ∆G of glucose fermentation to acetate and H2 was increased, and to propionate was decreased, by elemental Mg supplementation, along with the observed elevated dH2. These results were in agreement with our previous study in dairy cows fed four types of carbohydrates( Reference Wang, Wang and Xie 5 ). The magnitude of the effects of elemental Mg supplementation on ∆G of most rumen pathways at +2·5 h relative to the commencement of the morning feeding were rather small, which suggested that stimulation of pathways incorporating H2 might have taken place through more favourable kinetics of H2 uptake rather than through thermodynamic changes.
Changes in the composition of the rumen microbial community when gH2 accumulates as a consequence of methanogenesis inhibition have been demonstrated( Reference Martinez-Fernandez, Denman and Yang 6 , Reference Mitsumori, Shinkai and Takenaka 7 , Reference Shinkai, Enishi and Mitsumori 36 ). In dairy cows fed four types of carbohydrates, greater dH2 was associated with less acetate and lower number of H2 producers such as anaerobic fungi( Reference Wang, Wang and Xie 5 ). In that study, however, the concentration of dH2 was a consequence of the type of carbohydrate supplemented, and therefore changes in variables other than dH2 resulting from the dietary changes could have also influenced the abundance of microbial groups. To our knowledge, the present study was the first investigation on how elevated dH2 resulting from a non-fermentative source affected rumen microbiota, and we found that elevated dH2 generated by elemental Mg supplementation greatly decreased (−63·6 %) the copy numbers of fungi. Decreased fungal numbers did not adversely affect fibre degradation, which might indicate that other fibrolytic organisms, such as R. flavefaciens, might have compensated for the negative effects of elemental Mg on fungi. R. flavefaciens produces H2, but also succinate as a major product( Reference Stewart, Flint and Bryant 37 , Reference Philippeau, Lettat and Martin 38 ), and it would be possible that R. Flavefaciens shifted fermentation towards succinate production under elevated H2 resulting from elemental Mg supplementation. That said, the relative abundance of R. flavefaciens was small, which casts doubt on the influence it might have had on fibre digestion.
The impact of increased dH2 generated by supplementation with elemental Mg on the rumen bacterial community composition was further analysed using high-throughput sequencing. Supplementation with elemental Mg increased species richness in terms of Ace and Chao 1 diversity, although total copy numbers of bacteria were similar for both treatments. However, the PCoA score plot indicated that ruminal bacterial community was not distinctly different between both treatments. In a previous study, elevated H2 was associated with increased Bacteroidetes:Firmicutes ratio when methanogenesis was inhibited by chloroform–cyclodextrin( Reference Fonty, Joblin and Chavarot 39 ). However, in the present study, elemental Mg supplementation decreased the abundance of family Bacteroidaceae, but did not alter the abundance of Bacteroidetes and Firmicutes. An interesting result was that genus Succiniclasticum, which belongs to family Firmicutes and is involved in converting succinate to propionate( Reference Scheifinger, Linehan and Wolin 40 ), was 2-fold more abundant in goats that received elemental Mg supplementation. This result was in agreement with the increased propionate molar percentage in the rumen when dH2 concentration was increased by elemental Mg supplementation. In contrast, an unexpected observation was that elemental Mg supplementation and elevated dH2 decreased the abundance of S. ruminantium, an important propionate-producing bacterium in the rumen( Reference Sawanon, Koike and Kobayashi 41 ). The important increase in Succiniclasticum abundance might have compensated for the decreased abundance of S. ruminantium to produce propionate in this study, if in goats supplemented elemental Mg succinate conversion to propionate limited propionate production.
H2 and CO2 are the main substrates for methanogenesis in the rumen, and the majority of H2 produced from carbohydrate fermentation is used in CH4 production( Reference McAllister and Newbold 42 ). In our study, we found that elemental Mg supplementation increased dCH4 concentration, the copy number of methanogens and CH4 emissions, indicating that increased dH2 generated by elemental Mg supplementation promoted CH4 production in the rumen of goats. Olijhoek et al.( Reference Olijhoek, Hellwing and Weisbjerg 11 ) also reported that short-term infusion of H2 into the rumen increased CH4 production. Furthermore, we estimated that elemental Mg supplementation decreased ∆G of methanogenesis at +2·5 h after the commencement of the morning feeding, indicating that elevated dH2 would have thermodynamically favoured growth and metabolism of methanogens at that time point. Dissolved H2 concentration was positively correlated with copy numbers of methanogens, but not with CH4 emissions. This suggests that ruminal dH2 might have exceeded the capacity of methanogens to take up H2 in the treatment with elemental Mg supplementation, so that methanogenic population and activity might have been limiting for CH4 generation in the rumen.
In summary, goats adapted to elevated ruminal dH2 generated by elemental Mg supplementation had lower rumen total VFA concentration, copy number of fungi and greater propionate molar percentage and CH4 emissions when compared with goats fed the control diet supplemented with Mg(OH)2. Elevation of ruminal dH2 concentration seemed to inhibit rumen fermentation and altered fermentation pathways and some microbial groups, without affecting total tract digestibility. Effects of extra H2 obtained by supplementing elemental Mg were short-lived, because elevated dH2 had returned to control concentration by +6 h relative to the commencement of the morning feeding. Furthermore, the extent of elevated ruminal dH2 concentration caused by elemental Mg supplementation varied widely among the ten goats, and the goat with the greatest dH2 concentration (17 mmol/l) also had the greatest methanogen population and the lowest VFA concentration, acetate molar percentage, acetate:propionate ratio and copy number of fungi. This study provides insights on the effects of elevated dH2 on fermentation in the rumen and methanogenesis, although high dietary Mg concentration in both treatments might have altered the availability of other minerals and exerted some postabsorptive effects in goats. More studies with sustained and consistent elevated dH2 are needed to further elucidate how elevated dH2 concentration affects the rumen ecosystem, fermentation and digestion.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant nos 31561143009, 31320103917 and 31472133), National key research and development program of China (grant no. 2016YFD0500504), Youth Innovation Promotion Association CAS (2016327), China Agriculture Research System (grant no. CARS-37) and Hunan province science and technology plan (grant no. 2015WK3043).
M. W., R. W., H. X. M., D. L. L. and X. M. Z. conducted the research; M. W., R. W. and E. M. U. analysed the data; and M. W., E. M. U., K. A. B and Z. L. T. wrote the paper. M. W. and Z. L. T. designed the research and had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002161










