Obesity is a global health challenge linked to diabetes and CVD. Overweight patients have followed a wide variety of therapeutic methods, of which dietary restriction involving fat is considered most important(Reference Matsuo and Takeuchi1). However, more and more lipids found in daily diets consist of long-chain fatty acids (LCFA), which are preferentially stored as body fat. In contrast, medium-chain fatty acids (MCFA), composed of C8 (caprylic acid) and C10 (capric acid), are directly transported to the liver without resynthesis of TAG and subjected predominantly to β-oxidation, accumulating poorly as adipose tissues(Reference Takeuchi, Sekine and Seto2, Reference Jeukendrup and Aldred3). Research on both animals(Reference Nagata, Kasai and Watanabe4) and human subjects(Reference St-Onge, Bourque and Jones5) has demonstrated that consumption of MCFA may be useful in the dietary treatment or prevention of weight gain associated with the development of obesity(Reference Aoyama, Nosaka and Kasai6). Nevertheless, human subjects could not consume high-MCFA diets on a long-term basis, due to the lack of palatability and because of adverse gastrointestinal and other symptoms (nausea and osmotic diarrhoea)(Reference Marten, Pfeuffer and Schrezenmeir7).
Some approaches have been taken to solve the side effects of MCFA, including formulating of a new type of oil composed of medium- and long-chain TAG(Reference Zhang, Liu and Wang8), and development of water-in-oil MCFA microemulsions(Reference Constantinides, Welzel and Ellens9). However, these approaches are somewhat ineffective on normal or obese hypertriacylglycerolaemic subjects, or display limited biocompatibility. Nanoliposomes (NL), a self-assembling colloidal particle with sizes 50–150 nm, in which a lipid bilayer encapsulates a fraction of the surrounding aqueous medium(Reference Lasic and Papahadjopoulos10), are a cell-resembled and biocompatible nano-encapsulation system. They can provide protection and control the release of the entrapped ingredient(Reference Kuldarni, Greiser and O'Brien11). To date, their applications have mainly focused on gene delivery(Reference Kaasgaard and Andresen12), cancer therapy(Reference Tan, Choong and Dass13), and protein and peptides carriers(Reference Smith, Jaime-Fonseca and Grover14). Little use of liposomes in suppressing body fat accumulation or body-weight control has been reported.
There are several traditional methods to produce liposomes, such as thin-layer dispersion, freeze-dried rehydration, ethanol injection and ultrasonication evaporation methods. Nevertheless, these methods are lack of continuous production process and need extensive use of organic solvents. Dynamic high-pressure microfluidisation (DHPM) is a technology employing the combined forces of high-velocity impact, high-frequency vibration, instantaneous pressure drop, intense shear, cavitation and ultra-high pressures up to 200 MPa(Reference Liu, Liu and Xie15). This method can produce toxic solvent-free liposomes with reduced particle size. There have been limited studies on the large-scale preparation of NL from edible lipids by using DHPM from the viewpoint of developing the formulations into an efficient carrier of nutritional materials. In our previous research(Reference Liu, Yang and Liu16), thin-layer dispersion, ethanol injection and reverse-phase evaporation were used to prepare MCFA liposomes, and a new method of thin-layer dispersion combined with DHPM was developed to efficiently prepare MCFA NL with easy-energy-supply property to mice(Reference Liu, Liu and Liu17).
In the present study, apart from the thin-layer-DHPM method, the DHPM combined with freeze–thawing method was used for the first time to produce MCFA NL, because the freeze–thawing method may lead to the formation of unilamellar liposomes more readily(Reference Mayer, Hope and Cullis18). In addition, the particle size and size distribution, zeta potential, permanent stability, entrapment efficiency (EE) and drug loading (DL) of these two NL were investigated. Furthermore, the study focused on the effects of MCFA NL in diets on body fat accumulation in growing mice. The preclinical studies including short-term and long-term experiments were carried out to estimate the body fat reduction as well as its correlative parameters (body weight, food and energy consumption, liver weight, total cholesterol (TCH) and TAG) in mice. In particular, NL without MCFA (blank NL), MCFA non-entrapped in NL (MCFA) and LCFA were used as comparisons.
Materials and methods
Materials
MCFA were kindly provided by a USA company (UPMC, Pittsburgh, PA, USA). Rapeseed oil (as a source of LCFA) was purchased commercially (Wal-mart Supermarket, Nanchang, China). Soyabean phosphatidylcholine was provided by Merya's Lecithin Company Limited (Beijing, China). Cholesterol was obtained from Tianjin Damao Chemical Reagent Company Limited (Tianjin, China). TAG and TCH kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals and reagents used were of analytical purity or higher quality, and were obtained commercially.
Preparation of medium-chain fatty acid nanoliposomes
Medium-chain fatty acid nanoliposome-1 prepared by the thin-layer dispersion-dynamic high-pressure microfluidisation method
First, the thin-layer dispersion method developed by Bangham's technique(Reference Bangham, Standish and Watkins19) was applied to prepare crude MCFA liposome suspension. Briefly, the mixture of soyabean phosphatidylcholine, cholesterol, Tween-80, MCFA and vitamin E (6:1:1·8:1·125:0·12, mass ratio) were dissolved in absolute alcohol and then evaporated to a thin film in a rotary evaporator at 40°C. After further drying the lipid film for 6 h under vacuum, the dried lipid film was rehydrated with PBS (pH 7·4) to make the crude MCFA liposome suspension.
Second, to obtain MCFA NL, the crude suspension was treated with DHPM at ambient temperature. The DHPM treatment was carried out continuously or recycled with a microfluidiser (M-7125; Microfluidic Corporation, Newton, MS, USA), which worked on the principle of dividing a pressurised stream into two parts, passing each part through a fine orifice, and having an impact on or colliding against each other inside the interaction chamber of microfluidiser(Reference Liu, Liu and Liu20). MCFA NL (NL-1), with the final concentration up to 8 %, were treated for four cycles at a pressure of 120 MPa. A blank NL sample (blank NL) was obtained by the same procedure.
Medium-chain fatty acid nanoliposome-2 prepared by the dynamic high-pressure microfluidisation combined with freeze–thawing method
The freeze–thawing procedure was based on Mayer et al. (Reference Mayer, Hope and Cullis18) with a slight modification. Briefly, NL-2 was prepared generally the same way as NL-1, with a slight modification with addition of 3 % maltose (w/v) as the cryoprotectant. The freeze–thawing procedures were as follows: the samples were frozen in an ultra-cold freezer at − 80°C for 30 min and then heated in a water-bath at 40°C for 10 min, respectively. The process was repeated for three cycles.
Characterisation of medium-chain fatty acid nanoliposomes
Average size and zeta potential
The mean diameter and size distribution (polydispersity index) of NL-1 and NL-2 were determined by the dynamic laser light scattering technique at 25°C with an angle of 90° using a Nicomp 380 ZLS (Santa Barbara, CA, USA). Samples were diluted with PBS in order to obtain a satisfactory signal in the detector. The zeta potential, which is an indirect measurement of the liposome surface charge, was measured using Nicomp 380 ZLS. All the samples were measured at least three times to obtain the average.
Determination of entrapment efficiency and drug loading
EE is defined as the percentage of MCFA entrapped in NL relative to the total amount of MCFA initially added in the mixture, while DL is the mass ratio of MCFA entrapped in NL relative to the total weight of lipids. EE and DL were analysed using GC (Agilent 6890 Series GC System; Agilent Technologies, Santa Clara, CA, USA) and the measurement method were described by Liu et al. (Reference Liu, Liu and Liu17).
Stability of medium-chain fatty acid nanoliposomes
NL-1 and NL-2 were stored at 4°C for 180 d in a sealed condition. The average diameter and drug EE were determined once every month.
Preclinical experiments for body fat reduction and correlative parameters
Animals and diet
Male Kunming mice were obtained from Animal Experimentation of Nanchang University (Nanchang, China) and housed for at least 1 week in polycarbonate cages to be adapted to the new environment before the experiments. The study followed the Guidelines for Animal Care and Use by National Institutes of Health, and all experimental procedures were approved by the Ethics Committee for the Use of Experimental Animals. Water and standard mouse food (formula: maize, puffing bean, soyabean, rice starch, trace element, vitamin, lysine, carrier; digestive energy: 16 067 kJ/kg, metabolic energy: 11 987 kJ/kg) were accessed freely by the mice under the following environmental conditions: 23 ± 1°C, 60 (sd 5) % relative humidity, and a 12 h light–12 h dark cycle.
Short-term experiment
Mice were randomly separated into six groups: the control group (normal saline group), LCFA group, MCFA group, blank NL group, NL-1 group and NL-2 group, with eight mice for each group equally based on body weight before the test. The LCFA, MCFA, NL-1 and NL-2 groups were administered at concentrations of 1000 mg/kg body weight, which were calculated according to the doses of fatty acids in the samples. The control and blank NL groups were given the same volume suspension as the other four groups. The mice were observed for 4 h after administration, and once a day for the following 14 d. Body weights and food intake were obtained on the day (1, 2, 3, 7 and 14) of test administration.
Long-term experiment
After maintaining for at least a week, the mice were divided into six groups as described previously. The percentage of fatty acids contained in the intragastric administration was calculated to be approximately equal to a daily consumption at 500 mg/kg per d LCFA or MCFA, respectively. The control and blank NL groups were given the same volume suspension as the other four groups.
Following administration of the study diet for 5 weeks, weight gain, food and energy intake were obtained. The mice were anaesthetised with diethyl ether and euthanised via decapitation. Blood was collected and preserved for at least 20 min at 37°C before centrifugation for 10 min at a speed of 3000 g to separate the serum. In the serum, the concentrations of TAG and TCH were determined according to the recommended procedures provided by the kits. The fat mass was expressed as surrounding the epididymis, the kidneys and the mesenteric adipose tissue. Additionally, liver weight was determined for each mouse.
Statistical analysis
Comparison of means was conducted using a one-way ANOVA with the Bonferroni-adjusted post hoc test. The values are expressed as means and standard deviations. A P value < 0·05 was considered statistically significant and P < 0·01 as highly significant.
Results
Physical–chemical properties of medium-chain fatty acid nanoliposomes
Both of the average diameter and size distribution were affected by the DHPM and freeze–thawing treatment. As shown in Fig. 1, the average diameter of MCFA NL treated with DHPM only (NL-1) was 77·6 (sd 4·3) nm, with the polydispersion index 0·193 (sd 0·016), while NL after freeze–thawing (NL-2) showed a larger mean diameter 120·6 (sd 10·1) nm and a narrower polydispersion index 0·180 (sd 0·021).

Fig. 1 Medium-chain fatty acid nanoliposome-1 (NL-1) and NL-2: average diameter and size distribution.
MCFA NL of varying treatments yielded a slightly different surface potential. The zeta potential of NL-1 and NL-2 were about − 40·8 (sd 1·7) and − 34·5 (sd 2·1) mV, respectively.
To produce liposomes containing MCFA with high EE, the critical factor is the preparation process. In the present study, the novel modified thin-film dispersion-DHPM and DHPM-freeze–thawing methods were used to prepare MCFA NL. The higher EE and DL values were observed for NL-1, with 73·3 (sd 16·1) and 9·8 (1·2) %, respectively. However, after further treatment with freeze–thawing, NL-2 exhibited lower EE and DL, which were 55·2 (sd 12·2) and 7·1 (sd 0·8) %, respectively.
Results in Fig. 2 showed that the size of NL-1 was more stable than NL-2, because it only increased slightly after 120 d storage. After 180 d, the former was still smaller than the latter. In addition, the two samples showed a similar tendency in the EE decrease.

Fig. 2 Physical stability of medium-chain fatty acid nanoliposome-1 (■) and -2 (○). Values are means, with standard deviations represented by vertical bars. EE, entrapment efficiency.
Short-term preclinical study in mice
All mice that were administered short-term doses of LCFA, MCFA, blank NL, NL-1 and NL-2 at 1000 mg/kg survived, and no abnormal clinical signs or gross pathological abnormalities were observed in any tissue or organ all over the mouse body. The body weight of the MCFA (30·6 (sd 5·1) g), NL-1 (31·5 (sd 4·7) g) and NL-2 (29·4 (sd 3·9) g) groups were lower than both of the control (33·5 (sd 3·5) g) and LCFA (33·8 (sd 3·1) g) groups (Table 1). However, the body-weight gain of all groups has no significant differences compared with the control group throughout the 2-week experiment. Furthermore, Fig. 3 showed the different food consumption of mice. Compared with the control group (711·64 (sd 47·4) g), the food consumption of the LCFA (795·58 (sd 34·00) g, P = 0·008), MCFA (606·48 (sd 44·7) g, P = 0·001) and blank (591·68 (sd 42·2) g, P = 0·001) groups (at approximately 1000 mg/kg per d) showed a remarkable difference. In addition, the NL-1 (698·66 (sd 52·1) g, P = 0·028) and NL-2 (747·58 (sd 48·2) g, P = 0·039) groups showed high significant increases in food intake in contrast to the MCFA group, and there was no difference between the control and NL-1, NL-2 groups.
Table 1 Average changes in weight gain in mice short-term administered long-chain fatty acids (LCFA), medium-chain fatty acids (MCFA), blank nanoliposomes (NL), MCFA NL-1 and NL-2
(Mean values and standard deviations, n 8)
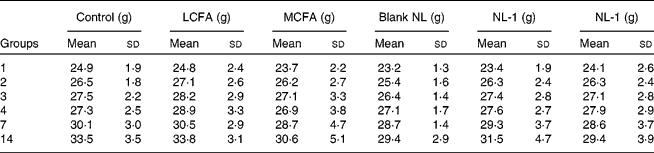

Fig. 3 Effect of long-chain fatty acids (LCFA), medium-chain fatty acids (MCFA), blank nanoliposomes (NL), MCFA NL-1 and NL-2 on food intake in the short-term preclinical study of mice. Values are means, with standard deviations represented by vertical bars (n 8). * Mean values were highly significantly different when compared with the control group (P < 0·01). † Mean values were highly significantly different when compared with the MCFA group (P < 0·01).
Long-term preclinical study in mice
A short-term of consumption of MCFA NL might be the reason why no significant reduction in body weight was found. However, the effect of smaller amount of MCFA NL was unknown. So, in this part, a longer-term nutrition research was performed (Fig. 4 and Table 2). Compared with the control group (weight gain, 15·9 (sd 5·5) g; food intake, 251·2 (sd 10·4) g; energy intake, 3015·5 (sd 124·7) kJ; liver weight, 1·86 (sd 0·23) g; body fat weight, 2·11 (sd 0·82) g; TCH, 2·99 (sd 0·48) mmol/l; TAG, 0·94 (sd 0·37) mmol/l, respectively), the MCFA group significantly induced body-weight loss (11·2 (sd 3·4) g, P = 0·036), reduced food (225·1 (sd 4·9) g, P = 0·048) and energy (2701·9 (sd 59·0) kJ, P = 0·044) consumption, suppressed body fat accumulation (1·46 (sd 0·49) g, P = 0·026), decreased TCH (2·42 (sd 0·43) mmol/l, P = 0·029) and TAG (0·57 (sd 0·20) mmol/l, P = 0·031) levels, although no liver weight (1·58 (sd 0·33) g) difference was observed. In addition, the body fat of the LCFA group (2·79 (sd 0·33) g) was observed to be remarkably heavier than that of the control group (P = 0·038), while the adipose tissue weight of the NL-1 (1·54 (sd 0·30) g, P = 0·039) and NL-2 (1·58 (sd 0·69) g, P = 0·041) groups sharply decreased. In addition, the TCH of the NL-1 (2·33 (sd 0·44) mmol/l, P = 0·021) and NL-2 (2·29 (sd 0·38) mmol/l, P = 0·015) groups were significantly lower than those of the control group, and the TAG of the NL-1 (2·29 (sd 0·38) mmol/l, P = 0·045) group also showed a large difference. Nevertheless, no significant increase or reduction in the other parameters was detected between the LCFA, blank NL, NL-1 and NL-2 groups and the control group.

Fig. 4 Effect of long-chain fatty acids (LCFA), medium-chain fatty acids (MCFA), blank nanoliposomes (NL), MCFA NL-1 and NL-2 on TAG and total cholesterol (TCH) in the long-term preclinical study of mice. Values are means, with standard deviations represented by vertical bars (n 8). * Mean values were significantly different when compared with the control group (P < 0·05). Mean values were significantly different when compared with the MCFA group: † P < 0·05, †† P < 0·01.
Table 2 Effect of long-term administered long-chain fatty acids (LCFA), medium-chain fatty acids (MCFA), blank nanoliposomes (NL), MCFA NL-1 and NL-2 on the weight, food and energy intake, body fat and liver weight of mice
(Mean values and standard deviations, n 8)

* Mean values were significantly different from the control group (P < 0·05).
† Mean values were significantly different from the MCFA group (P < 0·05).
On the other hand, MCFA NL were compared with the MCFA group (Fig. 4 and Table 2). We found that the food and energy intakes of the NL-1 (250·6 (sd 8·6) g, P = 0·047 and 718·6 (sd 24·7) g, P = 0·026, respectively) and NL-2 (256·2 (sd 5·0) g, P = 0·039 and 734·7 (sd 14·3) g, P = 0·035, respectively) groups were remarkably different from the MCFA group. However, the weight gain (12·6 (sd 3·2) and 12·9 (sd 2·5) g, respectively), liver weight (1·65 (sd 0·30) and 1·60 (sd 0·30) g, respectively), body fat weight, TCH and TAG levels of the NL-1 and NL-2 groups were similar to the MCFA group. In addition, the LCFA group showed a significant increase in TCH (3·11 (sd 0·30) mmol/l, P = 0·008) and TAG (0·96 (sd 0·31) mmol/l, P = 0·014) in contrast to the MCFA group. There was no significant difference between the NL-1 and NL-2 groups in all indices.
Discussion
MCFA NL, which were prepared by the DHPM and DHPM-freeze–thawing methods, were used in the present study to evaluate the body fat suppression property in mice. No significant difference in weight and fat gain, TCH and TAG was found between the MCFA NL and MCFA groups after the short-term and long-term administration.
The data revealed that the freeze–thawing procedure gave rise to a larger average size and a better distribution. Sriwongsitanont & Ueno(Reference Sriwongsitanont and Ueno21) stated that mean diameters of the liposomes, which had originally small size, increased with the increase in the number of freeze–thawing cycles. Therefore, it might be the repetitive freezing and thawing procedures that might have led to reconstitution of the vesicles, formation of larger particles and homogenisation of lipid composition(Reference Gruner, Lenk and Janoff22). It is well known that small liposomes might release nutrients readily when attacked by blood components(Reference Nagayasu, Uchiyama and Kiwada23), and a narrow particle size distribution exhibits high absorption efficiency(Reference Ding, Zhang and Hayat24). Accordingly, compared with NL-2, NL-1 released MCFA more quickly while exhibiting a slightly lower absorption efficiency.
Generally, a higher zeta potential (the absolute value higher than 30 mV) might contribute to a higher physical stability of the nanoparticles by reducing the rate of aggregation and fusion, due to the repulsion between particles reducing their aggregation(Reference DeLuca, Kaszuba and Mattison25, Reference Crommelin26). Thus, the results suggested that NL-1 treated with DHPM was more stable than NL-2.
As the hydrophobic core of the carrier, liposome is the reservoir of the hydrophobic substance(Reference Liu and Park27), such as MCFA. The EE and DL of MCFA might be affected by the diameter or specific surface area of the liposomes. Although some researchers have suggested that the EE of multilamellar vesicles could be enhanced due to the freeze–thawing procedure(Reference Ohsawa, Miura and Harada28), there is still a report that has indicated that using DHPM for the production of small unilamellar vesicles, high EE can be obtained(Reference Masson29). Furthermore, it has been found that the small vesicles prepared by freezing and thawing could cause themselves to rupture(Reference Labbe, Crowe and Crowe30), resulting in the disruption of NL-1 ( < 80 nm) as well as cell membranes during the following repeated treatment. Therefore, the EE and LD of NL-2 were considerably lower than those of NL-1.
Freeze–thawing is often considered the major cause of the destabilisation of the plasma membrane during cell cryopreservation(Reference Labbe, Crowe and Crowe30). Liposomes exhibit a similar structure to the cell and after reducing to a small and presumably uni- or paucilamellar state, they would rupture by the freeze–thawing treatment(Reference MacDonald, Jones and Qiu31), with lower stability during storage.
Many studies(Reference Zhang, Liu and Wang8, Reference Geliebter, Torbay and Bracco32) have reported that body weight of animals fed MCFA was lower than that of animals fed LCFA diets. However, the effect of dietary MCFA NL on body weight is unclear. Moreover, whether MCFA NL would induce satiety of the animals has not been examined. In the present short-term study, we have shown that body weight was slightly lower in mice fed the MCFA, NL-1 and NL-2 diets, though no significant difference was observed. The findings in the present experiment are consistent with the observations by Max et al. (Reference Max, Bach and Pallier33) and Yost & Eckel(Reference Yost and Eckel34), who demonstrated that ingestion of a large amount of MCFA (1000 mg/kg as in the present study) might fail for weight loss. On the other hand, the two NL groups seemed to have overcome the nausea and gastrointestinal discomfort induced by MCFA, for the food consumption did not decrease sharply when compared with the control group. However, because of the repellent odour and taste, MCFA had a dramatic influence on the normal food intake of the mice. It has been suggested that medium-chain TAG could enhance satiety and alter energy intake in rats(Reference Lasekan, Rivera and Hironen35) and human subjects(Reference Krotkiewski36). These results suggested that consumption of MCFA NL has the same effect as MCFA on reducing the body weight of mice, with gastric emptying and non-interference feed intake.
In the present long-term study, the body fat accumulation and body weight of the MCFA group and the two NL groups were lower, which might be related to the mechanism of the fast fat oxidation effect of MCFA. MCFA and LCFA have different metabolic rates, which may account for the difference in the reduction of body weight and body fat weight. MCFA enter the mitochondria of liver cells independently of CoA-carnitine, which is necessary for the transport of LCFA(Reference Papamandjaris, MacDougall and Jones37). This results in the rapid metabolism of MCFA and enhance modulate lipolysis(Reference Jeukendrup and Aldred3). Similar results were documented by Nosaka et al. (Reference Nosaka, Maki and Suzuki38), who found that medium-chain TAG could significantly decrease body fat weights v. long-chain TAG after a 12-week double-blind test.
In animals and human subjects, MCFA may stimulate the secretion of cholecystokinin, and the strong goat-like odour and repellent taste can also be a cause of a lower feed intake(Reference Molimard, Le Quéré and Spinner39). Decuypere & Dierick(Reference Decuypere and Dierick40) developed the MCFA containing TAG to avoid these side effects. However, because of the more prolonged retention time in the stomach and slower absorption rate, lower fat suppression effect of MCFA containing TAG might occur. Our present findings showed that MCFA encapsulated in NL did not differ from MCFA on controlling body weight and its correlated parameters. Meanwhile, the food and energy intake of the NL-1 and NL-2 groups was maintained as normal as the control group. These may attribute to the well encapsulation capacity and rapid absorption ability of NL.
Moreover, in our blood clinical investigation, the TCH and TAG levels were lower in the NL-1 and NL-2 groups than in the control group. Nevertheless, there have been few reports about the decreasing effect of MCFA or related agent on TCH and TAG so far. These researchers stated that intake of MCFA promoted energy expenditure and fat oxidation in both liver and adipose tissue(Reference Asakura, Lottenberg and Neves41, Reference Roynette, Rudkowska and Nakhasi42). However, some results were supported by our present findings. According to Geelen et al. (Reference Geelen, Schoots and Bijleveld43), the concentration of TCH decreased following the feeding of a medium-chain TAG diet. In addition, Xue et al. (Reference Xue, Liu and Wang44) demonstrated that an adequate consumption of medium-chain TAG oil may reduce the blood TAG. The causes of the dropping in TAG concentration were explained as (1) the hepatic secretion of TAG reduced in the form of the VLDL, and/or lipoprotein lipase increasingly cleared TAG(Reference Glisic, Arrigo and Alavantic45) or (2) TAG was removed increasingly without a change in the hepatic secretion of TAG, or (3) the hepatic production and peripheral clearance of TAG were increased(Reference Xue, Liu and Wang44). Collectively, MCFA are rapidly catabolised into ketones and CO2 and are not easily esterified, thus the present findings suggested that MCFA NL in fact decreased TCH and TAG.
Conclusion
In summary, MCFA NL prepared by the thin-layer dispersion-DHPM and DHPM combined with freeze–thawing methods showed good physical–chemical properties. The preclinical study demonstrated for the first time that MCFA NL could suppress body fat accumulation and reduce the concentration of TCH and TAG. More importantly, MCFA NL improved the poor palatability and gastrointestinal upset of MCFA, and maintained the normal appetite of mice. Therefore, MCFA NL could be the potential candidates in the diet for suppressing body fat accumulation.
Acknowledgements
The present study was supported by the National High Technology Research and Development Program of China (863 Program) (no. 2007AA100403), the key project of ‘863’ (no. 2008AA10Z330) and the Objective-Oriented Project of State Key Laboratory of Food Science and Technology, Nanchang University (no. SKLF-MB-201004). W. L. and C.-M. L. developed the original study aims; W.-L. L., H.-J. Z. and K.-M. S. conducted the experiments; S.-B. Y. and J.-H. L. carried out the data analyses; W.-L. L. wrote the manuscript. None of the authors has any conflicts of interest.








