The prevalence of energy imbalance and related obesity is rapidly increasing worldwide, including in a number of Asian countries( Reference Popkin 1 – Reference Misra and Khurana 3 ). This is linked to an increase in the prevalence of non-communicable diseases such as diabetes, CVD and hypertension( Reference Popkin 1 ). It is estimated that 300 million people will have diabetes by the year 2025 and that among them more than 100 million people live in Asia( Reference King, Aubert and Herman 4 ). However, diabetes in Asia has developed in a shorter time period (3–5-fold increase within 30 years), in a younger age group (45–64 years old) and in people with a lower BMI compared with that in Western countries( Reference Yoon, Lee and Kim 5 ).
Compared with whites with the same BMI and of the same age and sex, Asians have been found to have a higher body fat percentage and a lower fat-free mass (FFM)/appendicular skeletal muscle mass, as has been reported recently by us( Reference Wulan, Westerterp and Plasqui 6 ) and by others( Reference Deurenberg and Deurenberg-Yap 7 – Reference Rush, Freitas and Plank 13 ). Among Asians, South Asians (people from the Indian subcontinent) have the most pronounced difference in body fat compared with whites( Reference Deurenberg-Yap, Schmidt and van Staveren 14 ). Metabolic consequences, as revealed by the prevalence of the metabolic syndrome, are also greater than those in other Asian countries( Reference King, Aubert and Herman 4 , Reference Yoon, Lee and Kim 5 ). Raji et al. ( Reference Raji, Seely and Arky 15 ) reported that even at a normal BMI Asian Indians exhibit clustering of cardiovascular risk factors such as insulin resistance, dyslipidaemia and procoagulant state.
A strong gene–environment interaction, which is triggered by lifestyle changes due to modernisation, is suggested to be the cause of the rapid increase in the prevalence of diabetes in Asia( Reference Misra and Khurana 3 , Reference Ramachandran, Ma and Snehalatha 16 ). Furthermore, migrant Asian groups have also been reported to have higher susceptibility to adverse environmental influences than co-inhabitants of different races( Reference Ramachandran, Ma and Snehalatha 16 ). Major dietary changes such as a large increase in the consumption of fats and added sugars in the diet have accelerated in Asia in recent decades( Reference Popkin 17 ). Distinct genetic background represented partly by differences in body composition between South Asians and whites may contribute to the differences in metabolic responses when they are exposed to a high-fat diet challenge. It has been shown that a high-fat diet results in liver fat accumulation in a short time period( Reference van der Meer, Hammer and Lamb 18 ), leading to metabolic complications( Reference Brons, Jacobsen and Hiscock 19 ). In the present study, South Asians and whites were matched for body fat percentage, which is one of the confounding factors affecting metabolic profiles in individuals. The objective was to determine the effect of short-term overfeeding with a high-fat diet on the metabolic profile of South Asians and whites.
Subjects and methods
Subjects
A total of ten healthy non-diabetic South Asian men and ten white men were included in the study. They were matched for body fat percentage on an individual basis; as a consequence, the variation in body fat percentage was comparable between the groups. The number of subjects was determined based on a previous study that detected a significant reduction in insulin sensitivity after an intervention with a high-fat diet in ten white subjects( Reference Westerbacka, Lammi and Hakkinen 20 ). Subject characteristics are given in Table 1. Asian subjects had four grandparents from South Asia, while white subjects were non-Hispanic Europeans. Subjects were selected based on the following inclusion criteria: healthy; not having metabolic diseases (diabetes or CVD); not using medications; aged between 20 and 40 years with BMI ranging between 18 and 29 kg/m2 for South Asians and 22 and 33 kg/m2 for whites; having a stable body weight (as defined by weight change < 5 kg) for the last 3 months before participating in the study; not being on a diet; not being an athlete. Verbal and written information about the study was given to all subjects before obtaining their consent. Written informed consent was obtained from all subjects. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Ethics Committee of Maastricht University Medical Centre, MEC No. 10-3-013, and registered in the public trial registry (www.ccmo.nlno.NL31217.068.10).
Table 1 Subject characteristics (Mean values and standard deviations)
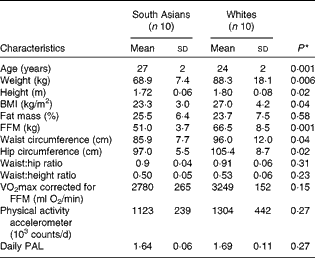
FFM, fat-free mass; PAL, physical activity level.
* Differences between the groups were assessed using an independent t test.
Experimental protocol
The present study was a dietary intervention study carried out in a free-living condition. Body composition was measured before the start of the dietary intervention to ensure that the body fat percentage of the two ethnic groups matched. Cardiorespiratory fitness (defined as VO2max corrected for FFM) was measured before the start of the dietary intervention. Daily physical activity levels were measured for seven consecutive days with an accelerometer. Energy requirements of a weight-maintenance diet for 3 d were calculated based on FFM and daily physical activity levels of each subject (see below). The metabolic profiles were determined just before and after overfeeding with the high-fat diet for 4 d. All measurements were carried out at the Metabolic Research Unit Maastricht (MRUM), Maastricht University, Maastricht, The Netherlands.
Determination of body composition
Body composition was determined according to a three-compartment model based on body weight, body volume and total body water. Body weight and body volume were determined in the morning in a fasting state. Body volume was determined by hydrodensitometry with simultaneous measurement of residual lung capacity using the helium dilution technique. Total body water was determined using the 2H dilution method according to the Maastricht protocol( Reference Westerterp, Wouters and van Marken Lichtenbelt 21 ). Body composition was calculated from body density and total body water using the equation of Siri( Reference Siri 22 ).
Assessment of cardiorespiratory fitness
Physical fitness was assessed with an incremental test on a bicycle ergometer using the protocol of Kuipers et al. ( Reference Kuipers, Keizer and Verstappen 23 ). During the test, VO2 and CO2 production were measured continuously and heart rate was monitored (Polar heart rate monitor; Polar Electro Oy). After 5 min of warm-up at 100 W, the workload was increased by 50 W every 2·5 min. When the heart rate reached a value of 35 beats/min below the age-predicted maximal heart rate (220 beats/min − age) or the respiratory quotient (RQ = CO2 production/VO2) exceeded 1, the workload was increased by 25 W every 2·5 min until exhaustion. VO2max was determined by averaging the last few points of maximum VO2. Cardiorespiratory fitness was defined as VO2max corrected for FFM.
Measurement of daily physical activity levels
Daily physical activity levels were measured using a Direct Life triaxial accelerometer for movement registration (Tracmor-D; Philips NewWellness Solutions; http://www.directlife.philips.com). The device is a small (3·2 × 3·2 × 0·5 cm), lightweight (12·5 g) instrument. The accelerometer was attached to the lower back of the subjects by means of an elastic belt. It registered accelerations minute by minute, in the mediolateral (x-axis), longitudinal (y-axis) and anterioposterior (z-axis) positions of the trunk as described elsewhere( Reference Bonomi, Plasqui and Goris 24 ).
The subjects were instructed to wear the accelerometer for seven consecutive days during waking hours, except during water activities. The subjects were advised to maintain their habitual physical activity levels during the dietary intervention period. Tracmor-D output is expressed as activity counts/min. To determine the average Tracmor-D counts/d, Tracmor-D activity counts/min were summed over the entire monitoring period and divided by the number of monitoring days.
Daily physical activity levels (PAL) were calculated based on the activity counts/d using the following formula( Reference Bonomi, Plasqui and Goris 24 ):
Total energy expenditure (TEE) was calculated using the formula of Bonomi et al. ( Reference Bonomi, Plasqui and Goris 24 ), by including activity counts/d (from the accelerometer) and FFM:
Determination of energy intake
The energy balance (weight-maintenance) diet to be consumed at home for 3 d before the baseline measurement was calculated on the basis of TEE of each subject as calculated above. The macronutrient composition of the energy balance diet before the baseline measurement was 30 % fat, 55 % carbohydrate and 15 % protein.
The high-fat diet was formulated to have 50 % excess energy above the TEE of subjects when on the balance diet( Reference Joosen, Bakker and Zorenc 25 ). The macronutrient composition of the high-fat diet was 60 % fat, 25 % carbohydrate and 15 % protein( Reference Schrauwen-Hinderling, Kooi and Hesselink 26 , Reference Schrauwen, van Marken Lichtenbelt and Saris 27 ). The fatty acid composition of the diet was 40 % SFA and 60 % unsaturated fatty acids.
A written instruction was given to prepare the diet at home. During the balance diet period, subjects were provided with amounts of the diet that exceeded their TEE and were allowed to eat more or less than the amounts prescribed, according to their preference (ad libitum). Any additional intake from the prescribed foods was recorded. All unfinished foods were collected and returned to the university to calculate the actual energy intake. During the overfeeding period, the subjects were asked to completely consume all the foods prescribed, but on failing to do so, to record their intake and return the unfinished foods. The diet consisted of normal ready-to-eat foods combining typical Western and Asian diets. Foods were selected by reviewing the ingredient content to ensure that there was no (or only a minimal) effect of certain ingredients on fat oxidation (such as spices). During the overfeeding period, the subjects were also provided with decaffeinated coffee and fruit tea, as caffeine has been reported to increase fat oxidation. Alcohol intake was limited during the dietary intervention period (only one serving/d when needed). During the dietary intervention period, the subjects were asked to wear the accelerometer and to maintain their habitual physical activity levels; this allowed us to monitor the energy balance of the subjects during the entire dietary intervention period.
Oral glucose tolerance test
A 2 h oral glucose tolerance test (OGTT) was carried out before and after overfeeding with the high-fat diet. After an overnight fast, the subjects underwent an OGTT test. A fasting blood sample was collected (t= 0), after which the subjects drank a glucose solution (82·5 g glucose monohydrate dissolved in 600 ml water) within 5 min( Reference Sievenpiper, Jenkins and Josse 28 ). This method is a modification of the American Diabetes Association (ADA) guidelines( 29 ), as it has been reported to improve overall tolerability and have only a minor impact on the intra-subject variation of plasma glucose and insulin responses with no effect on the diagnostically relevant 2 h endpoint irrespective of the body composition( Reference Sievenpiper, Jenkins and Josse 28 ). Venous blood samples were collected every 15 min in the first hour (t= 15, 30, 45 and 60) and every 30 min in the second hour (t= 90 and 120). Plasma glucose concentrations were measured to determine glucose tolerance according to the ADA criteria( 30 ).
Venous plasma glucose and insulin concentrations determined during the OGTT were used to assess pancreatic β-cell function and insulin sensitivity. These parameters were assessed using the updated homeostasis model assessment( Reference Levy, Matthews and Hermans 31 ) and the oral glucose insulin sensitivity index( Reference Mari, Pacini and Murphy 32 ), respectively. The oral glucose insulin sensitivity index is a measure of insulin sensitivity derived from glucose clearance in the OGTT. It is expressed as glucose clearance (ml/min) per m2 body surface by taking into account the subject's body weight and height (body surface), the dose of glucose solution, the concentration of plasma glucose at time 0, 90 and 120 min, and the concentration of insulin at time 0 and 90 min( 29 ).
Blood analysis
Blood samples were collected in tubes containing 30 μl of 0·2 m-EDTA. Plasma samples were immediately centrifuged at 3000 rpm for 10 min, frozen in liquid N2 and stored at − 80 °C until analysis. Glucose (Roche) concentrations were determined enzymatically. Insulin concentrations were determined using a RIA (Linco Research). Fasting blood lipid profiles were measured as follows: NEFA using the Wako Nefa C test kit; TAG with correction for free glycerol (Sigma Diagnostics); total cholesterol (Roche) using the oxidase phenol 4-aminoantipyrine peroxidase method. HDL-cholesterol concentrations were measured using the precipitation method, while LDL-cholesterol concentrations were determined using the Friedewald equation( Reference Friedewald, Levy and Fredrickson 33 ).
Statistical analysis
Data are presented as means and standard deviations. Statistical comparison of the subjects' baseline characteristics, energy intake and macronutrient composition of the diet consumed between the ethnic groups was made using an independent-samples t test. Repeated-measures ANOVA were carried out to assess within-group and between-group differences in parameters before and after overfeeding with the high-fat diet as well as the interaction between ethnicity and diet. The SPSS program version 16 (SPSS) was used for statistical analysis, and the statistical significance was set at P< 0·05.
Results
Subject characteristics
South Asian subjects were of Indian (n 8) and Pakistani (n 2) origins. White subjects were of Dutch (n 3), German (n 2), French (n 1), British (n 1), Danish (n 1), Polish (n 1) and Icelander (n 1) origins. For South Asian subjects, measurements were carried out within 3 years (n 5) and within 1 year (n 5) of their stay in The Netherlands. Subject characteristics are given in Table 1. South Asian subjects were slightly older than white subjects. South Asian subjects had a significantly lower BMI than white subjects (23·3 (sd 3·0) v. 27·0 (sd 4·2) kg/m2; P= 0·04), but there was no difference in body fat percentage (25·5 (sd 6·4) and 23·7 (sd 7·5) % for South Asian and white subjects, respectively; P =0·58). The FFM in South Asian subjects was lower than that in white subjects (P= 0·001). White subjects had significantly higher waist circumference and hip circumference than South Asian subjects, resulting in no difference being observed in the waist:hip ratio between the groups (P= 0·31). Cardiorespiratory fitness, defined as VO2max corrected for FFM, did not differ between the ethnic groups (P= 0·15), and there was no difference in daily physical activity counts (P= 0·27). Additionally, in the South Asian group, of the ten subjects, a family history of diabetes, dyslipidaemia and CVD was found in four, five and four subjects, respectively. In the white group, of the ten subjects, a family history of diabetes, dyslipidaemia and CVD was found in three, five and five subjects, respectively.
Energy intake and macronutrient composition during the dietary intervention period
Compliance with the dietary intervention is summarised in Table 2. White subjects had a significantly higher energy requirement due to a higher FFM and therefore had a significantly higher energy intake. During the overfeeding period, South Asian subjects consumed 143 (sd 12) % of their energy requirement, whereas white subjects consumed 152 (sd16) % of their energy requirement (P= 0·16). There was no difference in the macronutrient composition of the diet consumed between South Asian and white subjects. The percentage of fat intake (as % energy intake) during the balance diet period was 29 (sd 1·0) and 28·8 (sd 0·5) % (P= 0·33) for South Asian and white subjects, respectively. The percentage of fat intake during the overfeeding period was 59·3 (sd 1·3) and 58·8 (sd 2·3) % (P= 0·53) for South Asian and white subjects, respectively. There was no difference in the fatty acid composition of the diet consumed between South Asian and white subjects (P= 0·49).
Table 2 Energy intake and macronutrient composition during the weight-maintenance and dietary intervention periods (Mean values and standard deviations)
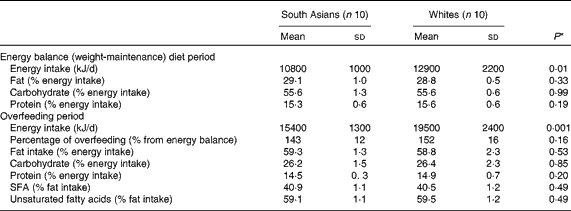
* Differences between the groups were assessed using an independent t test.
Plasma parameters
Plasma parameters are given in Table 3. Short-term overfeeding with the high-fat diet decreased fasting TAG concentrations (P= 0·008), with no difference being observed between the ethnic groups (P= 0·24), and increased HDL-cholesterol concentrations (P= 0·001), which tended to be higher in white subjects (P= 0·06). There was a significant interaction between diet and ethnicity with regard to the changes in total cholesterol (P= 0·01) and LDL-cholesterol (P= 0·007) concentrations, which trended towards a larger increase in South Asian subjects than in white subjects, whereas fasting NEFA concentrations remained unaffected.
Table 3 Plasma parameters* (Mean values and standard deviations)

OHFD, overfeeding with a high-fat diet; chol, cholesterol; HOMA, homeostasis model assessment; OGIS, oral glucose insulin sensitivity.
* Plasma parameters were determined in a fasting condition, except for the OGIS index, which was derived during the 2 h oral glucose tolerance test (OGTT).
† Differences in the changes in parameters within the groups (P diet) and between the groups (P ethnicity) and the interaction between diet and ethnicity were assessed using repeated-measures ANOVA.
‡ For the OGIS index (glucose clearance, ml/min per m2 body surface), data were based on seven South Asians and seven whites, matched for body fat percentage (25·9 (sd 7·1) and 24·4 (sd 8·4) %, P= 0·72, for South Asians and whites respectively), who had completed the OGTT.
There was no effect of overfeeding with the high-fat diet on fasting glucose and fasting insulin concentrations and hence there were no differences in the homeostasis model assessment index before and after overfeeding with the high-fat diet (P= 0·94) and no differences between the ethnic groups (P= 0·84). Overfeeding with the high-fat diet decreased insulin sensitivity (P= 0·004), expressed as the oral glucose insulin sensitivity index, which was calculated from glucose clearance in 2 h OGTT (ml/min) per m2 body surface. There was no difference in the changes in glucose clearance between the ethnic groups (P= 0·18). Fig. 1(a) and (b) shows that the glucose response during the 2 h OGTT was not affected by the diet (P= 0·16) and did not differ between the ethnic groups (P= 0·6), whereas the insulin response (Fig. 1(c) and (d)) increased significantly with overfeeding (P= 0·046), but no difference was observed between the ethnic groups (P= 0·23).

Fig. 1 Glucose (a) and insulin (c) responses during overfeeding with a high-fat diet (OHFD) in South Asians (before, ○; after, ●) and whites (before, □; after, ■) and the corresponding AUC of glucose (b) and insulin (d) during 2 h oral glucose tolerance test (OGTT) (□, before OHFD; ■, after OHFD). Values are means and standard deviations represented by vertical bars. Differences within and between the groups after the dietary intervention were assessed using repeated-measures ANOVA. * Mean value obtained after OHFD was significantly different from that obtained before OHFD for South Asian subjects. † Mean value obtained after OHFD was significantly different from that obtained before OHFD for white subjects. Data were available from fourteen subjects, seven subjects in each group matched for body fat (25·9 (sd 7·1) % and 24·4 (sd 8·4) % for South Asian and white subjects, respectively; P= 0·72), who had completed the OGTT: AUC glucose – P diet= 0·16, P ethnicity =0·60, and P diet × ethnicity= 0·53; AUC insulin – P diet= 0·046, P ethnicity= 0·23, and P diet × ethnicity= 0·53.
Discussion
In the present study, two ethnic groups were matched for body fat percentage, confirming the evidence that South Asian men have a lower BMI for the same body fat percentage when compared with white men. Despite a similar body fat percentage, South Asian men had a more adverse lipid profile, i.e. a larger increase in total cholesterol and LDL-cholesterol concentrations as a response to overfeeding with the high-fat diet, whereas glucose clearance (insulin sensitivity) decreased similarly in both ethnic groups.
Cross-sectional studies comparing South Asians and whites have consistently reported an unfavourable metabolic profile such as dyslipidaemia in South Asians when matched or adjusted for BMI, sex and age( Reference Forouhi, Jenkinson and Thomas 34 – Reference Chandalia, Lin and Seenivasan 36 ), which is expected, given that the South Asians have a higher body fat percentage at the same BMI than whites. In the present study, subjects were matched for body fat percentage, and the baseline metabolic profile measured before the overfeeding period was comparable between the ethnic groups, except for the HDL-cholesterol:total cholesterol ratio, which tended to be lower in South Asian subjects. Another study has reported comparable fasting TAG, total cholesterol, HDL-cholesterol and LDL-cholesterol concentrations but higher plasma NEFA concentrations in South Asian Indians compared with body fat percentage-matched white men( Reference Abate, Chandalia and Snell 37 ).
In the present study, fasting plasma NEFA concentrations were unaffected by overfeeding with the high-fat diet, and there was no difference between the ethnic groups. In the fasting state, lipolysis is activated and fatty acids released by the action of hormone-sensitive lipase are directed primarily into the venous plasma( Reference Frayn, Shadid and Hamlani 38 ). In the fed state, lipoprotein lipase (LPL) is activated and the fatty acids released from circulating TAG are directed into the tissue for esterification and storage in white adipose tissue( Reference Frayn, Shadid and Hamlani 38 ). In the overfeeding condition, the postprandial (fed) state may be slightly extended as a result of extended meal ingestion; the release of NEFA from subcutaneous fat presumably declines due to the prolonged presence of high insulin concentrations. However, it is a common observation either during infusion of TAG emulsions or after ingestion of high-fat meals( Reference Karpe, Olivecrona and Walldius 39 ) that the plasma NEFA concentration is relatively maintained, despite an elevation in insulin concentrations( Reference Frayn, Shadid and Hamlani 38 ). In addition, after the consumption of a high-fat meal, both the inward flux of fatty acids from the plasma into adipose tissue and the release of LPL-derived NEFA into the venous plasma are greater than those after the consumption of a lower-fat mixed meal( Reference Frayn, Coppack and Fielding 40 ). LPL appears to operate continuously, generating a pool of fatty acids, of which some are always released into the venous plasma, while a proportion are diverted into the tissue for esterification and storage in the postprandial state( Reference Frayn, Shadid and Hamlani 38 ).
We observed a decrease in fasting TAG concentrations after short-term overfeeding with the high-fat diet, but the decrease did not differ between the ethnic groups. Diets that replace fat with carbohydrate isoenergetically have been consistently observed to worsen certain elements of the plasma lipid profile( Reference Hellerstein 41 ), particularly TAG( Reference Parks and Hellerstein 42 , Reference Merchant, Anand and Kelemen 43 ). In a multiethnic population study in Canada, Merchant et al. ( Reference Merchant, Anand and Kelemen 43 ) reported that South Asian Indians consumed large amounts of carbohydrate followed by Europeans, Aboriginals and Chinese. In that study, higher carbohydrate intake was found to be associated with higher fasting TAG concentrations and those with the highest percentile of carbohydrate intake were found to have the highest fasting TAG concentrations. In the present study, reduction in fasting plasma TAG concentrations may be attributed to a lower proportion of carbohydrate in the high-fat diet (25 % energy from carbohydrate in the overfeeding period equals 37·5 % carbohydrate compared with 55 % carbohydrate in the balance diet period), as also reported by others( Reference Sharman, Gomez and Kraemer 44 – Reference Hu, Mills and Yao 46 ). High-carbohydrate diets are hypothesised to increase TAG concentrations by inducing fatty acid production in the liver and inhibiting the action of LPL through increased apo CIII production, particularly in the presence of insulin resistance( Reference Grundy, Abate and Chandalia 47 ). Higher fat intakes may increase circulating chylomicron concentrations, but do not affect LPL and the clearance of circulating TAG and hence do not affect TAG concentrations( Reference Grundy 48 ).
A significant interaction between diet and ethnicity was found with regard to the changes in total cholesterol and LDL-cholesterol concentrations, which trended towards a larger increase in South Asian subjects than in white subjects. In addition, overfeeding with the high-fat diet increased HDL-cholesterol concentrations, but the increase tended to be higher in white subjects than in South Asian subjects. Furthermore, the changes in HDL-cholesterol:total cholesterol ratio and LDL-cholesterol:total cholesterol ratio were different between the ethnic groups. Thus, overfeeding with the high-fat diet clearly resulted in a more unfavourable lipoprotein profile in South Asian subjects. The effect of a high-fat diet on lipoproteins varies considerably depending on the type of fat consumed, i.e. fatty acid composition of the diet( Reference Samaha 49 ). SFA increase the concentrations of both total cholesterol and LDL-cholesterol, while unsaturated fatty acids lower the concentrations of total cholesterol and LDL-cholesterol( Reference Samaha 49 ). Both SFA and unsaturated fatty acids increase HDL-cholesterol concentrations, although this increase is greater with SFA( Reference Samaha 49 ). A recent review by Hooper et al. ( Reference Hooper, Summerbell and Thompson 50 ) has reported that reduced and modified fat intakes decrease total cholesterol and LDL-cholesterol concentrations, with no effect on HDL-cholesterol concentrations. In the present study, during the overfeeding period, the subjects were given a diet containing 60 % energy from fat, of which 40 % of the fat consumed was from SFA and 60 % was from unsaturated fatty acids. No differences in the adherence to the diet and in fat intake and fatty acid composition were observed between the ethnic groups. Given that the subjects in the present study had a comparable range of body fat percentages and comparable metabolic profiles at baseline except for HDL-cholesterol, which tended to be present at lower concentrations in South Asian subjects, the unfavourable lipoprotein profile in South Asian subjects as a response to the high-fat diet needs to be investigated further. This may possibly be due to differences in the rate of LDL clearance and production( Reference Sniderman, De Graaf and Couture 51 ). Furthermore, data obtained from thirty-one study cohorts of twelve countries revealed that South Asian Indian men and women have the lowest HDL-cholesterol concentrations compared with Central and Northern Europeans, Japanese and Chinese across the glucose level categories( Reference Zhang, Qiao and Tuomilehto 52 ).
Overfeeding( Reference Samocha-Bonet, Campbell and Viardot 53 , Reference Samocha-Bonet, Campbell and Mori 54 ) and a high-fat diet( Reference Bachmann, Dahl and Brechtel 55 ) have been reported to be associated with disturbances in glucose metabolism and insulin sensitivity. As expected, short-term overfeeding with the high-fat diet increased the insulin response (AUC insulin) during the 2 h OGTT. We also calculated the glucose clearance (ml/min) per m2 body surface, which allowed us to correct for the differences in body height between South Asian and white subjects (indirectly FFM). The result remains that short-term overfeeding with the high-fat diet decreased glucose clearance (ml/min) per m2 body surface, with no difference being observed between the ethnic groups. In response to a meal, insulin suppresses lipolysis and the release of NEFA into the circulation and stimulates skeletal muscle glucose uptake( Reference Magkos, Fabbrini and Conte 56 ). In vivo, elevation of NEFA concentrations induced by intralipid plus heparin infusion has consistently been shown to reduce glucose oxidation and glucose uptake in the skeletal muscle( Reference Lewis, Carpentier and Adeli 57 ). Others have suggested the importance of CD36 in NEFA transport and ectopic TAG accumulation; incomplete oxidation generates intermediates that can impair insulin signalling( Reference Fabbrini, Magkos and Mohammed 58 ).
A limitation of the present study is the low number of subjects, which may not reflect the general population of South Asians and whites. Additionally, the short-term dietary intervention may reflect an early response to overfeeding with a high-fat diet. Although an early response may precede the metabolic complications in the long term, it cannot be simply extrapolated to assess the response in a longer term. However, this is a well-controlled dietary intervention study and, to our knowledge, no such studies comparing two ethnic groups have been carried out.
In summary, we observed a more adverse lipid profile in South Asian men compared with white men as a response to short-term overfeeding with a high-fat diet, whereas insulin sensitivity decreased similarly. Although the ethnic groups differed with respect to age in the present study, a regression analysis to assess the predictors of baseline metabolic profiles by including age, fat mass percentage and ethnicity revealed that age did not have a significant effect in the groups studied, presumably because all of them were young and the age difference between the groups was small. The differences in the lipid profile may be associated with differences in body fat distribution towards a more central fat deposition( Reference Misra and Vikram 59 ) in South Asians. Goel et al. ( Reference Goel, Misra and Vikram 60 ) reported that subcutaneous abdominal fat is associated with the metabolic syndrome independent of visceral fat in Asian Indians. These results may partly explain the high prevalence of the metabolic syndrome in South Asians, which is triggered by lifestyle changes, i.e. increased consumption of high-energy-density foods.
Acknowledgements
The authors thank Jos Stegen, Loek Wouters and Paul Schoffelen for their technical assistance and help with the analyses. They also deeply appreciate and thank all subjects who participated in the study.
The present study was partly funded by a scholarship from the Directorate General of Higher Education (DGHE), the Ministry of Education and Culture of the Republic of Indonesia, to S. N. W. The Ministry of Education and Culture of the Republic of Indonesia had no role in the design and analysis of the study and in the writing of this article.
The authors' contributions are as follows: S. N. W. conducted the research, carried out the data analysis and wrote the manuscript under the supervision of K. R. W. and G. P., who designed the study, interpreted the data and reviewed the manuscript.
None of the authors has any conflicts of interest to declare.






