Maternal fish consumption during pregnancy has been suggested to affect pregnancy and birth outcomes(Reference Olsen and Joensen1–Reference Olsen, Grandjean and Weihe4). Fish contains various nutrients considered to be beneficial for fetal growth and development, including polyunsaturated n-3 fatty acids, protein, selenium, iodine and vitamin D(Reference Philibert, Vanier and Abdelouahab5–Reference Uauy, Calderon and Mena7). In particular, the n-3 fatty acids DHA and EPA have been associated with higher birth weight in both randomised controlled trials and observational studies(Reference Szajewska, Horvath and Koletzko8, Reference Olsen, Osterdal and Salvig9). n-3 Fatty acids are hypothesised to affect eicosanoid synthesis. The down-regulation of PG2 production, which is related to initiation of the parturition process, has been suggested to increase pregnancy duration(Reference Olsen10, Reference Hansen and Olsen11). A shift of the prostacyclin/thromboxane A balance to a more anti-aggregatory and vasodilator state might increase placental flow and as a consequence fetal growth(Reference Sorensen, Olsen and Pedersen12, Reference Olsen13). However, fish consumption is also a well-known route of exposure to pollutants such as methyl mercury, dioxins and polychlorinated biphenyls (PCB), which may adversely affect fetal growth and birth outcomes(Reference Mahaffey14–Reference Halldorsson, Thorsdottir and Meltzer17). In a large study among Danish pregnant women, high maternal fish consumption was associated with lower birth weight, smaller birth length and head circumference (HC)(Reference Halldorsson, Meltzer and Thorsdottir18). Thus far, results from studies focused on the associations between maternal fish consumption and birth outcomes have not been consistent(Reference Olsen, Osterdal and Salvig9, Reference Rogers, Emmett and Ness19–Reference Oken, Kleinman and Olsen22). Differences in the results may be explained by specific effects of different types of fish, such as lean fish, fatty fish and shellfish. Fatty fish contains larger amounts of beneficial n-3 fatty acid and, as with shellfish, higher levels of contaminants(Reference Ramon, Ballester and Aguinagalde16, Reference Guldner, Monfort and Rouget23). Besides, previous studies have mainly focused on birth outcomes as a measure of fetal growth and development. However, similar birth weights might be the result of different fetal exposures or growth patterns. Assessing fetal growth characteristics in different trimesters of pregnancy may provide information about specific critical periods.
Therefore, we examined the associations of first-trimester maternal lean-fish, fatty-fish and shellfish consumption with fetal growth characteristics in the second and third trimesters and at birth and the risks of neonatal complications in a population-based prospective cohort study among 3380 mothers and their children.
Methods
Study design
The present study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life until young adulthood in the city of Rotterdam, The Netherlands. The present study was designed to identify early environmental and genetic determinants of growth, development and health from fetal life until young adulthood, and has been described in detail previously(Reference Jaddoe, van Duijn and van der Heijden24, Reference Jaddoe, Bakker and van Duijn25). Assessments during pregnancy included physical examinations, fetal ultrasounds, biological samples and questionnaires, and were planned in the first, second and third trimesters to collect information about fetal growth and its main determinants. The present study was performed in Dutch participants. Of the total group of Dutch mothers (n 4057), 98 % (n 3979) enrolled in the first or second trimester of pregnancy, and 87 % (n 3456) fully completed the FFQ including all questions referring to fish consumption. Twin pregnancies (n 49), pregnancies leading to intra-uterine death (n 24) or without known birth outcomes (n 3) were excluded from the study population. The analyses were performed in the remaining 3380 subjects (Fig. 1). The study was approved by the Medical Ethics Committee of the Erasmus Medical Centre, Rotterdam. Written informed consent was obtained from all parents. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki.
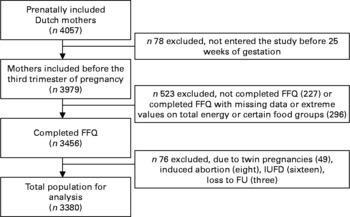
Fig. 1 Flow chart of participants included for analysis in the Generation R Study, Rotterdam, The Netherlands. IUFD, intrauterine fetal death; FU, follow-up.
Dietary assessment
We assessed maternal dietary intake, including fish consumption, at enrolment in the study (median 13·5 weeks of gestation, total range 5·4–24·9 weeks) using a modified version of the validated semi-quantitative FFQ of Klipstein-Grobusch et al. (Reference Klipstein-Grobusch, den Breeijen and Goldbohm26). This FFQ considered food intake over the prior 3 months, thereby covering dietary intake within the first trimester of pregnancy. The FFQ consists of 293 items structured to meal patterns. Questions include consumption frequency, portion size, preparation method and additions. Portion sizes were estimated using Dutch household measures and coloured photographs of foods showing different portion sizes(Reference Donders-Engelen, Van Der Heijden and Hulshof27). To calculate average daily nutritional values, the 2006 version of the Dutch food composition table was used(28). Frequency of fish consumption was assessed for total-fish consumption and different types of fish. Based on the nutrient content and information from previous studies, we assessed consumption of different fish types by seven categories: lean fish (codfish, plaice, catfish, sole fish, tuna, whiting and haddock), moderately fatty fish (trout, anchovy and gurnard), fatty fish (salmon, herring, mackerel, eel, sardines, halibut and bloater), shellfish (crab, lobster, shrimps and mussels), processed fish (fish fingers, fish burgers, crumbed and fried fish), fish derived from liver (haddock liver) and roe (soft and hard roe)(Reference Halldorsson, Meltzer and Thorsdottir18). For the analysis of total fish, we aggregated all fish consumed, and for the analysis of fatty fish, we aggregated moderately fatty fish and fatty fish. Processed fish, roe and fish derived from liver were not further analysed separately.
Fetal growth characteristics
Fetal ultrasound examinations were carried out at one of the two research centres in each trimester of pregnancy. Median of these visits was 12·9 (total range 7·7–18·0), 20·5 (total range 18·0–25·0) and 30·4 (total range 25·8–37·0) weeks of gestation for the first, second and third trimesters, respectively. These fetal ultrasound examinations were used for both establishing gestational age and assessing fetal growth characteristics(Reference Jaddoe, van Duijn and van der Heijden24). Since gestational age was established by the first fetal ultrasound examination, these ultrasounds were not used to assess fetal growth. In the second and third trimesters of pregnancy, we measured HC, abdominal circumference (AC) and femur length (FL) to the nearest millimetre using standardised ultrasound procedures(Reference Verburg, Steegers and De Ridder29). Estimated fetal weight (EFW) was calculated by means of the formula from Hadlock et al. (Reference Hadlock, Harrist and Sharman30) using HC, AC and FL:
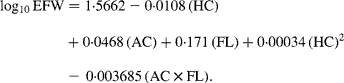
Neonatal complications
Information about offspring sex, gestational age, weight, length and HC at birth was obtained from medical records and hospital registries. Since HC and length at birth were not routinely measured at birth, missing birth measures were completed with data from the first month visit at the routine child health centre. Of all measurements, 25 and 16 % were based on the first month visit for HC and birth length, respectively. No differences in mean maternal fish consumption were observed between children with measurements at birth and those without measurements at birth (t tests: P = 0·46 for HC and P = 0·94 for birth length). The regression models with neonatal HC and length as outcome were adjusted for postconceptional age (gestational age for measurements at birth or gestational age+postnatal age for measurements from the child health centres) and for the method of measurements (birth or child health centre)(Reference Bakker, Steegers and Obradov31). Pre-term birth was defined as a gestational age of less than 37 weeks at delivery. Low birth weight was defined as birth weight below 2500 g. Small size for gestational age at birth was defined as a gestational age and sex-adjusted birth weight below the 5th percentile in the study cohort. We assessed both low birth weight and small for gestational age since it is important to differentiate between newborns having a low birth weight, independent of gestational age, and those who are fetally growth restricted. Both outcomes are independent risk factors of neonatal complications and development of diseases in adulthood(Reference Pena, Teberg and Finello32, Reference Barker33).
Covariates
Information about educational level, parity and periconceptional folic acid supplement use was obtained by a questionnaires at enrolment in the study. Maternal smoking and alcohol habits were assessed by questionnaires in each trimester. Maternal and paternal anthropometrics, including height (m) and weight (kg), were measured without shoes and heavy clothing, and BMI was calculated (weight/height2 (kg/m2)) in the first, second and third trimesters during visits at the research centre. Information about maternal weight just before pregnancy was obtained by questionnaires. As enrolment in the present study was in pregnancy, we were not able to measure maternal weight before pregnancy. However, in our population for analysis, 56 and 85 % of all women enrolled before a gestational age of 14 and 18 weeks, respectively. Correlation of pre-pregnancy weight obtained by questionnaires and weight measured at enrolment was 0·97 (P < 0·001). Since using weight measured at enrolment instead of pre-pregnancy weight obtained by questionnaires did not change the present results(Reference Ay, Kruithof and Bakker34), and considering the better data quality, we decided to use weight measured at enrolment in the analyses. Maternal age was registered at enrolment.
Statistical analysis
Based on the distribution of fish consumption and the number of subjects, we created five categories of total-fish consumption (0, 1–69, 70–139, 140–209 and>210 g/week), four categories of lean-fish consumption (0, 1–34, 35–69 and>70 g/week), four categories of fatty-fish consumption (0, 1–34, 35–69 and>70 g/week) and three categories of shellfish consumption (0, 1–13 and>14 g/week). We used the category of ‘no fish consumption’ as the referent for all analyses. Analysis of total-fish consumption and consumption of lean fish, fatty fish and shellfish in quartiles and quintiles did not change the results. We used t tests and χ2 tests to compare maternal characteristics in different categories of total weekly fish consumption. We analysed the associations of total weekly fish consumption with fetal growth characteristics in the second and third trimesters and at birth using multivariate linear regression models. In our first analyses, models were only adjusted for gestational age and fetal sex (model A). Subsequently, we considered confounders based on previous studies(Reference Ramon, Murcia and Ballester15, Reference Ramon, Ballester and Aguinagalde16, Reference Halldorsson, Meltzer and Thorsdottir18, Reference Rogers, Emmett and Ness19, Reference Drouillet, Kaminski and De Lauzon-Guillain21, Reference Oken, Kleinman and Olsen22, Reference Bakker, Steegers and Obradov31). These confounders included maternal total daily energy intake, age, BMI, weight gain, parity, marital status, educational status, smoking, alcohol use, coffee consumption, nausea, vomiting, periconceptional folic acid use and paternal height. Potential confounders were included in the models if the effect estimates changed more than 5 % in exploratory analyses. Using this approach, weight gain and parity were not included in the final multivariate analysis (model B). We performed similar analyses (models A and B) separately for consumption of lean fish, fatty fish and shellfish. We used logistic regression models to analyse the associations of total-fish, lean-fish, fatty-fish and shellfish consumption categories with the risks of neonatal complications (pre-term birth, low birth weight and small size for gestational age). Tests for trends were performed by using weekly consumption of total fish, lean fish, fatty fish and shellfish as a continuous variable in the multivariate linear and logistic regression analyses. Multiple imputation was used to complete missing data on the covariates BMI (0·4 %), weight gain (3·3 %), educational status (1·9 %), marital status (0·6 %), parity (0·2 %), smoking (7·6 %), alcohol use (7·3 %), nausea (7·8 %), vomiting (8·1 %) and folic acid supplement use (17·4 %). Since there were no differences in the observed results between analyses with imputed missing data or complete cases only, solely results including imputed missing data are presented. All measures of association are presented with their 95 % CI. P values are two-sided. Statistical analyses were performed using the Predictive Analytic Software version 17.0 for Windows (PASW, Inc., Chicago, IL, USA).
Results
Maternal age ranged from 15·7 to 46·3 years, with a mean of 31·4 years (Tables 1 and 2). The median of total weekly fish consumption was 75 (total range 0–600) g. The median of weekly consumption of lean fish, fatty fish, shellfish and processed fish was 24 (total range 0–338) g, 32 (total range 0–360) g, 0 (total range 0–93) g and 0 (total range 0–261) g, respectively.
Table 1 Maternal and fetal characteristics according to maternal fish consumption during pregnancy in the Generation R Study Cohort, Rotterdam, The Netherlands
(Mean values and standard deviations or percentages)
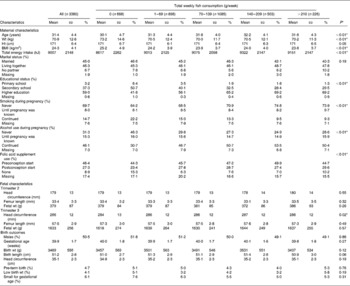
* P values obtained by ANOVA for continuous variables and the χ2 test for categorical variables.
Table 2 Associations between weekly maternal total-fish consumption and fetal growth and growth characteristics at birth in the Generation R Study Cohort, Rotterdam, The Netherlands*
(Differences and 95 % confidence intervals)
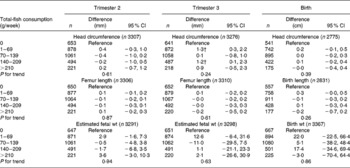
* Values were based on multivariate linear regression models and reflect the difference and 95 % CI for each level of total weekly fish consumption compared with the reference group (0 g/week). All models were adjusted for maternal energy intake, age, BMI, marital status, education, smoking, alcohol use, nausea, vomiting, use of folic acid supplements, gestational age at measurement, paternal height and fetal sex. Tests for trend were performed by including total weekly fish consumption as a continuous term in the regression model.
† P < 0·05.
Maternal older age, higher educational level, adequate folic acid supplement use, alcohol use and not smoking were associated with higher fish consumption (P < 0·01). In the total cohort, the mean offspring birth weight was 3489 (sd 556) g, and the median gestational age at birth was 40·3 weeks (total range 26·7–43·4) weeks. Of all births, 4·7 % were born pre-term, 4·0 % had a low birth weight and 6·1 % were small for gestational age at birth. Fetal growth characteristics measured during the second and third trimesters of pregnancy were available in 97·8 and 97·9 % of the mothers, respectively.
Results from the gestational age and sex-adjusted regression models focused on the associations between fish consumption and fetal growth characteristics (model A) are given in the Supplementary materials (the supplementary material for this article can be found at http://www.journals.cambridge.org/bjn). Higher consumption levels of total fish were associated with a larger third-trimester HC and birth length and a lower risk of low birth weight. Consumption of lean fish was positively associated with the third-trimester HC and birth weight and inversely associated with the risk of small for gestational age. A higher level of fatty-fish consumption was also inversely associated with the risk of small for gestational age. A higher shellfish consumption level was associated with larger FL in the second trimester.
After additional adjustment for confounders, most associations disappeared. The differences between model A and model B were largely explained by various factors including smoking, educational status and folic acid supplement use in the regression models. The results are shown in Tables 3–6. As compared to no fish consumption, weekly maternal consumption of 0–69 and 140–209 g of total fish was associated with larger HC measured in the third trimester (both P values < 0·05). The test for trend, however, was NS. No associations were observed between maternal total-fish consumption and other fetal growth characteristics in the second and third trimesters or at birth. Weekly consumption of lean fish and fatty fish was not associated with fetal growth characteristics in the second and third trimesters or at birth. Weekly consumption of >14 g of shellfish was associated with lower birth weight (P = 0·04). Shellfish consumption was not associated with other fetal growth characteristics. Maternal consumption of total fish, fatty fish, lean fish or shellfish was not consistently associated with the risk of children born pre-term, with a low birth weight or small size for gestational age.
Table 3 Associations between weekly maternal lean-fish consumption and fetal growth and growth characteristics at birth in the Generation R Study Cohort, Rotterdam, The Netherlands*
(Differences and 95 % confidence intervals)
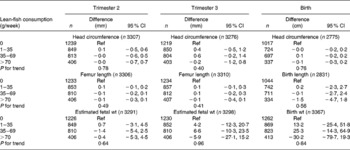
Ref, reference.
* Values were based on multivariate linear regression models for each level of weekly lean-fish consumption compared with the reference group (0 g/week). All models were adjusted for maternal energy intake, age, BMI, marital status, education, smoking, alcohol use, nausea, vomiting, use of folic acid supplements, gestational age at measurement, paternal height and fetal sex. Tests for trend were performed by including weekly lean-fish consumption as a continuous term in the regression model.
Table 4 Associations between weekly maternal fatty-fish consumption and fetal growth and growth characteristics at birth in the Generation R Study Cohort, Rotterdam, The Netherlands*
(Differences and 95 % confidence intervals)
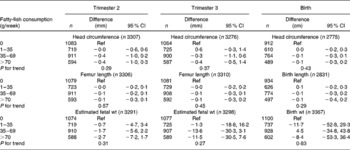
Ref, reference.
* Values were based on multivariate linear regression models for each level of weekly fatty-fish consumption compared with the reference group (0 g/week). All models were adjusted for maternal energy intake, age, BMI, marital status, education, smoking, alcohol use, nausea, vomiting, use of folic acid supplements, gestational age at measurement, paternal height and fetal sex. Tests for trend were performed by including weekly fatty-fish consumption as a continuous term in the regression model.
Table 5 Associations between weekly maternal shellfish consumption and fetal growth and growth characteristics at birth in the Generation R Study Cohort, Rotterdam, The Netherlands*
(Differences and 95 % confidence intervals)
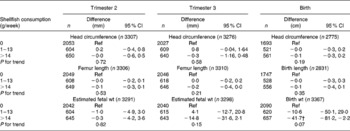
Ref, reference.
* Values were based on multivariate linear regression models for each level of weekly shellfish consumption compared with the reference group (0 g/week). All models were adjusted for maternal energy intake, age, BMI, marital status, education, smoking, alcohol use, nausea, vomiting, use of folic acid supplements, gestational age at measurement, paternal height and fetal sex. Tests for trend were performed by including weekly shellfish consumption as a continuous term in the regression model.
† P < 0·05.
Table 6 Associations between fish consumption and the risks of neonatal complications in the Generation R Study Cohort, Rotterdam, The Netherlands*
(Odds ratios and 95 % confidence intervals)
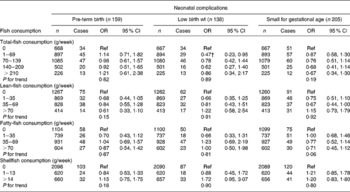
Ref, reference.
* Values were based on multivariate logistic regression models for pregnancy complications for each level of fish consumption compared with the reference group (0 g/week). All models were adjusted for maternal energy intake, age, BMI, marital status, education, smoking, alcohol use, nausea, vomiting, use of folic acid supplements, gestational age at measurement, paternal height and fetal sex. Tests for trend were performed by including weekly fish consumption as a continuous term in the regression model.
† P < 0·05.
Discussion
Main findings
In this cohort of pregnant women in The Netherlands, we found no consistent associations of total-fish, lean-fish or fatty-fish consumption with fetal growth characteristics during the second and third trimesters and at birth, after adjustment for potential confounders. Shellfish consumption was not associated with fetal growth characteristics during the second and third trimesters; however, some evidence was found for an association between shellfish consumption and lower birth weight. No consistent associations were observed between fish consumption and the risks of neonatal complications. The median of weekly fish consumption in the present study population was 75 g, which is higher than the median fish consumption presented in a Dutch cohort study (52 g/week) and measured by the Dutch National Food Consumption Survey (63 g/week) in a population of similar age(Reference de Goede, Geleijnse and Boer35, 36). These differences may be due to the differences in the time period, since data on fish consumption were collected between 1993 and 1998. Mean fish consumption in the present study was comparable to mean fish consumption in The Netherlands reported in a more recent study comparing fish consumption between European countries (13·4 g/d in the present study and 12·6–14·8 g/d in the European Investigation into Cancer and Nutrition study)(Reference Welch, Lund and Amiano37).
Interpretation of the main findings
To our knowledge, only one previous study has assessed the associations of total-fish consumption with fetal growth characteristics in the second and third trimesters(Reference Drouillet, Kaminski and De Lauzon-Guillain21). The present study among 1805 pregnant women in France did not show associations between total-seafood consumption and fetal growth characteristics. Many studies have assessed the associations of consumption of different types of fish with birth outcomes as measures for fetal development. In a large cohort in Denmark, no association was observed between lean-fish consumption and the risks of adverse birth outcomes. However, associations were observed between frequent consumption of fatty fish with lower birth weight and smaller birth length and HC(Reference Halldorsson, Meltzer and Thorsdottir18). Frequent fatty-fish consumption was also associated with a higher risk of small size for gestational age at birth. Also, higher total-fish consumption levels were associated with lower birth weight and smaller HC, which seems to be fully explained by the consumption of fatty fish. In the present study, we did not find consistent associations of total-fish or fatty-fish consumption with fetal growth characteristics. This difference in the results might be due to the differences in quality and quantity of fish consumption. In our population, mean total-fish consumption was much lower than in Denmark. A daily consumption of >40 or >60 g/d was reported by 14 and 6 % in the Danish population, as compared with 3 and 1 % in the present study population.
Differences in the quantity of types of fish consumed complicate a direct comparison of the results between countries(Reference Welch, Lund and Amiano37). A recent study in Spain showed an association between weekly consumption of ≥ 2 portions of large oily fish and a higher risk of small size for gestational age. The authors also described an association of high-lean-fish consumption with a lower risk of small size for gestational age(Reference Ramon, Ballester and Aguinagalde16). Mean consumption levels of large oily fish or lean fish were not presented, but the mean total-fish consumption in the present study was 65 g/d, compared with 13 g/d in our Dutch population. Another Spanish study showed that consumption of >1 portion of crustaceans per week was associated with a higher risk of small size for gestational age(Reference Mendez, Plana and Guxens38). A French study showed the same association with consumption of >2 portions of shellfish per week(Reference Guldner, Monfort and Rouget23). In the Spanish study, mean consumption of crustaceans was 6·3 and 13·1 g/d for other types of shellfish; in the French study, mean consumption of shellfish was 19·7 g/d. In the present study population, shellfish were barely consumed at all. Nevertheless, a tendency towards a negative association of shellfish consumption with birth weight was observed. This association has been reported previously. It is therefore important to explore which type of shellfish contributes to this effect. It is most likely that this association is driven by large crustaceans (crabs, lobster, etc.), since they are known to contain more dioxins and PCB. However, we were not able to separately analyse different types of shellfish, since we assessed shellfish by a predefined category. Therefore, this association needs to be studied in further detail.
Strong associations of higher fish consumption with increased growth measures at birth have mainly been described in ecological and cohort studies that collected data in the 1980s and 1990s in countries that have a high seafood consumption(Reference Olsen and Joensen1–Reference Olsen, Grandjean and Weihe4, Reference Olsen and Secher39, Reference Thorsdottir, Birgisdottir and Halldorsdottir40). Results from large cohorts conducted more recently have suggested the absence of any association or inverse associations. Since not only in the present study, but also in other cohort studies, high fish consumption was strongly related to a higher education level and more healthy lifestyle habits(Reference Ramon, Ballester and Aguinagalde16, Reference Halldorsson, Meltzer and Thorsdottir18, Reference Drouillet, Kaminski and De Lauzon-Guillain21, Reference Donahue, Rifas-Shiman and Olsen41), these positive associations between fish consumption and birth anthropometrics may be partly due to residual confounding by lifestyle-related characteristics. However, in a Danish study conducted in 2002, the association of fish consumption with a lower risk of pre-term birth and having a low birth weight remained significant after adjustment for confounders. Also, in a British study, the association of fish consumption with a lower risk of fetal growth retardation remained significant after adjustment for maternal education.
Contamination of fish has frequently been suggested as explanation for the inverse associations of fish consumption with birth outcomes(Reference Ramon, Ballester and Aguinagalde16, Reference Halldorsson, Meltzer and Thorsdottir18, Reference Mendez, Plana and Guxens38). In a study in Denmark, regular fatty-fish consumption was associated with increased maternal plasma concentrations of PCB(Reference Halldorsson, Thorsdottir and Meltzer17). These maternal plasma PCB concentrations were associated with lower birth weight in this Danish study, as well as in other studies conducted in Japan, Slovakia, the USA and Sweden(Reference Konishi, Sasaki and Kato42–Reference Rylander, Stromberg and Dyremark46). In The Netherlands, fish consumption contributes on average to up to 26 % of total PCB intake and 12 % of dioxin intake(Reference Baars, Bakker and Baumann47, Reference De Mul, Bakker and Zeilmaker48). In line with the results from previous studies, PCB concentrations in maternal and cord blood have been associated with lower birth weight in The Netherlands as well(Reference Patandin, Koopman-Esseboom and de Ridder49). The present study, however, was conducted in 1998, when PCB levels were considerably higher. Contrary, in the study of Mendez et al. (Reference Mendez, Plana and Guxens38), adjustment for blood levels of Hg and PCB did not change, and thus explained the inverse associations between fish consumption and birth outcomes. Some other studies also did not observe associations of maternal plasma PCB concentrations with birth weight(Reference Grandjean, Bjerve and Weihe50–Reference Weisskopf, Anderson and Hanrahan52). Direct comparison of the effects of contamination by either Hg, dioxins or PCB is complicated due to different levels of contamination between countries, areas and within fish species(Reference De Mul, Bakker and Zeilmaker48). Dioxin and PCB concentrations in mussels, shrimps, mackerel and cod in The Netherlands are comparable with reported levels in other northern European countries, but higher as compared with levels in Spain(Reference van Leeuwen, Leonards and Traag53). Furthermore, there seems to be a downward trend in concentrations of contaminants. A continuous decline, although levelling off, of dioxin and PCB concentrations has been shown in both the Dutch river systems, food and breast milk(Reference Baars, Bakker and Baumann47, Reference De Mul, Bakker and Zeilmaker48, Reference van Leeuwen, Leonards and Traag53). The latter might reflect concentrations in the human body(Reference De Mul, Bakker and Zeilmaker48, Reference Polder, Thomsen and Lindstrom54). Also, it appeared recently that when measuring plasma dioxin and PCB concentrations, timing of blood sampling around the time of conception plays an important role(Reference Murphy, Gollenberg and Buck Louis55). Furthermore, differences in the results between studies may be explained by specific congeners assessed, since it is suggested that especially anti-oestrogenic PCB are associated with lower birth weight(Reference Murphy, Gollenberg and Buck Louis55).
Methodological considerations
The strength of the present study is that we assessed fetal growth by actually measuring fetal growth characteristics in the second and third trimesters of pregnancy instead of using only birth outcomes as a proxy for fetal growth. We prospectively collected detailed information on consumption of different fish types, which enabled us to separate the analyses. Also, from our questionnaires, we were able to extensively collect information on many potential confounding variables. A limitation of the study is that we did not have biomarkers on Hg or other chemical exposures. Therefore, we were not able to separate harmful effects of pollutants from potential beneficial effects of fish consumption. Also, since we assessed fish consumption by categories of fish types, we were not able to assess separate effects of consumption of each different fish species or to recombine specific fish species. Furthermore, since 87 % of eligible mothers completed the FFQ, we may have introduced some bias by missing data. Effect estimates could be biased if the associations of fish consumption with fetal outcomes differ between mothers included and not included in the study population. This seems unlikely, but cannot be excluded.
Conclusions and future studies
In a population which is relatively low in exposure to fish consumption, we observed no consistent associations of total-fish consumption and consumption of different types of fish with fetal growth characteristics or the risk of neonatal complications. Further studies are required focusing on the association between fish consumption and fetal growth measured by ultrasound. Monitoring contaminants in fish and analysis of additional contaminants, especially congeners having anti-estorgenic activity, might help to further elucidate potential associations.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and the Faculty of Social Sciences at the Erasmus University, Rotterdam, the Municipal Health Service, Rotterdam area, and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (Star-MDC), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam. The general design of the Generation R Study was made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University, Rotterdam, the Dutch Ministry of Health, Welfare and Sport, and The Netherlands Organization for Health Research and Development (ZonMw). H. T. was supported by a research grant from the European Community's 7th Framework Programme (FP7/2008–2013) under grant agreement no. 212 652 (NUTRIMENTHE Project ‘The Effect of Diet on the Mental Performance of Children’). V. W. V. J. received an additional grant from The Netherlands Organization for Health Research and Development (ZonMw 90700303). None of the authors had a financial or personal conflict of interest related to the content of the study. All authors have made substantial contribution to the present study and to the writing and editing of the manuscript. Additional contributions are as follows: D. H. M. H. was involved in the planning of the study, statistical analyses, interpretation of the data and drafted the manuscript; S. T. and H. d. B. contributed to the acquisition of the data; V. W. V. J. contributed to the design of the study, supervision, interpretation of the data and critical review of the manuscript; E. A. P. S., H. T. and S. T. had critical input into the manuscript; A. H. conceptionalised the Generation R study and participated in its design and conduction. All authors read and approved the final manuscript.









