Fatty acids in membrane phospholipids determine the structural and physiological functions of the cell membrane(Reference Clandinin, Cheema and Field1). Since dietary fats can modify the fatty acid composition of membrane phospholipids, they can perturb membrane-mediated functions of immunocompetent cells(Reference Calder2). Long-chain (LC) PUFA serve as precursors for inflammatory lipid mediators. Arachidonic acid (AA; 20 : 4), an n-6 LCPUFA, gives rise to pro-inflammatory mediators, namely leukotriene B4 (LTB4) and PGE2, whereas EPA (20 : 5) and DHA, the n-3 LCPUFA, produce less inflammatory mediators such as leukotriene B5 and PGE3, resolvins and protectins(Reference Calder2, Reference Simopoulos3). n-3 LCPUFA have been reported to alter the lipid composition of the T-cell membrane(Reference Fan, McMurray and Ly4) and the signalling of IL-2 receptors(Reference Li, Wang and Tan5). n-3 LCPUFA have also been reported to decrease the expression of genes involved in eicosanoid synthesis, scavenger receptor activity, adipogenesis, NF-κB and hypoxia signalling in human blood mononuclear cells(Reference Bouwens, Rest and Dellschaft6). Therefore, n-3 LCPUFA may have a therapeutic value in acute and chronic inflammation and also in disorders involving impaired immune function.
Mammals can convert α-linolenic acid (ALA; 18 : 3n-3) to EPA and DHA; however, there are concerns regarding the efficiency of its conversion to n-3 LCPUFA(Reference Burdge, Jones and Wooton7, Reference Pawlosky, Hibbeln and Novotny8). It has been reported that a decrease in linoleic acid (LA; 18 : 2n-6) and an increase in ALA content in the diet can alter the levels of AA and EPA in the tissues of hamsters(Reference Morisea, Combeb and Bouéb9) and rats(Reference Diwakar, Dutta and Lokesh10, Reference Jeffery, Sanderson and Sherrington11). An ALA-rich diet has been reported to increase the levels of ALA and EPA in the phospholipid fraction of erythrocytes(Reference Coblijn, Murphy and Othman12), suppress the ex vivo proliferation of lymphocytes(Reference Kelley, Branch and Love13) and inhibit TNF-α and IL-1β production in human mononuclear cells(Reference Caughey, Mantzioris and Gibson14). ALA has been reported to modulate the T-helper 1/T-helper 2 balance when ingested at normal dietary concentrations(Reference Mizota, Kambara and Matsuya15). These studies have demonstrated that the efficacy of ALA to modulate the functions of immunocompetent cells may be of importance in altering the pathophysiological effects of inflammatory diseases.
Garden cress seed oil (GCO) is a good source of PUFA. It contains 32 % of ALA and 12 % of LA. GCO has a fairly balanced ratio of SFA:MUFA:PUFA (1:2·6:3) compared with flaxseed and perilla seed oil. GCO is a relatively stable oil due to the presence of a high concentration of natural antioxidants such as tocopherols (1330 mg/kg) and carotenoids (5·32 mg/kg)(Reference Diwakar, Dutta and Lokesh16). The major part of ALA in GCO is esterified at the sn-2 carbon of TAG(Reference Diwakar, Dutta and Lokesh16), hence it can be easily absorbed by the intestine. We have reported a significant increase in EPA and DHA levels in the serum, liver, heart and brain with a concomitant decrease in LA and AA in rats fed with a GCO diet(Reference Diwakar, Dutta and Lokesh10). The aim of the present study was to assess the modulatory effect of GCO on some key functions of immunocompetent cells such as proliferation and release of inflammatory mediators ex vivo.
Materials and methods
Materials
Garden cress seeds were purchased from a local market in Mysore. The seeds were identified and authenticated at the Department of Horticultural Sciences, University of Agriculture Sciences, Bangalore. Seeds were air-dried and flaked in a roller flaker (Kvarnmaskiner, Malmö, Sweden). Flaked garden cress seeds were pressed in a hydraulic press (B Sen Barry and Company, New Delhi, India), at a pressure of 98·1 kPa for 10 min, to expel the oil. Oil was collected, flushed with N2 and stored at − 20°C(Reference Diwakar, Dutta and Lokesh16).
All chemicals used were of analytical grade. Fatty acid methyl ester standards, B trifluoride, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide, Ca ionophore (A23187), concanavalin A (Con-A), phorbol 12-myristate-13-acetate (PMA), phytohaemagglutinin (PHA) and lipopolysaccharide (LPS) were procured from Sigma Chemical Company (St Louis, MO, USA). Roswell Park Memorial Institute (RPMI)-1640 medium and antibiotics were procured from Thermo Scientific Company (Rockford, IL, USA). Rat IL-2 ELISA kit was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). TNF-α was purchased from Koma Biotech (Seoul, South Korea).
Animals and diets
Weaned female Wistar rats (OUTB-Wistar, IND-cft (2c)) weighing 55–62 g, bred in the animal house facility at the Central Food Technological Research Institute, Mysore, India, were used in the present study. The experimental protocol adopted in the present study was approved by the Institute's animal ethical committee. Animals were housed individually in stainless-steel cages, in a room, where the temperature was maintained at 25 ± 2°C. Animals were divided into four groups, with twelve animals in each group. They were fed with a semi-synthetic diet containing sunflower oil (SFO)10 (SFO 10 g/100 g), GCO2·5 (GCO2·5 g+SFO 7·5 g/100 g), GCO5 (GCO 5 g+SFO 5 g/100 g) and GCO10 (GCO 10 g/100 g), respectively. The composition of the isoenergetic, semi-synthetic diet and its fatty acid profile are presented in Tables 1 and 2, respectively. Diets were prepared every week and stored at 4°C. Animals were given a fresh diet ad libitum for 8 weeks. Animals had free access to their respective diets and water at all times throughout the study. After 8 weeks of feeding, rats were fasted overnight and were euthanised under diethyl ether anaesthesia. Blood was drawn by cardiac puncture, and serum was separated by centrifugation at 600 g for 10 min (after allowing the blood to clot at room temperature for 2 h). Serum from animals of each group was pooled, heat inactivated at 54°C for 30 min, filtered through a 0·2 μm filter and used at a 2·5 % level in culture media for primary cell culture experiments.
Table 1 Composition of the diets
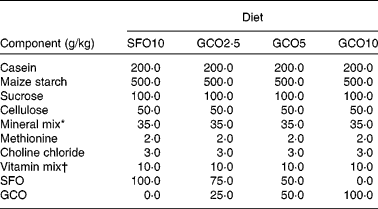
SFO10, sunflower oil (SFO) 10 g/100 g; GCO2·5, garden cress seed oil (GCO) 2·5 g+SFO 7·5 g/100 g; GCO5, GCO 5 g+SFO 5 g/100 g; GCO10, GCO 10 g/100 g.
* Bernhart Tommarelli salt mixture.
† AIN-76A containing per kg: 1·8 g vitamin A, 0·125 g vitamin D2, 22 g dl-α-tocopherol, 45 g ascorbic acid, 5 g inositol, 2·25 g menadione, 5 g p-aminobenzoic acid, 4·25 g niacin, 1 g riboflavin, 1 g pyridoxine hydrochloride, 1 g thiamine hydrochloride, 3 g calcium pantothenate, 0·02 g biotin, 0·09 g folic acid, 0·00135 g vitamin B12 and 833·46 g sucrose carrier.
Table 2 Fatty acid composition of the experimental diets*
(Mean values with their standard errors, n 4)

SFO10, sunflower oil (SFO) 10 g/100 g; GCO2·5, garden cress seed oil (GCO) 2·5 g+SFO 7·5 g/100 g; GCO5, GCO 5 g+SFO 5 g/100 g; GCO10, GCO 10 g/100 g.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05; ANOVA, a < b < c < d).
* For details of the diets, see Table 1.
Spleen lymphocyte preparation
Spleens were aseptically removed, dilacerated and passed through a sterile cell strainer to form a cell suspension. The cell suspension was centrifuged for 10 min at 150 g, and the resulting pellet was suspended in RPMI-1640 medium. Spleen lymphocyte (SL) were collected by centrifugation at 400 g for 30 min on Ficol Histopaq gradient, washed and re-suspended in complete media (RPMI-1640 containing 25 mm-HEPES supplemented with 2·5 % autologous serum, penicillin (60 mg/l)), streptomycin (100 mg/l), 2 mm-l-glutamine and 10 μm-2-mercaptoethanol). The number of viable cells was counted by the trypan blue exclusion method using a haemocytometer, and cell number was adjusted to 1·5 × 106 cells/ml.
Proliferation assay of rat spleen lymphocytes
Proliferation of SL was evaluated by the MTT assay(Reference Sladowski, Steer and Clothier17). SL were cultured at a cell density of 1·5 × 105cells/well in complete medium (RPMI-1640 containing 25 mm-HEPES supplemented with 2·5 % autologous serum, penicillin (60 mg/l), streptomycin (100 mg/l), 2 mm-l-glutamine and 10 μm-2-mercaptoethanol) in ninety-six-well flat-bottomed culture plates (Nunc, Roskilde, Denmark) in the presence or absence of 5 μg/ml of T-cell mitogens (Con-A and PHA) and a B-cell mitogen (LPS) with triplicate wells for each sample. Cells were incubated in a CO2 incubator at 37°C for 72 h. At the 69th hour, 20 μl of MTT (5 mg/ml) prepared in PBS (pH 7·4) were added and incubated for 3 h. The plates were spun at 500 g, the culture medium was discarded, the solublising reagent dimethyl sulfoxide (100 μl) was added and then the absorbance was measured at 550 nm.
IL-2 measurement
SL were cultured at a cell density of 2·5 × 105 cells/well in complete medium, as described earlier, and stimulated with 5 μg of Con-A or PHA for 48 h. Culture medium was aspirated and stored at − 80°C. Subsequently, the culture medium was thawed, vortexed and centrifuged (4°C) at 500 g for 5 min. An aliquot (50 μl) of the culture supernatant was analysed quantitatively for IL-2 levels using a commercially available ELISA kit (Quantakine, IL-2; R&D Systems, Inc.). Results are expressed as pg of IL/2·5 × 105 cells, and data are presented as means with their standard errors (n 4).
Isolation and activation of peritoneal macrophages
The GCO and SFO diets were fed to rats for 8 weeks. After 8 weeks, rats were injected intraperitoneally with 1 % sodium caseinate in PBS to elicit the migration of macrophages to the peritoneal cavity. After 4 d, rats were anaesthetised with diethyl ether, and peritoneal exudate cells were collected aseptically by irrigating the peritoneal cavity with chilled RPMI-1640 medium. Peritoneal exudates were centrifuged, washed and resuspended in complete RPMI-1640 medium. Cells were seeded at a density of 1 × 105 cells/well in ninety-six-well culture plates (Nunc) and incubated for 2 h at 37°C in a CO2 incubator. Non-adherent cells were removed by washing (three times) with serum-free medium, and adherent cells were cultured overnight in complete medium. More than 95 % of the adherent cell population was found to be peritoneal macrophages (PMΦ) as determined by morphology and non-specific esterase staining.
Stimulation of peritoneal macrophages for nitric oxide production
PMΦ (1 × 105 cells/well) were cultured for 12 h at 37°C in a CO2 incubator, and treated with either Con-A (10 μg/ml), PHA (1 μg/ml), LPS (10 μg/ml), A23187 (1 μg/ml) or PMA (500 ng/ml). Samples were drawn after 6, 12 and 24 h incubation and centrifuged, and the supernatant (100 μl) was transferred to ninety-six-well assay plates. Nitrite concentration in the cell-free culture supernatant was measured by a spectrophotometric assay based on the Griess reaction as described elsewhere(Reference Yaqoob and Calder18). Briefly, 100 μl of the culture supernatant were mixed with an equal volume of Griess reagent (one part of 0·1 % (w/v) naphthylethylenediamine dihydrochloride in distilled water and one part of 1 % (w/v) sulphanilamide in 5 % (v/v) H3PO4) at room temperature. After 15 min, the absorbance was measured using an ELISA plate reader at 540 nm. Nitrite content (μmol/105 cells) was quantified using a sodium nitrite standard curve. Determinations were carried out in triplicate (n 6).
Measurement of TNF-α
PMΦ (1 × 105 cells/well) in RPMI-1640 medium were treated with LPS (10 μg/ml) in ninety-six-well culture plates (Nunc). After 10 h incubation at 37°C in a CO2 incubator, the cell-free culture supernatant was removed and stored at − 80°C. An aliquot (50 μl) of the culture supernatant was analysed quantitatively for TNF-α using a commercially available ELISA kit (Koma Biotec). Results are expressed as pg of TNF-α/1 × 105 cells. Data are expressed as means with their standard errors (n 4).
Measurement of hydrogen peroxide release
H2O2 released in the culture medium by PMΦ was determined according to the method of Yaqoob & Calder(Reference Yaqoob and Calder18). PMΦ (1 × 105 cells/well) were incubated in 100 μl Hanks balanced salt solution containing 0·1 % (w/v) dextrose, 0·1 % phenol red and horseradish peroxidase (40 μg/ml). PMA (10 U/ml) was added and incubated for 2 h at 37°C in a CO2 incubator. The reaction was stopped by the addition of 10 μl of 0·1 m-NaOH, and the absorbance was measured at 610 nm. Results are expressed as nm of H2O2/1 × 105 PMΦ. H2O2 concentration was determined by the extinction coefficient E = 43·6 cm/m.
In vitro lysosomal phosphatase enzyme activity
Cellular lysosomal phosphatase enzyme activity was determined according to the method described by Manosroi et al. (Reference Manosroi, Saraphanchotiwitthaya and Manosroi19). Briefly, PMΦ (1 × 105 cells/well) were solubilised with 20 μl of 0·1 % Triton X-100, 100 μl of 10 mm-p-nitrophenyl phosphate solution and 50 μl of 0·1 m-citrate buffer (pH 5·0), and incubated for 30 min at 37°C. The reaction was terminated by adding 150 μl of 0·2 m-borate buffer (pH 9·8), and the absorbance was measured at 405 nm. Results are expressed as a percentage of lysosomal phosphatase activity.
where OD is optical density.
Measurement of leukotriene B4
LTB4 secretion by PMΦ in response to LPS stimulation was measured by the HPLC method(Reference Raghavenra, Diwakra and Lokesh20). PMΦ (3 × 106) were incubated with or without Ca ionophore (A23187) at a concentration of 5 μg/ml in a twenty-four-well culture plate (Nunc) for 90 min at 37°C in a CO2 incubator. The reaction was stopped by adding 30 μl of 1 m-HCl on ice. The supernatants were centrifuged at 2000 g for 20 min at 4°C and loaded onto Sep-Pack C18 cartridges (Waters Millipore Corporation, Milliford, MA, USA). Leukotrienes were eluted with ethyl acetate. The ethyl acetate fraction was evaporated to dryness, resuspended in methanol and stored at − 80°C. Identification and quantification of LTB4 was carried out by the HPLC method. A Shimadzu LC-10A HPLC system with a UV detector and a Hypersil reverse-phase C18 column (150 × 4·6 mm; 5 μm) was used. An isocratic elution program was employed using a mobile phase containing methanol–ammonium acetate (70:30, v/v). The flow rate was 0·5 ml/min, and the chromatogram was monitored at 280 nm. LTB4 content was quantified based on the calibration curve of standard LTB4 (Sigma Chemicals Company). Results are reported as ng of LTB4/3 × 106 cells. Determinations were done in duplicate (n 6).
Measurement of peritoneal macrophage 5-lipoxygenase activity
PMΦ were suspended in PBS and sonicated for 20–30 s at 20 kHz to release the cytosolic 5-lipoxygenase (5-LO) enzyme. This solution was centrifuged at 2000 g for 30 min at 4°C. The supernatant was used as the source of the enzyme. Protein was estimated by Lowry's method(Reference Lowry, Rosebrough and Farr21) using bovine serum albumin as standard. Assay of 5-LO was performed according to the method of Raghavenra et al. (Reference Raghavenra, Diwakra and Lokesh20). The standard reaction mixture for the 5-LO assay contained 100 mm-phosphate buffer (pH 7·4), 50 mm-dithiothreitol, 200 mm-ATP, 300 mm-CaCl2, 150 mm-AA and 5·0 mg protein. The enzymatic reaction was carried out at 28 ± 2°C. Enzyme activity was measured as 5-hydroperoxyeicosatetraenoic acid (5-HETE) formed at 234 nm using a Shimadzu spectrophotometer. The molar extinction coefficient of 25/mm cm was used to calculate the specific activity of 5-LO. Enzyme activity was expressed as μm-5-HETE/min per mg protein. Determinations were done in duplicate (n 6).
Lipid extraction and fatty acid analysis
Lipids were extracted from SL and PMΦ with a chloroform–methanol mixture (2:1, v/v). Fatty acid analysis was carried out according to the method of Morisson & Smith(Reference Morisson and Smith22). Extracted lipid samples were saponified for 1 h with 1 ml of 0·7 m-methanolic KOH at 60°C, followed by neutralisation with 1 ml of methanolic HCl (0·7 m). The resulting NEFA were extracted with hexane and evaporated to dryness. The fatty acids were methylated using boron trifluoride (14 % in methanol) and 0·2 ml benzene. The fatty acid methyl esters were extracted with hexane, washed with water and evaporated to dryness. Fatty acid analysis was performed using a gas–liquid chromatograph (Shimadzu, GC-14B; Shimadzu Corporation, Kyoto, Japan) fitted with a fused silica capillary column (BP 21, 30 m in length, 0·30 mm in inner diameter). The gas–liquid chromatograph was equipped with a flame ionisation detector and a Clarity Lite 420 integrator. The column temperature was set at 220°C, the injector temperature at 230°C and the detector temperature at 240°C. N2 was used as the carrier gas, and the flow rate was 1 ml/min. Individual fatty acids in the sample were identified by comparison with retention times of standard fatty acid methyl esters. Fatty acid determinations were carried out in duplicate (n 6).
Statistical analysis
Results were analysed using SPSS-10 software (SPSS, Inc., Chicago, IL, USA). The significance of mean differences between the groups was determined by one-way ANOVA, followed by Duncan's multiple-range test as a post hoc test for multiple comparisons whenever a significant F value emerged. Significance was set at P < 0·05.
Results
Fatty acid composition of lymphocytes and peritoneal macrophages
The fatty acid composition of lipids extracted from diets is presented in Table 2. The n-6:n-3 ratio was greatly affected by the addition of GCO in diets. The n-6:n-3 ratio was 114·5 in the SFO10 diet. The ratio decreased to 4·6, 1·9 and 0·3, respectively, in the GCO2·5, GCO5 and GCO10 diets.
The fatty acid composition of lipids extracted from SL and PMΦ is presented in Table 3. SFA (16 : 0+18 : 0) content in SL and PMΦ did not change among the dietary groups. However, an increase of 51·4 and 27·9 % was observed in MUFA levels in both SL and PMΦ, respectively, in the GCO10 group compared with the SFO10 group. ALA, EPA and DHA contents increased by 3·2, 1·72, 1·7 % in SL lipids and 0·97, 1·08, 1·6 % in PMΦ lipids, respectively, in the GCO groups compared with the SFO10 group. LA and AA content was decreased by 28·05 and 39·5 % in PMΦ, and 38·35 and 35·37 % in SL, respectively, in the GCO10 group compared with the SFO10 group (Table 3).
Table 3 Fatty acid composition of spleen lymphocyte (SL) and peritoneal macrophage (PMΦ) lipids isolated from rats fed with the experimental diets*
(Mean values with their standard errors, n 6)

SFO10, sunflower oil (SFO) 10 g/100 g; GCO2·5, garden cress seed oil (GCO) 2·5 g+SFO 7·5 g/100 g; GCO5, GCO 5 g+SFO 5 g/100 g; GCO10, GCO 10 g/100 g.
a,b,c Mean values within a row with unlike superscript letters were significantly different: (P < 0·05; ANOVA, a < b < c).
* For details of the diets see Table 1 and for procedures see Materials and methods.
Lymphocyte proliferation and IL-2 production
The effect of the experimental diets on SL proliferation is summarised in Fig. 1. SL proliferation induced by Con-A and PHA was significantly inhibited in the GCO groups. GCO at 2·5 % caused a 40 and 36 % reduction in the proliferation of SL induced by Con-A and PHA, respectively. An insignificant decrease was observed in the proliferation of SL induced by LPS in the GCO10 group (1·06 (sem 0·09)) compared with the SFO10 group (1·95 (sem 0·42)). A marginal decrease in IL-2 levels was observed in SL stimulated with Con-A in the GCO10 group (21·32 (sem 1·9) pg/2·5 × 105 cells) compared with the SFO10 group (22·5 (sem 1·0) pg/2·5 × 105 cells).
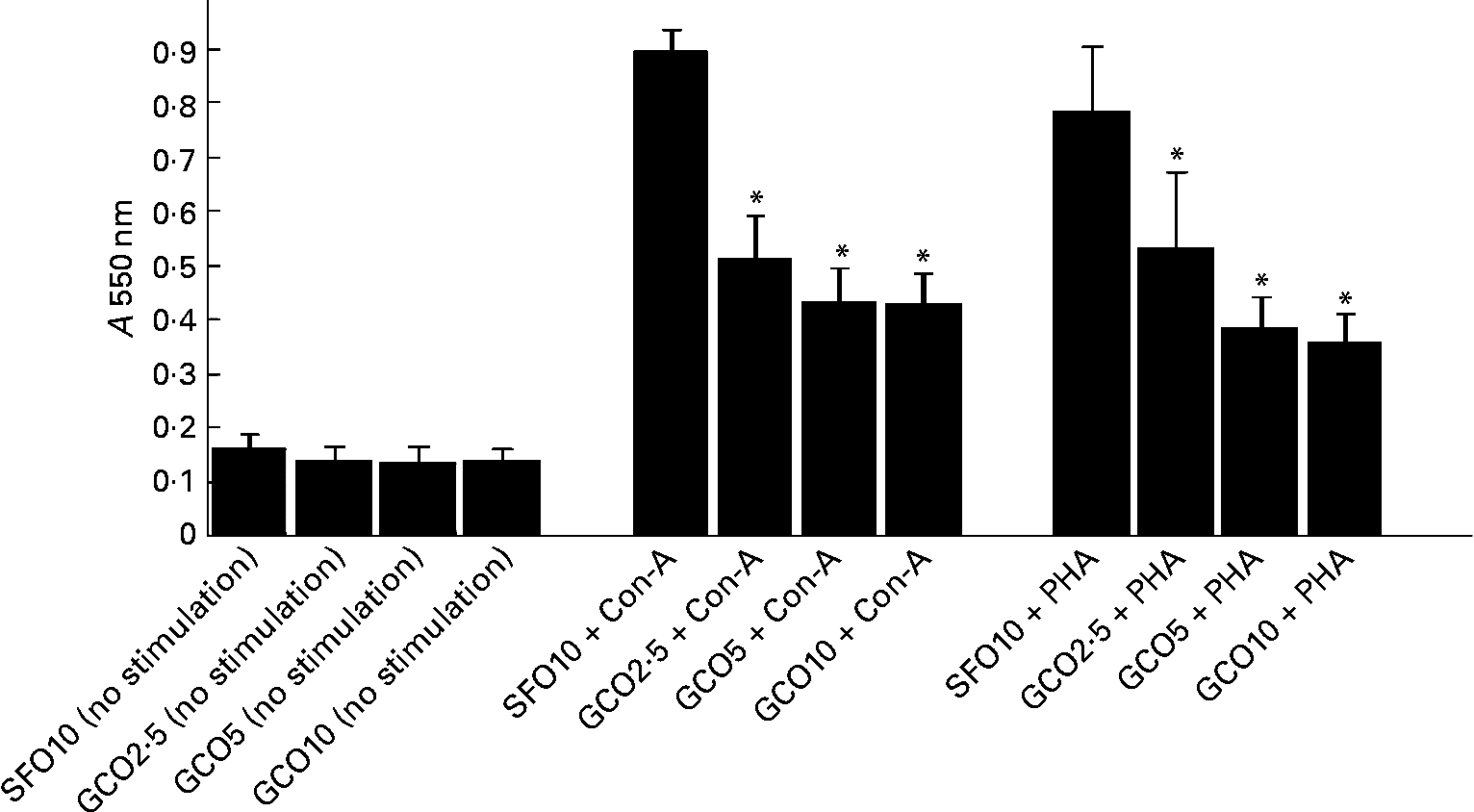
Fig. 1 Effect of dietary lipids on the proliferation of spleen lymphocytes stimulated with concanvalin-A (Con-A, 5 μg/ml) or phytohaemagglutinin (PHA, 5 μg/ml). Rats were fed with the sunflower oil (SFO)- and garden cress seed oil (GCO)-supplemented diets for 8 weeks as described in Materials and methods. Spleen lymphocytes (1·5 × 105 cells/well) were isolated and cultured for 72 h in Roswell Park Memorial Institute-1640 complete medium and 2·5 % autologous serum in the presence or absence of mitogens. The amount of formazan formed by the cells was measured spectrophotometrically at 550 nm. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different compared with the SFO 10 g/100 g group (P < 0·05). A, absorbance.
Nitric oxide release by peritoneal macrophages
A stable end product, nitrite, was determined to measure NO release in PMΦ. Nitrite content at different time intervals (6, 12 and 24 h) after post-stimulation of PMΦ with Con-A, LPS, A23187, PHA and PMA is presented in Fig. 2. In the GCO groups, a significant decrease in nitrite production was observed at 12 h post-treatment with Con-A, PHA and A23187 (Fig. 2(a)–(c), respectively) compared with the SFO10 group. However, a significant decrease in nitrite content was observed at 6, 12 and 24 h in PHA-treated PMΦ, in all GCO groups. A non-significant downward trend was observed in nitrite production in LPS- and PMA-stimulated PMΦ (Fig. 2(d) and (e), respectively) in GCO-fed rats.

Fig. 2 Effect of dietary lipids on nitric oxide produced by rat peritoneal macrophages (PMΦ). PMΦ from rats fed with the experimental diets for 8 weeks were stimulated ex vivo with (a) concanvalin-A (10 μg/ml), (b) phytohaemagglutinin (1 μg/ml), (c) calcium ionophore (1 μg/ml), (d) lipopolysaccharide (10 μg/ml) and (100 mg/l) (e) phorbol 12-myristate 13-acetate (0·5 μg/ml). Culture supernatants were removed after 6, 12 and 24 h post-treatment of mitogens. Concentration of nitrite in the medium was measured by the Griess method. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different compared with the 10 g/100 g sunflower oil group (SFO10); P < 0·05. □, SFO10; ■, garden cress seed oil (GCO)2·5; ![]() , GCO5;
, GCO5; ![]() , GCO10.
, GCO10.
TNF-α and leukotriene B4 release by peritoneal macrophages
Concentration of TNF-α released by PMΦ after 10 h post-stimulation of LPS did not differ much between the GCO10 (125 (sem 6·9) pg/1 × 105 cells) and SFO10 (145 (sem 3·0) pg/1 × 105 cells) groups. Concentration of LTB4 released by PMΦ in response to A23187 stimulation was measured using reverse-phase HPLC and is depicted in Fig. 3. LTB4 content was reduced significantly in the GCO5 and GCO10 groups compared with the SFO10 group.

Fig. 3 Effect of dietary lipids on leukotriene B4 production by peritoneal macrophages (PMΦ, 3 × 106 cells/ml) stimulated with calcium ionophore (A23187, 5 μg/ml). Rats were fed with the sunflower oil (SFO)- and garden cress seed oil (GCO)-supplemented diets as described in Materials and methods. PMΦ were isolated and incubated for 90 min in Roswell Park Memorial Institute-1640 complete medium with 2·5 % autologous serum in the presence or absence of A23187 (5 μg/ml). Leukotriene B4 in the culture supernatant was analysed by reverse-phase HPLC as described in Materials and methods. Values are means, with their standard errors represented by vertical bars (n 6). * Mean values were significantly different compared with the SFO group (10 g/100 g) (P < 0·05).
Leukotriene B4 release by peritoneal macrophages
Concentration of TNF-α released by PMΦ after 10 h of post-stimulation of LPS did not differ much between the GCO and SFO groups. Concentration of LTB4 released by PMΦ in response to A23187 stimulation was measured using reverse-phase HPLC and is depicted in Fig. 3. LTB4 content was reduced significantly in the GCO5 (17·0 (sem 0·9) ng/3 × 106 cells) and GCO10 groups (12·0 (sem 1·0) ng/3 × 106 cells) compared with the SFO10 group (20·0 (sem 1·2) ng/3 × 106 cells).
Hydrogen peroxide release by stimulated peritoneal macrophages
No significant difference was observed in H2O2 levels released by PMΦ stimulated with PMA in the GCO groups.
Acid phosphatase and 5-lipoxygenase activity in peritoneal macrophages
Acid phosphatase and 5-LO activities in PMΦ were unaltered among the different GCO groups.
Discussion
PMΦ and SL are key cells of the immune system involved in host defence, inflammation and immune response. Since the action of these cells is mediated by membrane-bound components, an alteration in the membrane lipid composition could modulate immune function. It is known that dietary lipids can affect the fatty acid composition of immunocompetent cells. In the present study, we demonstrated that partial or complete replacement of LA (18 : 2n-6) with ALA (18 : 3n-3) in diets through GCO can alter the fatty acid composition and modulate inflammatory mediators produced by PMΦ and SL.
Immunocompetent cells (PMΦ and SL) originate in the bone marrow. Lymphocytes mature in primary lymphoid tissues and stay in peripheral lymphoid organs to keep a vigil on invading microbes. Rapidly dividing promonocytes are progenitor cells of blood monocytes, which give rise to PMΦ. When stimulated, blood monocytes divide rapidly and move to the peritoneal cavity as PMΦ(Reference Furth and Colin23). The type and amount of fatty acids in the blood fatty acid pool during blastogenesis determine the fatty acid profile of PMΦ. Earlier studies have shown an increase in the ALA content of PMΦ and SL in rats fed with flaxseed and perilla oil. These oils contain 55–56 % of ALA, 13 and 18 % of LA and a n-6:n-3 ratio of 0·24 and 0·32, respectively(Reference Jeffery, Sanderson and Sherrington11, Reference Caughey, Mantzioris and Gibson14, Reference Kaku, Yunoki and Ohkura24). Lymphocytes are cells that specifically recognise and respond to foreign antigens. In response to stimuli, T-lymphocytes secrete cytokines and undergo cell division(Reference Abbas, Lichtman and Pober25). Proliferation of lymphocytes leads to an increase in the number of antigen-specific lymphocytes and the activation of B-lymphocytes, natural killer cells and macrophages. Therefore, proliferation is a key process in the regulation, amplification and memory of the cell-mediated immune response(Reference Abbas, Lichtman and Pober25). In the present study, a significant decrease was observed in the ex vivo proliferation of lymphocytes in response to Con-A or PHA stimuli in the GCO groups. Substitution of SFO with 50 % GCO in the diet significantly decreased the proliferation of SL. A further increase in GCO in the diet had no additive effect on the proliferation of lymphocytes. A similar effect has been observed when a small amount of SFO was replaced by linseed oil (n-6:n-3 PUFA ratio of 14·75) in the diet of rats(Reference Jeffery, Sanderson and Sherrington11). An ALA-rich diet has been reported to decrease the ex vivo proliferation of blood lymphocytes in response to mitogenic stimuli in poultry, rats and human subjects(Reference Kelley, Branch and Love13, Reference Wang, Field and Sim26–Reference Thies, Nebe-von-Caron and Powell29). The exact mechanisms by which n-3 PUFA modulate lymphocyte proliferation are far from clear, but certain mechanisms have been suggested. A decreased expression in IL-2 in activated lymphocytes by n-3 fatty acids has been suggested for the inhibition of lymphocyte proliferation. However, in the present study, we observed no significant change in the IL-2 of lymphocytes in GCO-fed rats. Earlier studies with fish oil (EPA and DHA) have reported a decrease in IL-2 levels(Reference Turek, Schoenlein and Clark30, Reference Wallace, Miles and Evans31) and or no effect on IL-2 production in lymphocytes of rats and human subjects(Reference Thies, Nebe-von-Caron and Powell29, Reference Meydani, Lichtenstein and Cornwall32–Reference Babu, Wiesenfeld and Collins35). However, a decreased expression in IL-2 receptors has been suggested to play a role in the suppression of lymphocyte proliferation(Reference Jolly, Mac Murray and Chapkin36). LCPUFA in fish oil have been reported to differentially modulate T-cell rafts and soluble membrane phospholipids, causing a displacement of acylated proteins from rafts(Reference Fan, McMurray and Ly4, Reference Stulnig, Huber and Leitinger37). When T-cells are activated, protein kinase Cθ, phospholipase C-γ, linker for the activation of T-cells are translocated to lipid rafts, which induce the proliferation of lymphocytes(Reference Ebinu, Stang and Teixeira38, Reference Bi and Altman39). The conditions that modify the integrity of T-cell rafts can disrupt the early steps of T-cell activation(Reference Xavier, Brennan and Li40). Therefore, the incorporation of n-3 PUFA into the membrane microdomain may influence signal transduction and modulate T-cell activation(Reference Fan, McMurray and Ly4).
PMΦ constitute a major group of phagocytic leucocytes, which are crucial to immune surveillance against invading pathogens. Activated PMΦ release an array of mediators including pro-inflammatory cytokines (TNF-α, IL-1 and IL-6), growth factors, bioactive eicosanoids (LTB4, PGE2 and thromboxane A2), hydrolytic enzymes (peroxidase and protease), reactive oxygen intermediates and NO. Each of the above secretory molecules is implicated in the pathogenesis of chronic inflammatory conditions and considered as pharmacological targets of inflammatory diseases. In the present study, we assessed the effect of dietary GCO on inflammatory mediators released by PMΦ. NO is considered as one of the principal effector molecules in PMΦ-mediated cytotoxicity(Reference Gordge41). The amount of NO released by PMΦ isolated from the GCO group of rats was significantly reduced in comparison with NO released by PMΦ of the SFO group. The percentage of reduction was found to be 57, 62 and 56 %, respectively, after 6, 12 and 24 h post-stimulation of PHA. NO release was also significantly reduced by 37 and 44 %, respectively, at 12 h post-stimulation with Con-A and A23187 in the GCO10 group compared with the SFO10 group. Previous studies have shown decreased(Reference Boutard, Fouquery and Philippe42, Reference Joe and Lokesh43), increased or no change in the production of NO by the PMΦ of animals fed with fish oil(Reference Yaqoob and Calder18, Reference Turek, Schoenlein and Clark30, Reference Renier, Skamene and de Sanctis44, Reference Chaet, Garcia and Arya45) and linseed oil(Reference Hubbard, Chapkin and Erickson46). Dietary LCPUFA have been reported to decrease the expression of inducible NO synthase in cancer cells and LPS-stimulated RAW 264.7 macrophages(Reference Narayanan, Narayanan and Simi47, Reference Aldridge, Razzak and Babcock48). Ren & Chung(Reference Ren and Chung49) reported that ALA markedly inhibits LPS-induced NO production and inducible NO synthase gene expressions by blocking NF-κB activation and also the phosphorylation of mitogen-activated protein kinases in RAW 264.7 macrophages. In the present study, macrophages were cultured with 2·5 % of autologous serum from the respective SFO- and GCO-fed rats. In a previous study, serum from GCO2·5-, GCO5- and GCO10-fed rats contained 2·2, 4·5 and 8·3 % of ALA, 0·8, 1·2 and 5·4 % of EPA and 1·72, 2·9 and 2·7 % of DHA, respectively(Reference Diwakar, Dutta and Lokesh10). Thus, the exogenous pool of n-3 PUFA added to PMΦ might play an important role in the reduction of NO observed in the present study. Furthermore, it has been reported that γ-tocopherol can also inhibit inducible NO synthase in activated macrophages(Reference Jiang, Schwab and Courtemanche50). Since GCO contains high amount of γ-tocopherol (87·74 mg/100 g)(Reference Diwakar, Dutta and Lokesh16), we hypothesise that the decrease in NO production in ex vivo-stimulated PMΦ can be attributed to the additive effect of ALA and γ-tocopherol present in GCO. However, further studies are needed to elucidate the mechanism of modulation of inducible NO synthase and NO production by physiological concentrations of ALA and γ-tocopherol.
The SFO (18 : 2n-6)-rich diet increased the levels of AA in PMΦ lipids. A concomitant increase in LTB4 was observed in A23187-stimulated PMΦ. On the contrary, the GCO10 diet reduced AA and LTB4 levels by 38 and 40 %, respectively, in PMΦ lipids. Leukotrienes are synthesised by the 5-LO pathway, and their activity was unaltered in the PMΦ of rats fed with the SFO and GCO diets. Therefore, the decrease in LTB4 observed in the present study can be attributed to the decrease in AA levels in the cellular lipids of PMΦ in GCO-fed rats. Since LTB4 is a potent chemotactic agent, recruits immune cells at the site of injury and orchestrates inflammatory events leading to tissue damage, modulating its levels by n-3 fatty acids may be beneficial in chronic inflammatory conditions.
Phagocytosis by polymorphonuclear leucocytes or PMΦ results in increased oxygen consumption and production of reactive oxygen intermediates, which contribute to the microbicidal action of these immunocompetent cells(Reference Allen51). Furthermore, lysosomal acid phosphatase activity is considered as a marker and correlates with the bactericidal action of macrophages(Reference Schnyder and Baggiolini52). Therefore, we investigated the effect of SFO or GCO on H2O2 release and in vitro lysosomal phosphatase enzyme activity in PMA-stimulated PMΦ. The ex vivo release of H2O2 and lysosomal phosphatase activity were not affected in the PMΦ of rats fed with SFO or GCO. This observation shows that GCO may not modulate the bactericidal activity of macrophages.
Conclusion
We report that dietary supplementation of GCO increased ALA, EPA and DHA levels in the membrane lipids of immunocompetent cells. GCO significantly suppressed the ex vivo proliferation of SL in response to stimulation by T-cell mitogens with little or no effect on IL-2 secretion. GCO suppressed the release of inflammatory mediators such as LTB4, NO and, to a lesser extent, TNF-α in activated PMΦ. Our data add further evidence that dietary supplementation of ALA alters the lipid composition of immune cells and modulates certain inflammatory mediators in normal rats. However, further studies are needed to understand the long-term effect of ALA in the modulation of inflammatory mediators in ulcerative colitis, arthritis, asthma and allergic disorders.
Acknowledgements
The authors thank Dr V. Prakash, Director, Central Food Technological Research Institute, and Dr P. V. Salimath, Head of the Department of Biochemistry and Nutrition, for their encouragement and support. K. A. N. gratefully acknowledges the financial assistance in the form of a project from the Indian Council of Medical Research, New Delhi. Financial support to B. T. D. through a Senior Research Fellowship from the Council for Scientific and Industrial Research, New Delhi, India, is also gratefully acknowledged. There are no conflicts of interest whatsoever among the authors. B. T. D. was responsible for conducting the experiments. K. A. N. supervised the experiments and prepared the manuscript. B. R. L. helped in the interpretation of the results and preparation of the manuscript.








