Flaxseed (Linum usitatissimum L.) is the richest source of α-linolenic acid and lignan secoisolariciresinol diglycoside (SDG)( Reference Carter 1 ). Flaxseed has been a focus of interest in the field of functional foods because of its potential health benefits, such as cardiovascular system protection, antioxidant activity and hypoglycaemic effects( Reference Ratnayake, Behrens and Fischer 2 – Reference Prasad 4 ).
However, some researchers have suggested caution when flaxseed is consumed during pregnancy and lactation( Reference Tou, Chen and Thompson 5 , Reference Collins, Sprando and Black 6 ). The risk of developing chronic diseases during adult life may be influenced by exposure to diets and/or bioactive food compounds in early life that may interfere with or act as hormones, which is called metabolic programming( Reference Moura, Santos and Lisboa 7 ).
We have previously demonstrated that maternal intake of flaxseed (25 %) during lactation leads to lower body fat mass, hyperleptinaemia, hypoinsulinaemia, increased insulin sensitivity, increased pituitary expression of leptin receptor, phosphorylated signal transducer and activator of transcription 3, and lower serum T3 concentrations in weaned pups. In adulthood, these animals were programmed for an increased adipocyte area in both visceral and subcutaneous adipose tissues, hyperglycaemia, hyperinsulinaemia, hypoadiponectinaemia and increased insulin resistance, lower serum T4 (thyroxine), type 1 deiodinase (D1) and type 2 deiodinase (D2) activities in the thyroid, higher D2 activity in brown adipose tissue, and increased expression of leptin receptor in the thyroid( Reference Figueiredo, de Moura and Lisboa 8 – Reference Figueiredo, Passos and Trevenzoli 10 ). Probably the majority of these effects were mediated by bioactive compounds present in flaxseed, such as α-linolenic acid and SDG.
As changes in adiposity and glucose homeostasis are dependent on normal adrenal function, we hypothesised that maternal flaxseed supplementation during lactation could affect adrenal function in the offspring later in life. Therefore, the present study aimed to evaluate the effects of maternal whole flaxseed intake during lactation on adrenal function in adult male offspring. We studied the hypothalamic–pituitary–adrenal (HPA) axis by evaluating the protein expression levels of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), corticosterone, glucocorticoid receptor (GR)-α in the hypothalamus, pituitary gland and liver and 11-β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in the liver, which converts 11-dehydrocorticosterone into the active hormone corticosterone in rats to supply local demand( Reference Livingstone, Kenyon and Walker 11 ). Furthermore, we studied catecholamine content in adrenal glands and adrenaline β2 receptor in the liver. As both hormones influence liver metabolism, the contents of cholesterol, TAG and glycogen in the liver were also measured.
Materials and methods
Animal experiment
Wistar rats, aged 3 months old, were maintained in a temperature-controlled room (25 ± 1°C) with a 12 h dark–12 h light cycle. Virgin rats (200–220 g) were mated, and each female was placed in an individual cage with free access to water and food until parturition. Animal use and experimental design were approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro (no. 230/2008).
At birth, sixteen lactating rats were randomly assigned to one of the following experimental groups: (1) control dams (CON), with free access to a diet containing 17 % protein, 52 % carbohydrate, 7 % lipid and 5 % fibre; (2) flaxseed dams (FLAX), with free access to a diet containing 17 % protein (12 % casein and 5 % flaxseed), 54 % carbohydrate, 10 % lipid exclusively from flaxseed and 5 % fibre exclusively from flaxseed (Table 1). We ground the whole flaxseed before adding to the diet. The diets were started at birth, which was defined as day 0 of lactation, and ended at weaning (day 21). After birth, litters were adjusted to six males per dam, as this has been shown to maximise lactation performance( Reference Fishbeck and Rasmussen 12 ).
Table 1 Composition of the control, flaxseed and standard diets
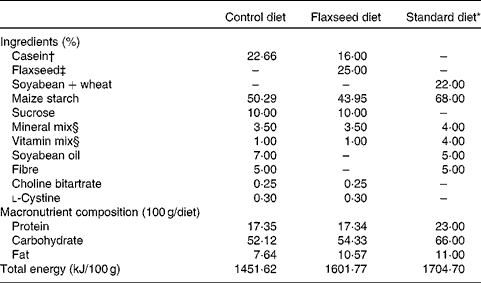
* Standard diet for rats (Nuvilab; Nuvital Nutrientes).
† Commercial casein was acquired from Kauffman Company.
‡ Armazén Produtos Naturais LTDA.
§ Formulated to meet the AIN-93G recommendations.
After weaning, two pups from each dam received a standard laboratory diet (Nuvilab; Nuvital Nutrientes) containing 22 % protein, 66 % carbohydrate and 11 % lipid until they were 180 d old (Table 1). All offspring were killed at 180 d of age with a lethal dose of pentobarbital (0·06 g/kg body weight). Blood was collected by cardiac puncture, and tissues (hypothalamus, pituitary gland, liver and adrenal gland) were stored at − 80°C until analysis.
Evaluation of nutritional status
Body weight and food intake of the offspring were evaluated once every 4 d after weaning until they were 180 d old.
Measurement of serum hormone concentrations
Blood samples were centrifuged to obtain serum, which was individually kept at − 20°C until assay. All measurements were performed in one assay. Serum corticosterone concentration was measured using a specific commercial RIA kit (ICN Biomedicals, Inc.) with an assay sensitivity of 50 ng/ml and an intra-assay CV of 7 %.
Quantification of tissue catecholamine content
Right adrenal glands were homogenised in 10 % acetic acid and centrifuged (1120 g , 5 min), and supernatants were kept frozen for later analysis. Total catecholamines (adrenaline and noradrenaline) were quantified by the trihydroxyindole method( Reference Trevenzoli, Valle and Machado 13 ). Adrenaline was used as the standard. Briefly, 50 μl of the standard/supernatant were mixed with 250 μl of 0·5 m-buffer phosphate (pH 7·0) and 25 μl of 0·5 % potassium ferricyanate, followed by incubation in an ice bath for 20 min. The reaction was stopped with 500 μl of 60 mg/ml of ascorbic acid per 5 m-NaOH (1:19). Fluorescence was measured at 420 nm (excitation) and 510 nm (emission) using a fluorometer (Victor 3; PerkinElmer).
Measurement of liver TAG, cholesterol and glycogen contents
Liver samples (50 mg) were homogenised in 1 ml isopropanol (Vetec) and centrifuged (5900 rpm, 10 min, 4°C). Total TAG and cholesterol levels were measured by a colorimetric method using a commercial kit (Bioclin)( Reference Peixoto-Silva, Conceição and Carvalho 14 ).
The glucose produced by glycogen hydrolysis was measured using a commercial kit (Glucox; Doles). Liver was homogenised with 4 ml TCA (10 %). After centrifugation (1000 g , 4°C, 10 min), 2 ml of supernatant were added to 5 ml of absolute ethanol and frozen. After 24 h, the mixture was centrifuged (1000 g , 4°C, 10 min), and the supernatant was discarded. Glycogen was hydrolysed by boiling the pellet for 30 min with 1 m-HCl. After addition of 1 ml of 1 m-NaOH to neutralise the mixture, glucose was measured in 200 μl of supernatant as described previously( Reference Casimiro-Lopes, Alves and Salerno 15 ).
Western blot analysis
For Western blot assays, samples of the liver, hypothalamus and pituitary gland were taken from rats and immediately frozen in liquid N2 ( Reference Figueiredo, Passos and Trevenzoli 10 ). Briefly, to obtain cell extracts, tissues were homogenised in ice-cold lysis buffer (50 mm-HEPES, 1 mm-MgCl2, 10 mm-EDTA, Triton X-100 1 %, pH 6·4) containing the following protease inhibitors: 10 mg/ml of aprotinin; 10 mg/ml of leupeptin; 2 mg/ml of pepstatin; 1 mm-phenylmethylsulfonyl fluoride (Sigma-Aldrich). β-Actin was used as an internal control. The total protein content in homogenates was determined by the BCA protein kit assay (Thermo Scientific), and cell lysates were denatured in sample buffer (50 mm-Tris–HCl, pH 6·8, 1 % SDS, 5 % 2-mercaptoethanol, 10 % glycerol, 0·001 % bromophenol blue) and heated at 95°C for 5 min. Samples (30 mg total protein) were separated by 10 % SDS–PAGE and transferred to polyvinylidene fluoride membranes (Hybond-P; Amersham Pharmacia Biotech). Molecular-weight markers (Amersham Biosciences) were run in parallel. Membranes were blocked with 5 % non-fat milk in Tween-Tris-buffered saline solution (20 mm-Tris–HCl, pH 7·5, 500 mm-NaCl, 0·1 % Tween-20). The following primary antibodies (Santa Cruz Biotechnology, Inc.) were used: anti-ACTH (1:500) in the pituitary gland; anti-CRH (1:500) in the hypothalamus; anti-11β-HSD1 in the liver; anti GR-α (1:500) in the liver, pituitary gland and hypothalamus; anti-adrenaline β2 receptor adrenergic receptors (1:500) in the liver. Membranes were then washed three times with Tween-Tris-buffered saline solution (0·1 %), followed by incubation for 1 h with an appropriate secondary antibody conjugated with biotin (Santa Cruz Biotechnology, Inc.). Then, the membranes were incubated with streptavidin-conjugated horseradish peroxidase (Caltag Laboratories). All Western blots were allowed to react with horseradish peroxidase substrate (ECLplus; Amersham Pharmacia Biotech) and then exposed to X-ray film for 10 s to 1 min. Images were obtained, and bands were quantified by densitometry using ImageJ software and normalised to the bands obtained for β-actin.
Statistical analysis
Data were analysed by the statistical program GraphPad Prism 5 (GraphPad Software, Inc.), and reported as means with their standard errors. Body weight and food intake were analysed using ANOVA. Other parameters were analysed by Student's unpaired t test. Differences were considered significant at P <0·05.
Results
After weaning, pups from FLAX mothers had significantly higher body weight at days 34, 38 and 42 (approximately 14 %, P <0·05), on days 46, 50 and 53 (approximately 10 %, P <0·05), on day 57 (approximately 15·5 %, P <0·05) and on days 84, 116, 128 and 144 (approximately 9 %, P <0·05) (Fig. 1(a)) . Similarly, pups from FLAX mothers had higher food intake at days 27–53 and days 72–132 (approximately 10 %, P <0·05; Fig. 1(b)). No changes were observed in weight gain between pups from FLAX and CON mothers (Fig. 1(c)).
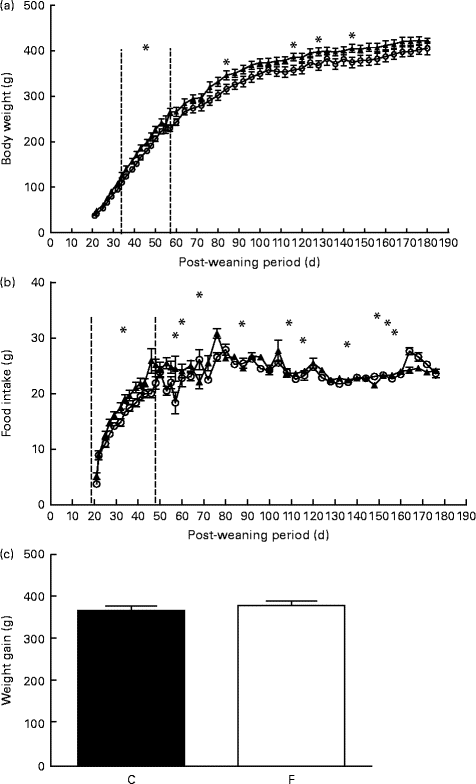
Fig. 1 (a) Body weight, (b) food intake and (c) weight gain of the offspring after weaning until 180 d old whose mothers were fed either a control (○, n 16; C) or flaxseed (▲, n 16; F) diet. Values are means of two pups from each dam per group after weaning, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
The adult offspring from FLAX mothers exhibited higher serum corticosterone (+48 %, P< 0·05; Fig. 2(a)) and lower adrenal catecholamine ( − 23 %, P< 0·05; Fig. 2(b)) contents compared with the offspring from CON mothers.
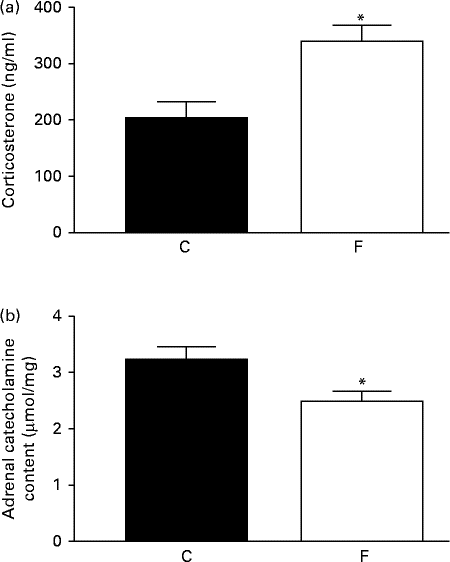
Fig. 2 (a) Serum corticosterone and (b) adrenal catecholamine contents of the offspring at 180 d of age whose mothers were fed either a control (C) or flaxseed (F) diet. Values are means of eight rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
The offspring from FLAX mothers exhibited lower glycogen ( − 30 %, P< 0·05; Fig. 3(a)), higher cholesterol (4-fold increase, P< 0·05; Fig. 3(b)) and higher TAG (3-fold increase, P< 0·05; Fig. 3(c)) contents in the liver compared with the offspring from CON mothers. A higher expression level of hepatic 11β-HSD1 (+62 %, P< 0·05) was observed in these offspring; however, there were no changes in hepatic GR-α expression and ADR-β3 (Fig. 4(a)–(d)) .
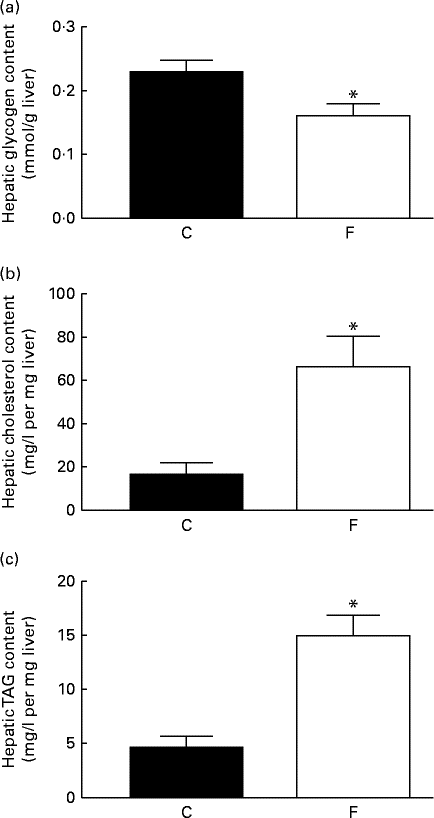
Fig. 3 (a) Glycogen, (b) cholesterol and (c) TAG contents in the liver of the offspring at 180 d of age whose mothers were fed either a control (C) or flaxseed (F) diet. Values are means of eight rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).

Fig. 4 (a) 11-β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1), (b) adrenaline β2 receptor (ADRβ2) and (c) glucocorticoid receptor-α (GR-α) protein expression in the liver of the offspring at 180 d of age whose mothers were fed either a control (C) or flaxseed (F) diet during lactation. The analysis was conducted by Western blot and expressed as arbitrary units (a.u.). β-Actin was loaded as a control, and data were normalised to β-actin by densitometry. (d) Representative bands are shown. Values are means of eight rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
There was no change in CRH expression in the hypothalamus of the offspring from FLAX mothers, but there was a higher expression level of GR-α (2-fold-increase, P< 0·05; Fig. 5). However, lower ACTH expression ( − 34 %, P< 0·05) was found in the pituitary gland of the offspring from FLAX mothers, but no changes in GR-α expression were observed (Fig. 6).
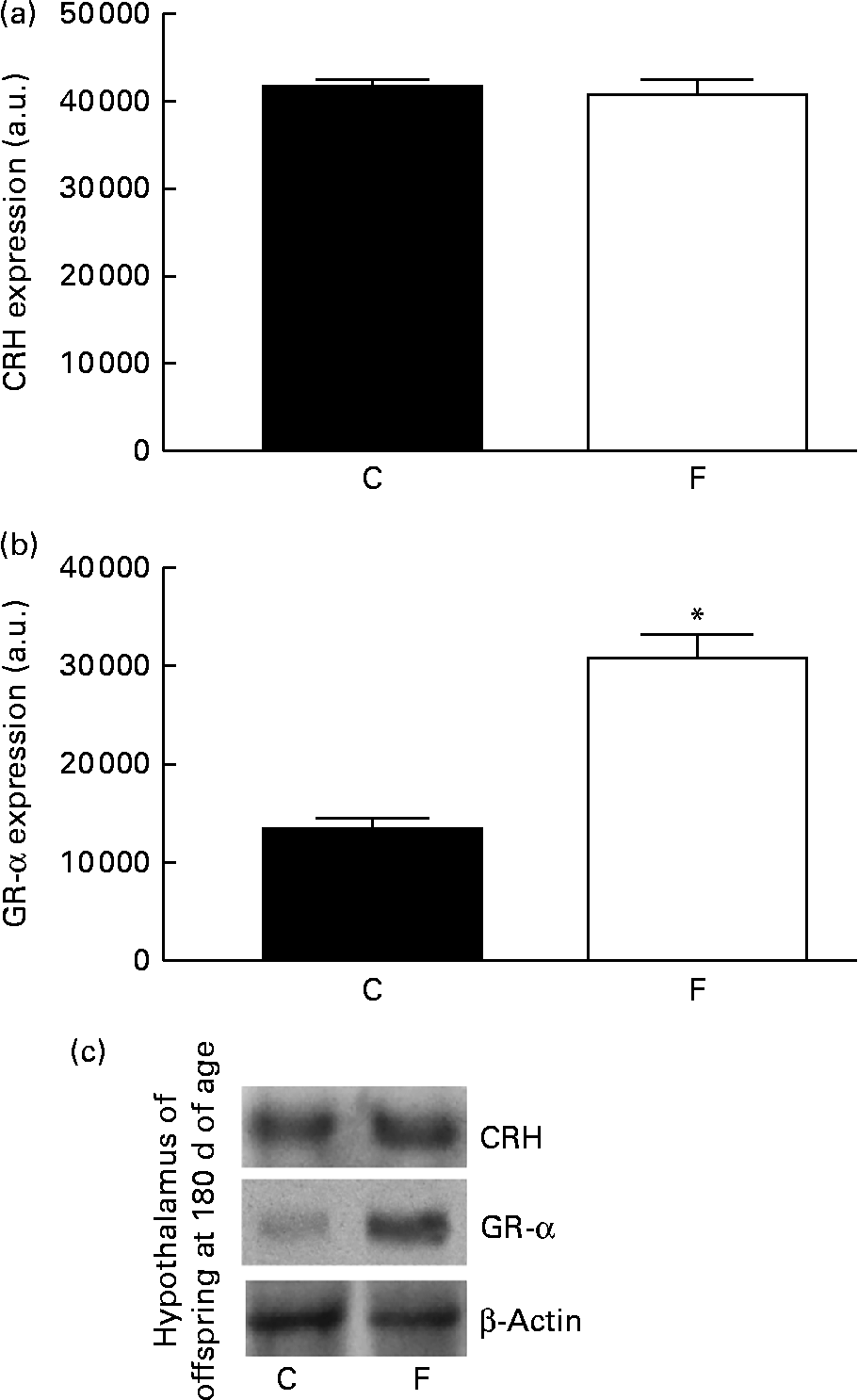
Fig. 5 (a) Corticotropin-releasing hormone (CRH) and (b) glucocorticoid receptor-α (GR-α) protein expression in the hypothalamus of the offspring at 180 d of age whose mothers were fed either a control (C) or flaxseed (F) diet during lactation. The analysis was conducted by Western blot and expressed as arbitrary units (a.u.). β-Actin was loaded as a control, and data were normalised to β-actin by densitometry. (c) Representative bands are shown. Values are means of eight rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
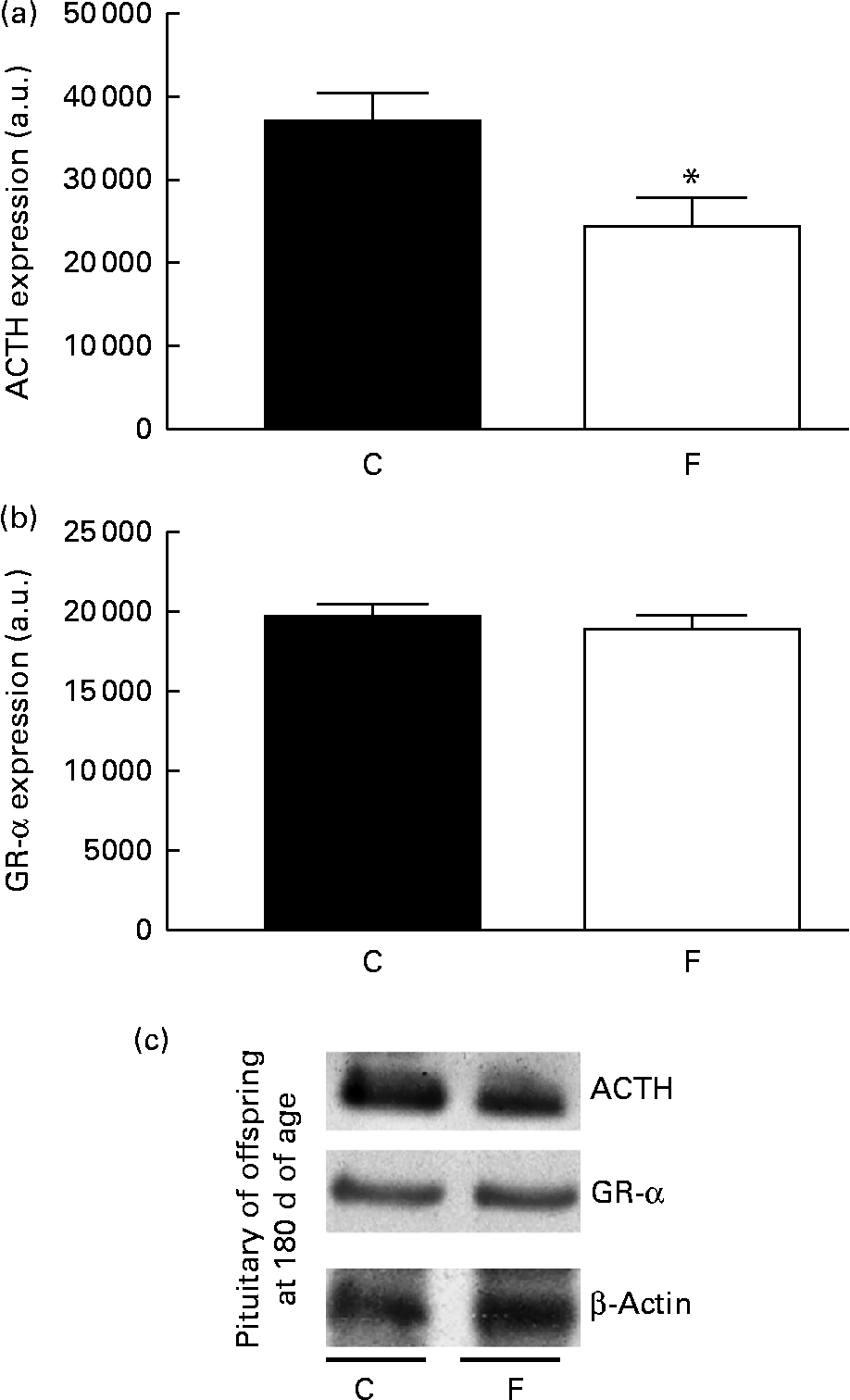
Fig. 6 (a) Adrenocorticotropic hormone (ACTH) and (b) glucocorticoid receptor-α (GR-α) protein expression in the pituitary gland of the offspring at 180 d of age whose mothers were fed either a control (C) or flaxseed (F) diet during lactation. The analysis was conducted by Western blot and expressed as arbitrary units (a.u.). β-Actin was loaded as a control, and data were normalised to β-actin by densitometry. (c) Representative bands are shown. Values are means of eight rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
Discussion
Flaxseed was added to the diet because it is usually ingested by humans in their food as part of a healthy lifestyle to provide fibre and α-linolenic acid( Reference Carter 1 ), especially during pregnancy, when fibre intake might be increased from 25 to 28 g/d, according to the United States Food and Nutrition Board (Food and Nutrition Board, 2005). The maternal flaxseed diet that we used here during lactation had flaxseed as the exclusive source of oil and fibre and represented a dose of 25 % flaxseed in the diet. This dose was based on a previous experimental study conducted only during gestation or from gestation until 90 d old, which used 20–40 % flaxseed( Reference Collins, Sprando and Black 6 ). Our experimental animals received roughly one-quarter of their diet as flaxseed, i.e. about 8·8 % oil, 5 % protein, 5 % carbohydrate, and 5 % fibre exclusively from flaxseed. For women who eat about 800 g of food per d, this would represent 200 g of flaxseed and would be equivalent to about nine spoonfuls of flaxseed per d. This amount is easily reached if women ate three spoonfuls of flaxseed with each main meal. Daily consumption of 200 g of flaxseed would represent 20 % of the recommended daily fibre intake (25 g/d) for women, and this amount was used in our previous experimental studies showing negative health effects in pups when they become adults( Reference Figueiredo, de Moura and Lisboa 8 – Reference Figueiredo, Passos and Trevenzoli 10 ). At adulthood, male offspring whose mothers received dietary flaxseed (25 %) exhibited higher body weight despite lower body visceral fat. These rats also exhibited lower total serum cholesterol and TAG levels. However, the offspring from FLAX mothers exhibited hyperleptinaemia and hypoinsulinaemia( Reference Figueiredo, de Moura and Lisboa 8 ). In another study, female offspring of the same age showed a similar phenotype; however, they had lower body weight at weaning( Reference Troina, Figueiredo and Moura 16 ).
Maternal flaxseed intake during lactation results in small differences in body weight and food intake in the adult offspring. The maternal flaxseed diet induces long-lasting changes in adrenal function. We observed higher serum corticosterone levels in the offspring from FLAX mothers, which can be due, at least in part, to an increase in hepatic 11β-HSD1 expression. The liver constitutes an important site for the intracellular production of glucocorticoids due to the conversion of 11-dehydrocorticosterone into the active hormone corticosterone to supply local demand( Reference Livingstone, Kenyon and Walker 11 ).
11β-HSD has a lipogenic activity. Chapagain et al. ( Reference Chapagain, Caton and Kieswich 17 ) showed that high 11βHSD content and activity are associated with increased hepatic expression of lipogenic genes as well as increased insulin resistance. This helps to explain the association of higher hepatic TAG and cholesterol contents with increased expression of 11β-HSD1 in adult FLAX offspring( Reference Chapagain, Caton and Kieswich 17 ). These data also help to explain why these animals exhibited hyperinsulinaemia and increased insulin resistance( Reference Figueiredo, de Moura and Lisboa 8 ).
The lower content of catecholamine may be due to lower production or higher secretion. In the liver, the lower glycogen content can be due to higher catecholamine action, as corticosterone is known to increase liver glycogen concentration( Reference Lang 18 ). No change was observed in the expression of ADRβ2 in the liver. One limitation of the present study was that we did not measure serum catecholamine concentrations. Thus, we can only suggest that serum catecholamine concentration could be higher due to the observed reduced concentration of liver glycogen.
Regarding the HPA axis, the negative feedback is disrupted at least in the hypothalamus, as high corticosterone levels suppress CRH; on the contrary, we found that despite the higher expression of GR-α, CRH was normal. In the pituitary gland, high serum corticosterone concentrations decreased the expression of ACTH, albeit GR-α expression was normal.
The regulation of GR-α by glucocorticoids is not straightforward. The pioneer study of Burnstein et al. ( Reference Burnstein, Bellingham and Jewell 19 ) reported a down-regulation in cultured lymphocytic cells. However, another study( Reference Breslin, Geng and Vedeckis 20 ) showed exactly the opposite, i.e. an up-regulation of the promoter of the human GR-α gene in immature T-lymphocytes. Furthermore, a more recent study( Reference Spiga and Lightman 21 ) showed that corticosterone up-regulates both the nuclear content of GR-α (translocation) and DNA binding in the hypothalamus, but not in the pituitary gland. Thus, the fact that we showed higher hypothalamic GR-α expression in the offspring from FLAX mothers could be consistent with these previous reports. However, it is puzzling how corticosterone did not decrease the levels of CRH, suggesting hypothalamic corticosterone resistance or the action of other stimulatory factors on CRH, such as catecholamines, serotonin, acetylcholine and the cytokines IL-1 and IL-6( Reference Kovács 22 ). However, we cannot overemphasise the measurement of GR-α, as it was evaluated in the total pituitary gland and hypothalamus, which may not reflect the expression in corticotroph cells and in the paraventricular nucleus, respectively.
Proulx et al. ( Reference Proulx, Clavel and Nault 23 ) showed that rats exposed to high leptin concentrations during the first 10 d of life exhibited a high ACTH response to dexamethasone and higher GR-α expression in the brain. Previously, we demonstrated that pups whose mothers received a flaxseed diet during lactation had hyperleptinaemia at weaning( Reference Figueiredo, de Moura and Lisboa 8 ). According to that study, we expect that GR-α expression remained elevated at least in the hypothalamus. Other factors, such as somatostatin( Reference Luque, Gahete and Hochgeschwender 24 ), dopamine( Reference Boschetti, Gatto and Arvigo 25 ) and oxytocin( Reference Legros 26 ), may suppress the levels of ACTH and could be increased in these programmed animals; however, we did not evaluate these neurohormones. Interestingly, for example, the level of dopamine can be increased in depressive patients( Reference Swaab, Bao and Lucassen 27 ). This situation of intense stress has been associated with glucocorticoid resistance in the hypothalamus that could explain feedback disruption.
It is known that intra-pituitary and intra-hypothalamic corticotrophin-binding globulin (CBG) (transcortin) is important for genomic and non-genomic glucocorticoid effects. Since flaxseed contains phyto-oestrogens that may permanently affect CBG synthesis, it is possible that even at high corticosterone levels, the amount of free corticosterone at the cellular level may be differentially affected in the hypothalamus and pituitary gland, helping to explain why corticosterone seems to better regulate the expression levels of pituitary ACTH but not CRH( Reference Viau and Meaney 28 – Reference Sivukhina, Schäfer and Jirikowski 30 ).
Some substances, such as arachidonate, could have a negative impact on GR-α through a possibly direct but weak binding at sites different from steroid binding sites on receptor molecules( Reference Kato 31 ). Gomez-Pinilla & Ying( Reference Gomez-Pinilla 32 ) demonstrated that dietary DHA supplementation has differential effects on several of these class proteins. In these experiments, the DHA diet reduced the levels of glucocorticoid receptors in the hypothalamus. However, in the present study, whole flaxseed had a higher amount of n-3 in the form of α-linolenic acid, which can convert into EPA and DHA; however, this increase in DHA levels did not decrease the GR-α content in the hypothalamus( Reference Gomez-Pinilla 32 ). Previously, Ma et al. ( Reference Ma, Wang and Zhao 33 ) demonstrated that ovariectomised mice treated with SDG for 21 d prevented the increase in the HPA axis response to chronic mild stress. Therefore, in the present study, SDG suppressed only the central regulation of cortisol; however, this dose of SDG present in whole flaxseed caused slight changes in the peripheral metabolism of cortisol in the liver.
Conclusion
The present findings suggest that the maternal intake of flaxseed through the diet during lactation leads to changes in adrenal function in the adult life of the offspring. Nutritional imprinting factors could programme the offspring for hypercorticosteronaemia and perhaps for higher adrenal catecholamine secretion. Thus, during the critical period of lactation, it is advised that women restrict their intake of flaxseed to prevent future modification of adrenal function in their progeny.
Acknowledgements
The authors are grateful to Mr Ulisses Siqueira and Ms Monica Moura for their technical assistance.
The present study was supported by the National Council for Scientific and Technological Development (CNPq), the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ) and the Coordination for the Enhancement of Higher Education Personnel (CAPES).
The authors' contributions are as follows: E. G. d. M. and M. S. F. designed the study and wrote the manuscript; P. C. L. and E. d. O. revised the manuscript; M. S. F. and E. P. S. d. C. were responsible for animal programming, and biochemical and hormonal analysis. All authors contributed to and approved the final manuscript.
The authors declare that they have no conflicts of interest.










