A layer of intestinal epithelial cells lining the gastrointestinal tract forms a selective barrier to the harsh environment of intestinal lumen(Reference Gewirtz, Liu and Sitaraman1,Reference Madara2) . Disturbances in intestinal barrier, characterised by increased mucosal permeability, allow luminal bacteria, toxins and antigenic agents to ‘leak’ across the epithelium, resulting in inflammation, diarrhoea and potentially systemic disease(Reference Berkes, Viswanathan and Savkovic3,Reference Livingston, Mosenthal and Deitch4) . Early life stress is a predisposing factor for the development of chronic intestinal barrier damage(Reference Farhadi, Keshavarzian and Van de Kar5,Reference Bennett, Tennant and Piesse6) . Immune stress in piglets resulting in sustained impairment in mucosal barrier is considered one of the major causes of diarrhoea and an economic loss case. The mechanism that immune stress-induced loss of intestinal barrier function is not fully understood but thought to be mediated by the release of pro-inflammatory cytokines such as TNF-α as well as interleukins, which are central mediators of intestinal inflammatory diseases(Reference Suenaert, Bulteel and Lemmens7,Reference Prasad, Mingrino and Kaukinen8) .
Micro-environmental hypoxia has been identified to be a feature of sites of chronic inflammation. Hypoxia-inducible factor-1α (HIF-1α) is an oxygen-dependent subunit and masters transcription responses to hypoxia(Reference Semenza9). HIF-1α activation plays a gut-injurious role associated with some conditions such as hypoxia and inflammation(Reference Feinman, Deitch and Watkins10,Reference Kannan, Colorado and Reino11) . Many findings have converged to suggest HIF-1α could be inducible in existence of pro-inflammatory cytokines, and the fact that its activation happens not only under hypoxia but also normoxic conditions(Reference Frede, Freitag and Otto12,Reference Belaiba, Bonello and Zahringer13) . Importantly, HIF-1α promoter contains an active NFκB binding in transcription start site(Reference van Uden, Kenneth and Rocha14), and pro-inflammatory cytokines like TNF-α (Reference Taylor, Dzus and Colgan15) and IL-6(Reference Matsui, Ihara and Fujio16) are among the target gene identified for hypoxia-induced NFκB. Theoretically, co-regulation of gastrointestinal NFκB and HIF-1α pathways by nutrition means and subsequent down-regulation of the pro-inflammatory cytokines genes may exert beneficial effects on the intestinal injury.
Natural plant-derived polysaccharides have been safely used mainly in nutritional and medical areas for a long period in many Oriental countries. Acanthopanax senticosus polysaccharides (ASPS) are the major active ingredients extracted from traditional herbal medicine Acanthopanax senticosus, and have been characterised by the bioactivity of immune regulation(Reference Han, Yoon and Ahn17,Reference Chen, Liu and Zhao18) . In our previous works(Reference Han, Bian and Liu19 – Reference Han, Li and Bai21), following the elucidation of down-regulation of the genes expressions of pro-inflammatory cytokines under inflammatory conditions with ASPS application, subsequent study identified the important role of ASPS on suppressing the toll-like receptor 4 (TLR4)/NFκB/myosin light chain kinase (MLCK) signalling pathway to sustain intestinal integrity in lipopolysaccharide (LPS)-challenged mice model. Given the intimate molecular link between NFκB and HIF-1α (Reference Taylor22), further identification of the role of dietary ASPS on the HIF-1α signalling pathway under inflammation condition is the point of interest.
Therefore, in the present study, we employed Escherichia coli LPS-induced immune stress model in piglet to test the hypothesis that dietary supplemented with ASPS could modulate the HIF-1α signalling pathway and concomitant amelioration of LPS-induced intestinal barrier dysfunction.
Methods
Preparation and analysis of Acanthopanax senticosus polysaccharides
ASPS were extracted from the root of A. senticosus by using a method of water extraction and alcohol precipitation with some modifications(Reference Zhang, Chen and Zhang23). Briefly, crushed roots were boiled to obtain filtrate, which was concentrated and precipitated with ethanol to collect precipitate by centrifugation. Following protein elimination from the precipitation by the Sevag method(Reference Staub24), recrystallisation and drying were employed to obtain a kind of tan powdery polysaccharide. About 96 % content of ASPS was determined by the phenol sulphuric acid method, and the composition analysis using HPLC with a diode-array-detector showed that it is a heteropolysaccharide composed of glucose, galactose, mannose, arabinose, glucuronic acid, rhamnose, galactosidonic acid and xylopyranose and fucose (online Supplementary Fig. S1).
Experimental design, feeding and lipopolysaccharide challenge
A total of thirty-six crossbred weaned piglets (Duroc × Large White × Landrace, weaned at 24–25 d of age) with an average initial body weight (BW) of 7·98 (sem 0·04) kg were kept in a house equipped 2·0 × 2·1 m2 stainless steel pens (three piglets per pen). Each pen is equipped with plastic floor and a self-feeder and a nipple drinker to allow ad libitum access to feed and water. The temperature in the inner house was controlled at 24°C approximately. The piglets were randomly assigned according to birth weight and sex into three treatment groups as follows: (1) basal diet + saline challenge (CONTR); (2) basal diet + LPS challenge (LPS); (3) basal diet with 800 mg/kg ASPS + LPS challenge (ASPS + LPS). The maize–soya bean-meal basal diet was formulated to meet or exceed the nutrient requirements recommended by Feeding Standard of Swine (25) of 8–20 kg BW for all nutrients except for Ca (Table 1). The crude protein, Ca and total P contents in diet were analysed according to the method of Association of Official Analytical Chemists (26). The ASPS dose in the present work was determined on the basis of our published studies indicating that it ameliorated intestinal injury of LPS-challenged mice(Reference Han, Liu and Yu20,Reference Han, Li and Bai21) . On days 15, 18 and 21 during the 21-d feeding trial, pigs in LPS and ASPS + LPS groups were challenged intraperitoneally with LPS (E. coli serotype 055:B5; Sigma Chemical Inc.) at 100 μg/kg BW dissolved in sterile saline, and the pigs in CONTR were given an equivalent amount of sterile saline. At 21 d, pigs were orally given d-xylose at the dose of 500 mg/kg BW prepared as 0·5 g/ml solution in deionised water at 1 h post-challenge to assess intestinal absorptive function in vivo.
Table 1. Ingredient composition and nutrient contents of the basal diet (%, as-fed basis)
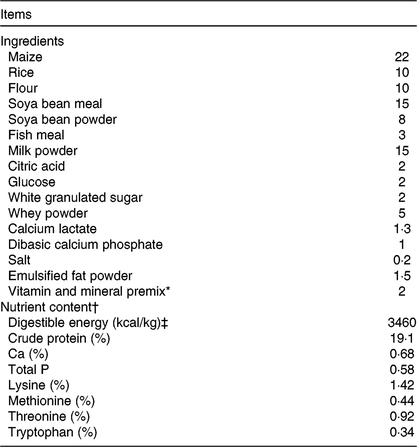
* Premix provided per kg of diet: vitamin A 3·6 mg; vitamin D3 0·82 mg; vitamin E 40 mg; vitamin K3 4·7 mg; thiamine 4·4 mg; riboflavin 10·2 mg; niacin 40 mg; pantothenic acid 32 mg; pyridoxine 3·2 mg; vitamin B12 0·01 mg; folic acid 1·2 mg; biotin 0·14 mg; choline 500 mg; Cu (copper sulphate) 8 mg; Zn (zinc sulphate) 84 mg; Fe (ferrous sulphate) 84 mg; Mn (manganese sulphate) 33 mg; iodine (calcium iodate) 0·6 mg; Se (sodium selenite) 0·3 mg.
† Crude protein, Ca and total P were analysed values. The digestible energy and amino acid contents were calculated values.
‡ To convert energy in kcal to kJ, multiply by 4·184.
The institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Shenyang Agricultural University Institutional Animal Care and Use Committee.
Sample collection
On day 21, piglets were deprived of feed at 3 h pre-injection except for ad libitum access to water in order to avoid the potential effects on the characteristics of blood of LPS-induced feed intake reduction. At 3 h after LPS challenge, whole blood samples were collected in pro-coagulant vacuum tubes by anterior vena cava. Plasma was then separated by centrifuging at 3500 g for 10 min, and stored at −20°C for further analysis of biochemical index. Following the blood collection, euthanasia and midline laparotomy were performed. After dissecting small intestine free of the mesenteric attachment, mid-ileum segments were then excised and flushed gently with ice-cold PBS and immediately immersed in 4 % chilled polyoxymethylene for histological evaluation and 2·5 % glutaraldehyde for electron microscopy identification, respectively. The remaining ileal mucosa was scratched with glass microscope slide and collected into sterile frozen tubes following longitudinal opening and ice-cold PBS rinsing, and then which was immediately snapped in liquid nitrogen and stored at −80°C until analysis.
Growth performance and faecal characteristics evaluation
Piglets were weighed individually at days 0, 14 and 21 of the trial to obtain the average BW of each pen and determine average daily gain (ADG). Surplus feed remaining in the feeder of each pen was cleared away and weighted to calculate average daily feed intake.
Faecal characteristics of piglets in each pen were assessed at 09.00 and 17.00 hours, and the degree of dehydration score was recorded twice daily during the entire LPS-challenged period (from 15 to 21 d). It was assigned a faecal score based on visual analysis of symptoms according to the following criteria(Reference Andersen, Sangild and Munch27 – Reference Zhang, Wang and Cui29), 0, no stools; 1, normal, solid faeces; 2, pasty faeces; 3, droplets of watery faeces/diarrhoea; 4, moderate diarrhoea and 5, severe diarrhoea; and the criteria of 3, 4 and 5 were considered as diarrhoea condition. Diarrhoea incidence and diarrhoea index were calculated according to the following equations:
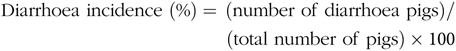 $$\eqalign{
& {\rm{Diarrhoea}}\;{\rm{incidence}}\;(\% ) = {\mkern 1mu} ({\rm{number}}\;{\rm{of}}\;{\rm{diarrhoea}}\;{\rm{pigs}}){\rm{/}} \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{total}}\;{\rm{number}}\;{\rm{of}}\;{\rm{pigs}}) \times {\rm{100}} \cr} $$
$$\eqalign{
& {\rm{Diarrhoea}}\;{\rm{incidence}}\;(\% ) = {\mkern 1mu} ({\rm{number}}\;{\rm{of}}\;{\rm{diarrhoea}}\;{\rm{pigs}}){\rm{/}} \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{total}}\;{\rm{number}}\;{\rm{of}}\;{\rm{pigs}}) \times {\rm{100}} \cr} $$
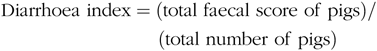 $$\eqalign{
& {\rm{Diarrhoea}}\;{\rm{index = }}({\rm{total}}\;{\rm{faecal}}\;{\rm{score}}\;{\rm{of}}\;{\rm{pigs}})/ \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{total}}\;{\rm{number}}\;{\rm{of}}\;{\rm{pigs}}) \cr} $$
$$\eqalign{
& {\rm{Diarrhoea}}\;{\rm{index = }}({\rm{total}}\;{\rm{faecal}}\;{\rm{score}}\;{\rm{of}}\;{\rm{pigs}})/ \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{total}}\;{\rm{number}}\;{\rm{of}}\;{\rm{pigs}}) \cr} $$
Intestinal barrier function parameters and inflammatory mediators in blood or intestinal mucosa
Intestinal function parameters, including enzymatic activity of intestinal DAO and lactase, invertase, maltase and the plasma concentration of d-xylose, as well as the levels of inflammatory cytokines involving TNF-α, IL-6 and IL-1β in intestinal mucosa, were quantified using enzyme-linked immunosorbent assay kits (Omnimabs). All procedures were carried out strictly according to the manufacturer’s protocols.
Intestinal mucosal histological assay
Villus height and crypt depth were measured on haematoxylin and eosin–stained and paraformaldehyde-fixed ileal histological slices. Detailed methods of these tests have been described previously(Reference Han, Liu and Yu20). Ileal villus and microvillus morphology were further researched by scanning electron microscope, and the processing was carried out as described elsewhere(Reference Ringø, Salinas and Olsen30). Briefly, ileal sections measuring 3 × 3 × 3 mm3 were cut and immediately transferred into 2·5 % glutaraldehyde for fixation. The segments were washed three times in phosphate buffer and then post-fixed in OsO4 (1 % in phosphate buffer) for 30 min. After a series of dehydration processes in alcohol at the concentration of 30, 50, 70, 80, 90 and 100 %, and substitution in a series of tert-butyl alcohol at the concentration of 50, 75, 90 and 100 %, the segments were dried in freeze dryer (IXRF, VFD-30) and sputter-coated with a layer of platinum (Hitachi, MC1000), and finally examined under Regulus 8100 scanning microscope (Hitachi). The morphology of intestinal epithelial tight junction was evaluated by testing mid-ileal sections employing transmission electron microscope (HT-7700; Hitachi) as previously described(Reference Han, Li and Bai21).
mRNA expression analysis by real-time PCR
The procedures of mRNA expression analysis were done according to our previous description(Reference Han, Liu and Yu20) with some modification. Total RNA of ileal mucosa was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instruction. The integrity of isolated RNA preparation was examined by electrophoresis on a 1 % agarose gel containing 0·5 mg/ml ethidium bromide, and the purity and concentration of RNA were quantified using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific) according to the optical dentistry at OD 260/280 readings. Total RNA was reverse-transcribed using the PrimeScript RT reagent kit (Takara Biotechnology). The primers for the quantitative RT-PCR of each gene transcript for TNF-α, HIF-1α, inducible nitric oxide synthase (iNOS) and housekeeping gene were designed by Invitrogen and available in online Supplementary Table S1. The quantitative RT-PCR analysis was performed using the ABI StepOnePlus (Applied Biosystems), and the condition was set up as follows: 95°C for 30 s, followed by forty cycles at 95°C for 5 s, 60°C for 40 s; Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. The 2−ΔΔCt method was used to analyse the relative changes in each target gene expression(Reference Livak and Schmittgen31) with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The change (Δ) in Ct values in each group was compared with the Ct value of GAPDH (ΔCt), where ΔΔCt = (Ct of the target gene − Ct of GAPDH) treatment − (Ct of the target gene − Ct of GAPDH) CONTR. All the samples were analysed in triplicates.
Immunofluorescence microscopy
Immunofluorescence straining was carried out as per our previous description(Reference Han, Li and Bai21) and Yue et al. (Reference Yue, Lin and Zhang32). Ileal segments were fixed with 4 % paraformaldehyde and then cut into 3 μm thick slices. The slices were dewaxed and dehydrated with xylene and ethanol, respectively. The tissue samples were blocked with 5 % solution of bovine serum albumin (BSA) for 30 min at room temperature following incubation in 3 % hydrogen peroxide diluted by methanol, and then incubation with anti-HIF-1α (1:500; Santa Cruz Biotechnology) and anti-cyclo-oxygenase-2 (COX-2) (1:500; LifeSpan Biosciences) antibodies diluted in PBS containing 5 % BSA at 4°C overnight. The resulting sections were washed with PBS and incubated with Alexa fluor 488-conjugated secondary antibody for 2 h in dark conditions. After 4′,6-diamidino-2-phenylindole (DAPI) treatment for 5 min and sealing, sample images were obtained using a biomicroscope (Axio Scope A1; Zeiss).
Western blot assay
The quantification of protein expression in ileal mucosa was done according to the procedures outlined by Han et al. (Reference Han, Liu and Yu20) with some modification. In brief, an equal amount of protein extract was electrophoresed on 8 % reducing polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. Immunoblots were blocked with 5 % BSA for 50 min at room temperature and incubated overnight at 4°C with specific primary antibodies involving rat anti-HIF-1α and NFκB p65 (1:1000; Santa Cruz Biotechnology) and rabbit anti-COX-2 (1:1000; LifeSpan biosciences) in Tris-buffered saline with Tween-20. Blots were washed and then incubated with the corresponding HRP-conjugated secondary antibodies for 40 min at room temperature. The relative abundance of each target protein was expressed as target protein:β-actin protein ratio. The protein expressions of all samples were expressed as fold changes, calculated relative to CONTR.
Statistical analyses
The sample size was calculated using software SAS (version 9.4; SAS Institute Inc.). A power test based on the data obtained from a recent study on gene expression and biochemical index of pigs(Reference Han, Bian and Liu19) showed that four replicates per treatment were needed to achieve 80 % power and α = 0·05 according to Kononoff & Hanford(Reference Kononoff and Hanford33), and statistical power of more than 80 % for a sample size of six could be expected in the present experiment, which enables the additional power to reject the null hypothesis (H0), if H0 was false (P = 1−β).
The mean of three pigs in each pen/replicate was used to analyse growth performance and diarrhoea because each pen was regarded as an experimental unit, whereas individual pig was used as an experimental unit for analysis of the other parameters. All data sets were tested for normal distribution using the Shapiro–Wilk test, and then parametric data were analysed using one-way ANOVA with dietary as the main effect, and differences among groups were compared using Duncan’s test in the case where the significant main effect of diet was found.
The data of diarrhoea index were first performed with log-transformation due to lack of normal distribution, and then analysed using ANOVA. Statistical analyses were performed using IBM SPSS Statistics (version 22), and all the data were expressed as means with their standard errors. P<0·05 was considered significant for all analyses.
Results
The effect of Acanthopanax senticosus polysaccharides on growth performance and diarrhoea of lipopolysaccharide-challenged piglets
As given in Table 2, ASPS did not affect growth performance including ADG and average daily feed intake prior to LPS challenge of weaned piglets (from day 1 to 14). LPS challenge (from day 15 to 21) resulted in suppressed ADG (P < 0·05) and average daily feed intake (P < 0·05) compared with the piglets in CONTR. In contrast, the values of ADG and average daily feed intake (P < 0·05) of LPS-challenged piglets were marked boosted by ASPS administration although the change of ADG was insignificant. In addition, the values of diarrhoea incidence (P < 0·05) and diarrhoea index (P < 0·05) in LPS-induced piglets were decreased by the inclusion of ASPS when compared with the LPS group, and their levels were comparable with the CONTR group.
Table 2. Effects of dietary Acanthopanax senticosus polysaccharides (ASPS) supplementation on growth performance and diarrhoea of lipopolysaccharide (LPS)-challenged piglets
(Means of four pens with three pigs per pen with pooled standard errors)
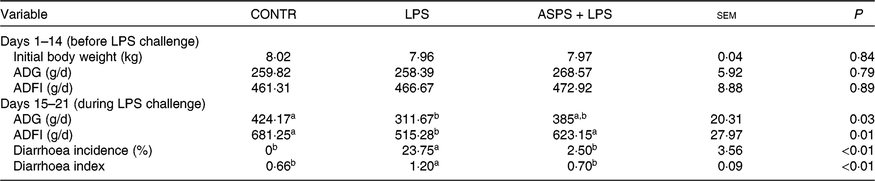
CONTR, non-challenged piglets fed basal diet (control); LPS, LPS-challenged piglets fed basal diet; ASPS + LPS, LPS-challenged piglets fed basal diet supplemented with 800 mg/kg ASPS; ADG, average daily gain; ADFI, average daily feed intake.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Changes in biochemical parameters in blood or intestinal mucosa
The piglets in LPS group exhibited the elevated level of intestinal mucosal TNF-α (P < 0·05) and IL-1β (P < 0·05), as well as decreased (P < 0·05) level in ileal enzyme activities of DAO (P < 0·05) and lactase (P < 0·05), and circulating d-xylose (P < 0·05) in LPS-challenged piglets when compared with CONTR (Table 3). Correspondingly, besides the significant normalising of aforementioned index (P < 0·05), the values of ileal activities of invertase and maltase, as well as ileal concentration of IL-6, were somewhere between CONTR and LPS groups although they were not significantly altered in the LPS-challenged piglets under ASPS application.
Table 3. The effects of Acanthopanax senticosus polysaccharides (ASPS) on intestinal damage and function parameters, and inflammatory mediators in blood or intestinal mucosa in lipopolysaccharide (LPS)-challenged piglets
(Mean values with pooled standard errors; n 6 per group)
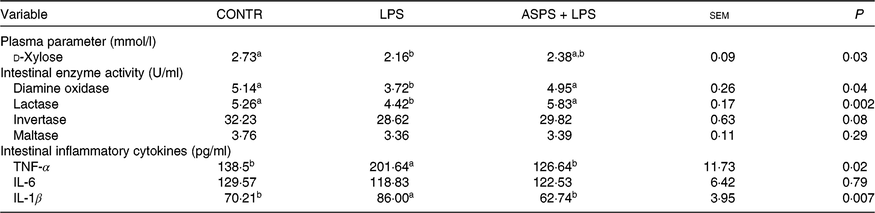
CONTR, non-challenged piglets fed basal diet (control); LPS, LPS-challenged piglets fed basal diet; ASPS + LPS, LPS-challenged piglets fed basal diet supplemented with 800 mg/kg ASPS.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Intestinal histological observation
Compared with CONTR group (Fig. 1(A)), ileal histological observation using scanning electron microscope showed obvious an destruction in intestinal epithelial morphology of villus and microvillus with irregular shape and arrangement in weaned piglets challenged with LPS (Fig. 1(B)). However, these pathologic changes were reversed drastically to form the normal intestinal epithelial appearance with the administration of ASPS (Fig. 1(C)), indicating that ASPS treatment prevented or repaired intestinal epithelial damage caused by LPS. Similarly, the evaluation for gut epithelial permeability using transmission electron microscope found that tight junction (TJ) ultra-structure characterised by intact structure and electron dense materials between the adjoining cells decreased following the LPS treatment (Fig. 1(E)) compared with CONTR group (Fig. 1(D)). As expected, ASPS inclusion significantly attenuated the negative changes induced by LPS challenge (Fig. 1(F)).
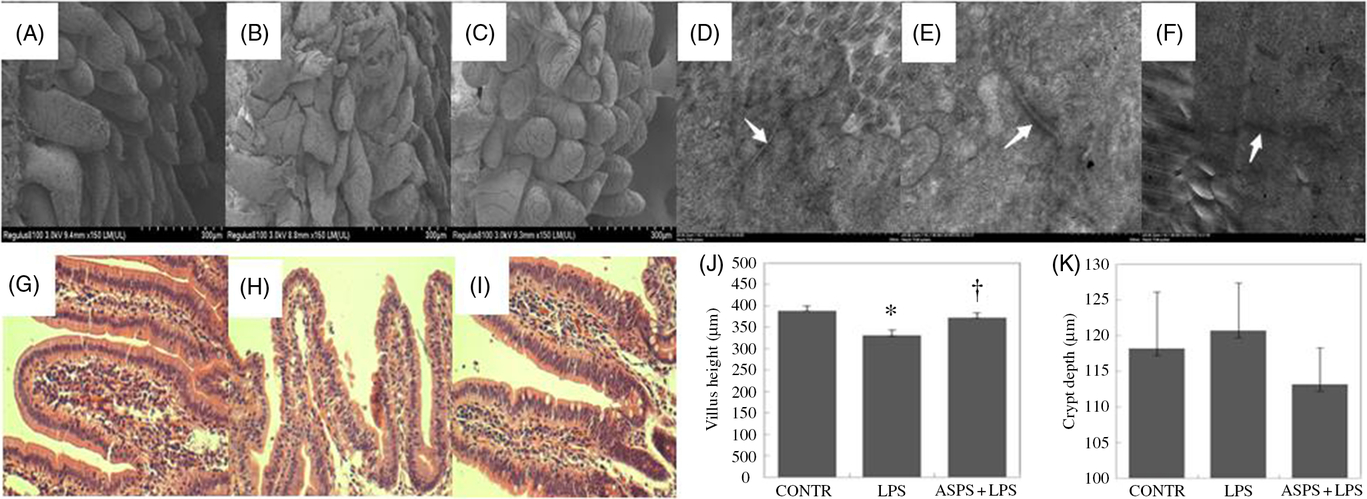
Fig. 1. Histological analysis of ileum influenced by Acanthopanax senticosus polysaccharides (ASPS) in lipopolysaccharide (LPS)-challenged piglets (n 6). (A–C) The representative photomicrographs of ileal villi from control (CONTR) (A), LPS (B) and ASPS + LPS (C) were observed by scanning electron microscope (×150). (D–F) The representative photomicrographs of ileal tight junctions from CONTR (D), LPS (E) and ASPS + LPS (F) were observed by transmission electron microscope (×20 000). (G–I) The representative photomicrographs of ileal segments stained with haematoxylin and eosin (×100 magnification) of CONTR (G), LPS (H) and ASPS + LPS (I). (J) Ileal mucosal villus height; (K) ileal mucosal crypt depth. Values are means with standard errors represented by vertical bars. * P < 0·05 v. CONTR; † P < 0·05 v. LPS.
Ileal haematoxylin and eosin staining showed obvious damage characterised by atrophic villi with a discontinuous brush border and irregular epithelium in LPS-injected piglets without ASPS supplementation (Fig. 1(G) and (H)). Correspondingly, these negative histologic changes were significantly alleviated by pretreatment with ASPS (Fig. 1(I)). As expected, compared with LPS group, dietary ASPS significantly increased villus height in ileum (P < 0·05) (Fig. 1(J)). However, no effect was observed on crypt depth following ASPS supplementation (Fig. 1(K)).
Gene expression
The data from mRNA expressions of TNF-α, iNOS and HIF-1α are shown in Fig. 2. Compared with the CONTR group, LPS challenge increased mRNA abundances of TNF-α (P < 0·05), HIF-1α (P < 0·05) and iNOS (P < 0·05) in ileal mucosa. As expected, the altered mRNA expressions of aforementioned genes were reversed with ASPS administration (P < 0·05).
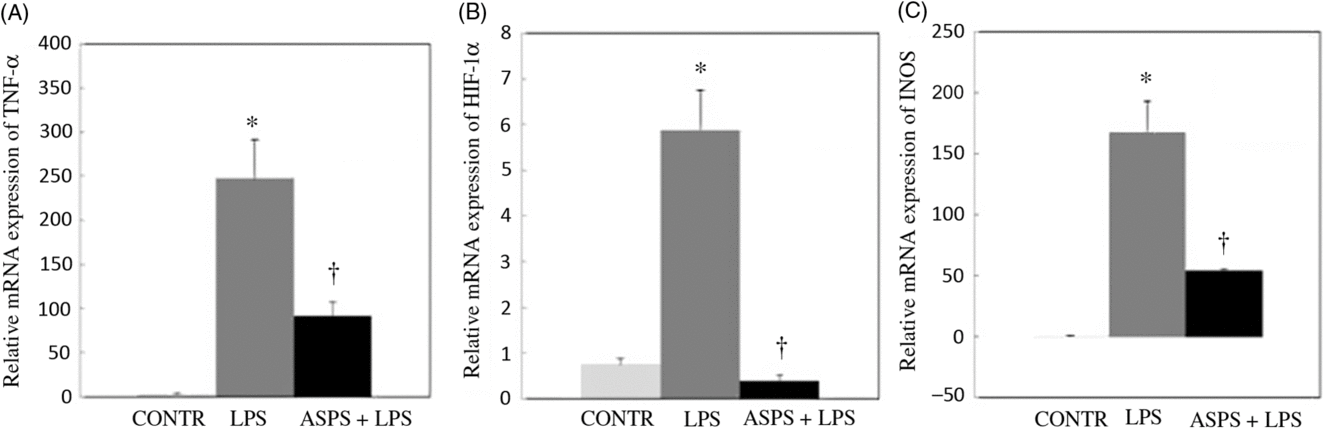
Fig. 2. Effects of dietary Acanthopanax senticosus polysaccharides (ASPS) supplementation on gene expression related to inflammation in lipopolysaccharide (LPS)-challenged piglets (n 6). Values are means of gene expression of TNF-α (A), hypoxia-inducible factor-1α (HIF-1α) (B) and inducible nitric oxide synthase (iNOS) (C), with standard errors represented by vertical bars. * P < 0·05 v. CONTR; † P < 0·05 v. LPS. CONTR, piglets receiving basal diet and injected with saline challenge (control); LPS, piglets receiving basal diet and injected with Escherichia coli LPS; ASPS + LPS, piglets receiving basal diet with 800 mg/kg ASPS and injected with LPS challenge.
Hypoxia-inducible factor-1α, cyclo-oxygenase-2 and NFκB p65 protein expressions in ileal mucosa by Western blotting and fluorescence microscopy
The localisation and expression of ileal HIF-1α and COX-2 proteins are evaluated by immunofluorescence presented in Fig. 3. Piglets in LPS group exhibited more distribution of HIF-1α and COX-2 staining with the presence of continuous bands along epithelial cell base compared with CONTR group (Fig. 3(A)). Correspondingly, ASPS pretreatment attenuated the HIF-1α and COX-2 expressions. In addition, immuno-blotting was further employed to measure the expressions of the two proteins (Fig. 3(B)). Unanimously, the protein expressions of HIF-1α, COX-2 and NFκB p65 were amplified in ileal epithelium in LPS-challenged piglets (Fig. 3(C)) (P < 0.05). Inclusion of ASPS reversed LPS-induced elevation of HIF-1α (P < 0.05), COX-2 (P < 0·05) and NFκB p65 (P < 0·05).
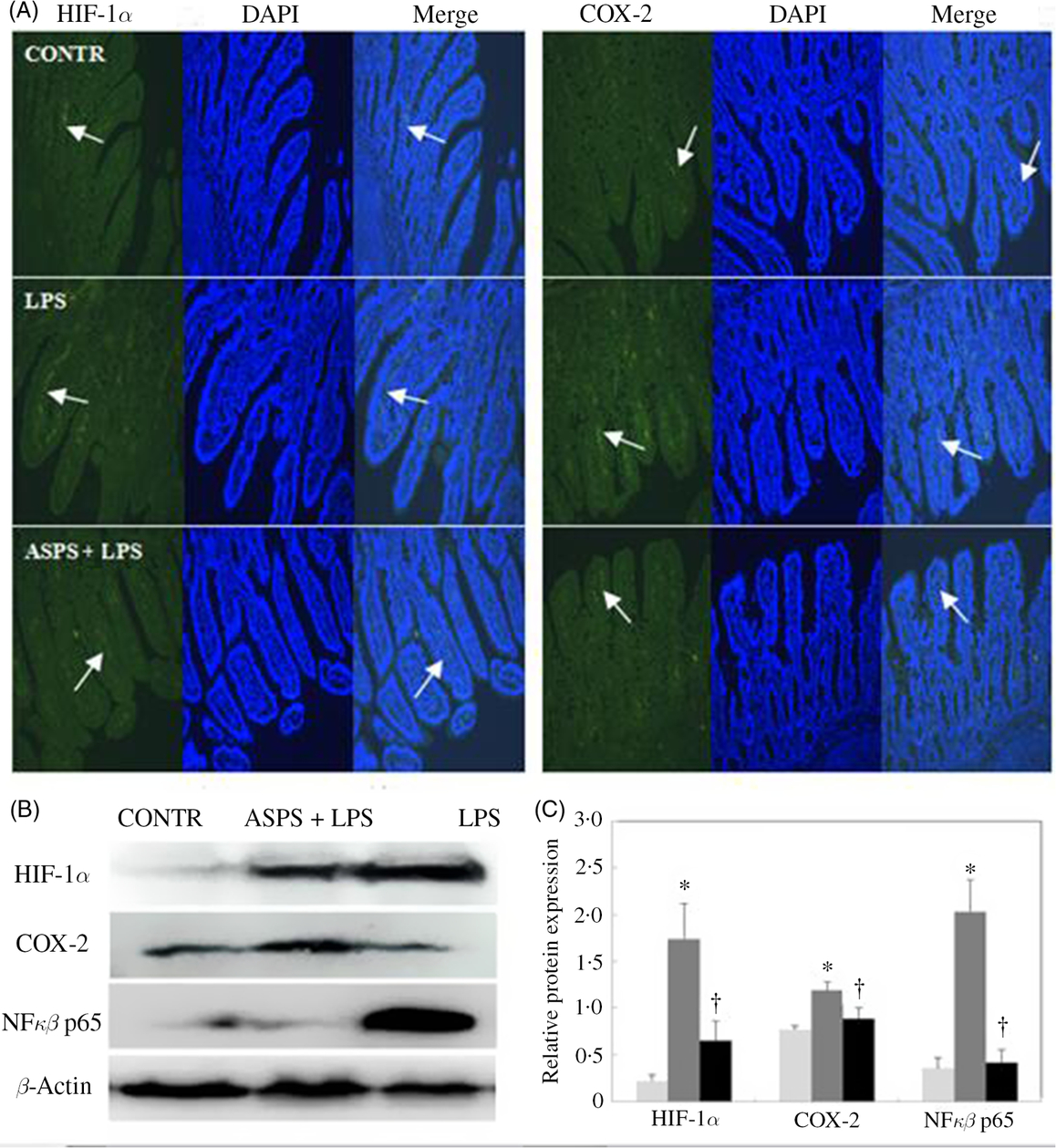
Fig. 3. Effects of Acanthopanax senticosus polysaccharides (ASPS) on the ileal expressions of hypoxia-inducible factor-1α (HIF-1α), cyclo-oxygenase-2 (COX-2) and NFκB p65 in lipopolysaccharide (LPS)-challenged piglets (n 6). (A) The representative photomicrographs of ileal segments at a ×100 magnification (green fluorescence) were observed by immunofluorescence. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue fluorescence). Arrows indicate the location of HIF-1α and COX-2 staining. (B) The bands are the representative Western blot images of HIF-1α, COX-2, NFκB p65 and β-actin in ileum. (C) Values are means of protein expression of HIF-1α and COX-2, with standard errors represented by vertical bars. * P < 0·05 v. CONTR; † P < 0·05 v. LPS. CONTR, piglets receiving basal diet and injected with saline challenge (![]() ); LPS, piglets receiving basal diet and injected with Escherichia coli LPS (
); LPS, piglets receiving basal diet and injected with Escherichia coli LPS (![]() ); ASPS + LPS, piglets receiving basal diet with 800 mg/kg ASPS and injected with LPS challenge (
); ASPS + LPS, piglets receiving basal diet with 800 mg/kg ASPS and injected with LPS challenge (![]() ).
).
Discussion
TLR recognise conserved pathogen-associated molecular patterns that are unique to micro-organisms(Reference Medzhitov and Janeway34). TLR4 is predominantly activated by LPS. The combination of LPS with TLR4 receptor can trigger downstream NFκB and result in the activation of immune response(Reference Rehli35). However, a high dose of LPS-inducible excessive activation of TLR4 signalling could lead to inflammatory damage in tissue(Reference Modlin36). In the present study, we employed an E. coli LPS-challenged immune stress model for inducing gut barrier inflammatory damage in weaned piglets to determine whether ASPS supplementation might modulate HIF-1α signalling response to hypoxia accompanied with inflammation and consequently contribute to improvement in loss of intestinal barrier. LPS as a toxin molecule present on the membrane of Gram-negative bacteria can induce systemic inflammatory and the various intestinal barrier disturbances induced by LPS injection in our current finding were coincident with many well-documented reports(Reference Kruzel, Harari and Chen37) and indicated an immune stress model built successfully in our study.
The present study validated that growth performance of piglets was similar among treatments despite the fact that ASPS was employed with lack of LPS challenge. Following LPS injection, ASPS treatment exhibited a positive role on feed intake elevation. However, the value of ADG of pigs fed ASPS was intermediate and no significance was obtained for ADG when comparing with the CONTR group. This finding was consistent with that in our previous report in weaned piglets(Reference Han, Bian and Liu19) and suggested that dietary ASPS preferred to alleviate the loss of growth performance under immunological stress than under normal condition. In addition, a noticeable decrease in diarrhoea incidence was observed in LPS-induced piglets with ASPS treatment and was tendentiously similar to those in CONTR. Intestinal barrier disturbance is characterised by a high incidence of diarrhoea and decreased performance in immune stress piglets(Reference Berkes, Viswanathan and Savkovic3). Improvement in gastrointestinal epithelial function may elaborate the cause of improved performance and decreased diarrhoea incidence due to ASPS application.
Deterioration in intestinal epithelial morphology and increased intestinal permeability are characterised by attenuation in absorption capacity of nutrients, fluids and electrolytes as a result of immune stress, which lead to fluid and electrolyte accumulation in the bowel and contribute to the development of diarrhoea(Reference O’Loughlin, Scott and Gall38). In the present study, dietary ASPS contributed to the irregular shape and arrangement of epithelial villus, as well as the elevation in villus height in ileum of LPS-challenged piglets in contrast to the challenged piglets without ASPS treatment. These signs of morphological improvement were further supported by biochemical parameters, including higher enzyme activity of ileal lactase and DAO. Lactase overlying the mucosal surface is responsible for decomposing disaccharides and facilitates mucosa maturation and digestive function(Reference Olsen, Li and Lioyd39). Likewise, DAO localised in the cytoplasm of apical villous cells of the small intestine with high activity should reflect the status of intestinal mucosa integrity(Reference Thompson, Burnett and Markin40,Reference Schmidt, Sattler and Hesterberg41) . Declined mucosal DAO activity as a biologic marker of boosted intestinal permeability under the condition of gut inflammation injury has been displayed in many research studies(Reference Liu, Chen and Odle42,Reference Chen, Zhang and Cheng43) . As expected, the activities of these two enzymes in the present study were normalised with the treatment of ASPS under challenged condition. The circulatory concentration of d-xylose was measured to evaluate the intestinal absorption function, and the value of it had been increased in ASPS treatment and no significance was observed when comparing with the CONTR and LPS groups in the present study. Collectively, the preceding changes serving as a relatively stable marker of intestinal mucosal status confirmed the alleviated loss of mucosal absorptive capacity and intestinal permeability with dietary ASPS, which was also in agreement with lower diarrhoea incidence.
The facts that interaction of inflammation and hypoxia results in a compromised intestinal barrier function have been identified in a number of studies(Reference Yang, Yu and Sun44,Reference Liu, Li and Wang45) . Hypoxia-inducible HIF-1α is critical in activating the master regulator of inflammatory response of NFκB and leads to NFκB-induced inflammation(Reference Koong, Chen and Giaccia46), and pro-inflammatory cytokines like TNF-α (Reference Taylor, Dzus and Colgan15), IL-6(Reference Matsui, Ihara and Fujio16) and iNOS(Reference Oliver, Taylor and Cummins47) are among the target genes identified for hypoxia-inducible NFκB. In turn, the existence of pro-inflammatory cytokines could induce HIF-1α (Reference Frede, Freitag and Otto12), and HIF-1α also could be the direct target for NFκB through binding the 197/188 location on the HIF-1α promoter(Reference Spirig, Djafarzadeh and Regueira48). To elucidate the mechanism where ASPS relieves inflammatory intestinal injury, our previous investigations using LPS-challenged mice have identified that ASPS supplementation caused the inhibition of gut TLR4/NFκB signalling pathway and concomitant intestinal epithelial improvement(Reference Han, Liu and Yu20,Reference Han, Li and Bai21) . However, the role of ASPS supplementation on HIF-1α regulation remains to be elucidated. Here, unanimous results of degressive HIF-1α expression were achieved in this work by the support of mRNA, protein and immune-fluorometric assay with ASPS treatment, and these changes were accompanied by a sharp decrease in intestinal mucosal expression and concentration of NFκB, TNF-α, iNOS, as well as IL-1β. Meanwhile, TNF-α is a central mediator in the predisposition and exacerbation of gastrointestinal inflammation(Reference Ye, Ma and Ma49), and it is thought to link with the inhibition of phosphorylation of the myosin light-chain mediated by the myosin light-chain kinase, leading to alternatively disrupted TJ stability and dysfunction of TJ protein expression(Reference Clayburgh, Barrett and Tang50). This observation can be extended to our study. Here, a drastic decline in the expression and concentration of ileal TNF-α in this work not only emphasised the important role of dietary ASPS in the regulation of TNF-α release and consequent improvement of gut epithelial barrier function of LPS-challenged piglets but also revealed that the down-regulation of TNF-α in ASPS-fed piglet is linked with HIF-1α change during immune stress. Our data were supported by our previous report in which the relief of releasing of TNF-α in the modulation NFκB/MLCK and the improvement of gut epithelial TJ by ASPS were observed in challenged mice(Reference Han, Li and Bai21). Given the crosstalk between HIF-1α and NFκB, these emerging evidences in this work are particularly relevant to explain, at least in part, that HIF-1α expression involved in the modulation of the NFκB signalling pathway by dietary ASPS resulted in the reduction of the previous inflammatory mediators and led to improved intestinal barrier function of piglets under inflammation condition.
Cyclo-oxygenase (COX)-2, an inducible key enzyme in the production of inflammatory prostanoids, could be up-regulated as the direct target for HIF-1α due to its direct binding to a specific location at −506 on the COX-2 promoter, and this highlights the biological significance of COX-2 up-regulation during hypoxia(Reference Kaidi, Qualtrough and Williams51). COX-2 overexpression has been described in the destruction of intestinal barrier in rat peritonitis model(Reference Short, Wang and Castle52). Therefore, identifying the regulatory role of ASPS in HIF-1α-inducible COX-2 up-regulation is crucial for further development of novel molecular target for diarrhoea prevention by ASPS. As expected, the decrease in gut mucosal HIF-1α expression accompanied with COX-2 activation by analysis detection of both protein and immunofluorescence following ASPS treatment in LPS-induced immune stress model of piglets was achieved in this work. In this regard, these emerging evidences suggested that up-regulation of COX-2 represented intestinal mucosa adaptive response to hypoxia, and the down-regulation of the HIF-1α/COX-2 signalling pathway may be a novel finding regarding the mechanism in which dietary ASPS relieved gut impair in LPS-challenged piglets. However, further research in vitro is needed to clarify whether the suppressed activation of the NFκB pathway with a sequential HIF-1α expression or modulation of HIF-1α-inducible NFκB is involved in ASPS work.
It still remains unclear that how the polysaccharides isolated from A. senticosus possibly affect the TLR4/NFκB signalling pathway. Due to share cell-type specificity of immune cell receptors with LPS, polysaccharide isolated from A. senticosus was reported to exert immunostimulation by the surface binding of the TLR4 and TLR2 receptors expressed on B cells and macrophages(Reference Han, Yoon and Ahn17), and natural plant-derived polysaccharides have been reported to be not connected with any tissue injuries at their biologically effective dose(Reference Mallick, Maiti and Bhutia53). In our relevant works, the possible reason why ASPS relieved the activation of TLR4/NFκB(Reference Han, Liu and Yu20) and NFκB/HIF-1α/COX-2 pathway and intestinal barrier dysfunction following LPS challenge may be connected with competing with the binding of TLR4 receptor with LPS and resulting in the reverse in excessive activation of this signalling. Notably, activated kinds of immune cells and the acted receptors on the surface of immune cells are differences among different compositions of polysaccharides due to diversities and complexity in the sequence and spatial structure of the monosaccharaides making up the polysaccharides(Reference Shso, Xu and Dai54,Reference Shao, Dai and Xu55) , which is likely connected with the difference in the extraction parts of plant, process and method. The ASPS used in the present research mainly consist of glucose residues (online Supplementary Fig. S1). Glucan, a polysaccharide consisting of glucose residues, is inferred to be likely the main active ingredient of ASPS to show the positive effect because glucan is also the main factor in functional additives of Astragalus polysaccharides(Reference Yuan, Piao and Li56) in China and many observations in previous reports suggesting the improved performance of pigs by dietary supplementation with β-glucan(Reference Schoenherr, Pollmann and Coalson57,Reference Dritz, Shi and Kielian58) . Nowadays, the herbal medicine of A. senticosus is widely used in China for modulating immune function although its polysaccharide products are under production. The further understanding on the action model of ASPS can help ASAP products to gain more attention.
In conclusion, the present study demonstrated that dietary supplementation with ASPS exerts alleviating role on inflammatory responses and intestinal barrier impairment and concomitant amelioration of diarrhoea incidence of immune stress piglets. These changes were possibly associated with a novel finding where down-regulation of HIF-1α/COX-2 is involved in NFκB-inducible release of inflammatory cytokines in immune stress piglets by ASPS supplementation.
Acknowledgements
The authors highly appreciate Mr Dongfeng Gao for assistance in the animal trial.
This study was jointly financially supported by the Natural Science Foundation of China (project no. 31702143; Beijing, P. R. China) and Key project of Liaoning Natural Science Foundation Plan (project no. 20170540796; Liaoning, P. R. China).
The authors contributed to this publication as follows: J. H. and X. J. L. designed the experiments; C. Y. F., F. Z., Y. S. L. and Q. T. X. participated in the animal experiment. J. H. and C. Y. F. discussed the results and wrote the paper. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001363









