An adequate amount of dietary fat is essential for health, particularly for pregnancy and lactation. Essential fatty acids play a major role during pregnancy. They provide the precursors for prostaglandins and leucotrienes and are present mainly in highly specialised membranes (retina and synapses). The consumption of essential fatty acids is deemed important for normal growth and development in infants. The interest in essential fatty acids in relation to pregnancy stems from both epidemiological observations(Reference Olsen, Hansen, Sommer, Jensen, Sørensen, Secher and Zachariassen1–Reference Olsen, Østerdal, Salvig, Kesmodel, Henriksen, Hedegaard and Secher7) and intervention studies(Reference Olsen and Secher8, Reference Olsen, Sørensen, Secher, Hedegaard, Henriksen, Hansen and Grant9). They showed longer gestation, larger babies and, in some cases, reduced numbers of pregnancy complications such as intra-uterine growth retardation, pregnancy-induced hypertension and pre-delivery in association with higher marine fatty acid (long-chain PUFA or n-3 fatty acids), fish or fish oil intake.
Several mechanisms have been suggested for explaining these associations. The first one is a delayed spontaneous delivery, resulting from altered balance between the prostaglandins involved in the initiation of the labour(Reference Hansen and Olsen10, Reference Olsen, Hansen, Sørensen, Jensen, Secher, Sommer and Knudsen11). The second one is an increased fetal growth rate, resulting from improved placental blood flow due to a lowered thromboxane:prostacyclin ratio(Reference Andersen, Andersen and Fuchs12) and blood viscosity(Reference Olsen, Olsen and Frische4). Moreover, marine fat could reduce the risk of preterm delivery(Reference Olsen, Secher, Tabor, Weber, Walker and Gluud13, Reference Olsen and Secher14) and of intra-uterine growth retardation(Reference Rogers, Emmett, Ness and Golding15).
However, results in the literature are not consistent. Indeed, in one study, Olsen et al. (Reference Olsen, Hansen, Secher, Jensen and Sandström16) could not detect any association between on the one hand the length of gestation, birth weight and length and on the other hand the intake of n-3 fatty acids in the second trimester of pregnancy, whether intake was quantified by a validated questionnaire or biochemical measurements. More importantly, another randomised controlled trial in pregnant women failed to detect effects of n-3 and n-6 fatty acid supplementation on gestational length, birth weight and length, head circumference or placental weight(Reference Helland, Saugstad, Smith, Saarem, Solvoll, Ganes and Drevon17). Nevertheless, several studies in both animals and human subjects have shown that deficiency of dietary n-3 PUFA is associated with biochemical changes in the brain and with disturbances in vision and other neurological parameters(Reference McCann and Ames18). The most vulnerable period of neural development is during embryonic and fetal growth. Essential fatty acids, especially DHA, are required for fetal brain, nervous system and retinal growth in late pregnancy. The maternal plasma concentration of individual fatty acids, and hence the composition of the maternal diet, may have large effects on long-chain PUFA delivery to the fetus.
In the ‘EDEN mother–child’ cohort study, we previously reported that a difference in pre-pregnancy seafood consumption from less than five to more than nine times per month was associated with a difference in birth weight of 5·0 % (from 3248 to 3412 g; P = 0·0006), in overweight women only(Reference Drouillet, Kaminski and De Lauzon-Guillain19). The mother's fat store is relevant to the maternal hormonal responses and to the nourishment of the embryo and fetus during pregnancy, and provides the basis for subsequent fat storage and utilisation during pregnancy(Reference Haggarty, Ashton, Joynson, Abramovich and Page20, Reference Haggarty21). We hypothesised that the association between seafood intake and fetal growth may be related to differences in the fatty acid contents of fat stored and was enhanced in overweight women because of a greater availability from fat stored.
The aim of the present analysis was, therefore, to study the relationship between fatty acid intake before and during pregnancy and fetal growth in the same French population and to evaluate whether it is a possible mediator of the observed association with seafood intake in overweight women(Reference Drouillet, Kaminski and De Lauzon-Guillain19).
Material and methods
Population and study design
Pregnant women seen for a prenatal visit at the departments of Obstetrics and Gynaecology of the University Hospitals of Nancy and Poitiers before 24 weeks of amenorrhoea were invited to participate. Enrolment started in February 2003 in Poitiers and September 2003 in Nancy; it lasted 27 months in each centre and ended up with the inclusion of 2002 women. Exclusion criteria were twin pregnancies, known diabetes before pregnancy, not being able to speak and read French, and planned moving away from the region. Among women who fulfilled these inclusion criteria, 55 % agreed to participate. The study was approved by the Ethics Committee of the Bicêtre Hospital. Written consents were obtained from the mother for herself at inclusion and for her newborn child after delivery.
At a visit performed between 24 and 28 weeks of amenorrhoea by midwife research assistants, maternal height was measured with a wall Seca 206 stadiometer (Hamburg, Germany) to the nearest 0·2 cm and maternal weight was measured using electronic Terraillon SL 351 scales (Hanson (UK) Ltd, Hemel Hempstead, Herts, UK) to the nearest 0·1 kg. Skinfolds were measured using a commercial Harpenden caliper (Chasmor Ltd, London, UK) three times in the following order: tricipital (posterior aspect of the arm, at midpoint between the acromion and the olecranon), bicipital (anterior aspect of the arm, at midpoint between the acromion and the olecranon), subscapular (1 cm below the lower angle at the scapula) and supra-iliac (1 cm over the iliac crest, at the mid-axillary line). After a 5 min rest, three measures of systolic and diastolic blood pressures were performed at 2 min intervals with an Omron M4I device (Omron Healthcare Europe, Hoofddorp, The Netherlands). Women came to the examination in a fasted state and received a 50 g glucose oral load. Glucose concentrations were measured on fasting and 1 h after the glucose challenge. Weight before pregnancy, educational level and smoking habits during pregnancy were obtained by interview. Pre-pregnancy BMI was computed as reported weight (kg)/measured height squared (m2). According to references of the International Obesity Task Force, overweight was defined as a BMI of 25 kg/m2 or more and obesity as a BMI of 30 kg/m2 and above. Average number of cigarettes consumed per d during pregnancy was computed.
A second visit was performed for newborns by the same research assistants on average 1·8 d after delivery. The mother's weight and skinfolds were obtained with the same protocol as above. Several anthropometric measurements were performed on the newborn. Circumferences were measured to the nearest 0·1 cm using a tape in duplicate: left arm circumference, measured at midpoint between the acromion and the olecranon; left wrist circumference, measured at the level of the styloid processes of the radius and ulna; head circumference, measured at the largest occipitofrontal circumference. Skinfolds were measured in triplicate using a commercial Holtain caliper (Chasmor Ltd) in the following order: tricipital skinfold, measured at the same level as the mid-arm circumference; subscapular skinfold, measured at the lower angle of the scapula.
Gestational age at delivery (determined from the date of the last menstrual period and early ultrasound assessment), newborn admission to a reanimation or neonatal unit, birth weight and length and placental weight (in Poitiers only) were extracted from clinical records. In the two obstetric departments, electronic Seca scales (Seca 737 in Nancy and Seca 335 in Poitiers; Hamburg, Germany) were used to measure infant weight and wooden somatometers (Testut, Béthune, France) to measure infant length.
Dietary assessment
Mothers completed two FFQ similar to the questionnaire developed for the French population in the Fleurbaix-Laventie Ville Santé Study (FLVS)(Reference de Lauzon, Romon, Deschamps, Lafay, Borys, Karlsson, Ducimetière, Charles and Fleurbaix Laventie Ville Sante Study Group22). This FFQ has been validated against a series of 24 h recalls(Reference Deschamps, de Lauzon-Guillain, Lafay, Borys, Charles and Romon23). The questionnaire used in the EDEN study is very close to that of the FLVS study with the addition of some questions for a more specific assessment of the intake of foods rich in folates, n-3 fatty acids and vitamin A and of seafood and trophallergic (allergen-containing) foods. It inquires about the intake of 137 different foods or food groups with a seven-item scale ranking from never to more than once per d.
The first-trimester FFQ (completed at recruitment, on average 15 weeks' gestation) concerned the usual diet during the year before pregnancy; the second FFQ (completed in the first few days following delivery) was related to food intake during the last 3 months of pregnancy. To compute energy and nutrient intakes, we multiplied, for each food, the intake frequency by the nutrient composition for a portion size. Portion size was determined using pictures for twelve food types (meats, French fries, pastas, vegetables, cakes, cheese, etc) on a three-level scale or were standard portions for the French adult population(24). We then summed contributions across all foods to obtain average daily total intake of energy and intake of various macro- and micronutrients. Food composition was obtained from the ‘SUpplementation en VItamines et Mineraux AntioXydants’ (SU.VI.MAX) nutrient composition database(25), which is based on a French nutrient composition database(Reference Favier, Ireland-Ripert, Toque and Feinberg26) and US Department of Agriculture publications and is continually incremented by other published sources and personal communications from laboratories and manufacturers(Reference Ireland-Ripert, Favier, Feinberg and CIQUAL27–29). Energy and nutrient intakes were not estimated when more than three items of the FFQ were missing. Moreover, women with estimated total energy intake under 4186·8 kJ/d (1000 kcal/d) or over 20 934 kJ/d (5000 kcal/d) were not included in the analyses. n-3 Fatty acids included linolenic acid, DHA, docosapentaenoic acid and EPA. The FFQ also gave information on the type of oil used for cooking or seasoning.
Variable description and statistical analyses
Mean consumptions of total lipid and different fatty acids (SFA, MUFA and PUFA), as well as n-3 and n-6 fatty acids, in g/d, were compared between centres by the Student's t test. Relationships with the sociodemographic characteristics of the women were studied by multiple linear regressions adjusted for centre and mother's age (in years).
We studied relationships between lipid consumption and fetal growth using the nutrient density method, i.e. we used the relative percentage of contribution of lipid intake to total energy intake, the relative percentage of contribution of SFA, MUFA or PUFA to total lipid intake or the relative percentage of contribution of n-3 fatty acids to total PUFA intake. Multiple linear regressions adjusted for different sets of confounding variables (centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex, BMI, and delay between birth and anthropometric measures) were performed to study these relationships.
As we had additional information on the type of fat used for cooking or seasoning, we used it to study whether this source of fatty acids was associated with our outcome variables. We defined a four-level variable from the answers to three questions asking for the type of oil used for cooking or seasoning. The first level corresponds to women who used with no preference any type of fat, the second level corresponds to women who used more often saturated fat (butter, hard margarine), the third level corresponds to women who used more often fat rich in n-6 fatty acids (sunflower-seed oil, maize oil) and the last level corresponds to women who used more often fat rich in n-9 fatty acids (olive oil, groundnut oil). Only three women consumed preferentially fat rich in n-3 fatty acids (colza oil), so they were classified in the four others groups according to the other type of fat they used. We tested whether the ‘type of fat used for cooking or seasoning’ variable modified the relationships between fatty acid intakes and fetal growth.
Separate analyses were performed for intake before and during the last 3 months of pregnancy. Interaction terms between fatty acid consumption and centre, gestational length, BMI (continuous then categorical variable), average cigarettes per d smoked during pregnancy and educational level were tested. A significant interaction was found for BMI (P < 0·05); therefore analyses were stratified according to overweight status (BMI < 25 v. ≥ 25 kg/m2). Several additional adjustments for educational level and maternal health variables (systolic or diastolic arterial pressure, fasting plasma glucose) were also made.
All analyses were performed with SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Subjects' characteristics
Analyses included the 1446 women who completed the two FFQ and for whom nutrient intake could be evaluated (sixty-seven were not included because of at least one missing FFQ and 374 because nutrient intake could not be estimated: 285 because more than three items of the FFQ were missing, fifty because total energy intake was under 4186·8 kJ/d and thirty-nine because total energy intake was over 20 934 kJ/d). Also, newborns for whom delay between birth and anthropometric measures was more than 7 d were not included (n 9). The main characteristics of included women (and their newborns), compared with the 450 excluded women, are shown in Table 1.
Table 1 Maternal and neonatal characteristics of the cohort (n 1896)
(Mean values and standard deviations or percentages)

*P < 0·05.
† Transfer to reanimation or neonatal unit.
Excluded women had less often reached a university level and were more often single than included women. The percentage of newborns transferred to reanimation or a neonatal unit was higher for excluded women. Mean birth weight was significantly lower for the offspring of excluded women compared with the others (3224 v. 3295 g). Mean maternal age was 29 years in both groups (range 17–45 years). For included women, mean pre-pregnancy BMI was 23 kg/m2, 9·5 % of women had a pre-pregnancy BMI < 18·5 kg/m2, 17·5 % were overweight and 7·7 % were obese. Overweight women had less often reached a university level and were less often single than non-overweight women (Table 2). Mean birth weight and length were significantly lower for the offspring of non-overweight women compared with the others (3269 v. 3362 g and 49·4 v. 50·0 cm respectively).
Table 2 Maternal and neonatal characteristics of included women according to their BMI
(Mean values and standard deviations or percentages)

*P < 0·05.
† Transfer to reanimation or neonatal unit.
The average intakes of energy, proteins, carbohydrates, lipids and different families of fatty acids (SFA, MUFA and PUFA) before and in the last 3 months of pregnancy are given in Table 3. Intakes were normally distributed except alcohol consumption. Significantly higher total energy intake and percentage of fat in total energy intake (with and without taking into account alcohol in total energy intake) were observed at the end of pregnancy compared with before pregnancy (9948 v. 9596 kJ/d, 39 v. 38 % and 39·2 v. 38·7 %, respectively; P < 0·001). Nevertheless, the proportion of PUFA in total lipid intake increased by 0·4 % during pregnancy (P < 0·0001), as well as the proportion of n-3 fatty acids in total PUFA intake, which decreased by 0·1 % (P = 0·08). The same trends were observed in both non-overweight and overweight women, except for the proportion of n-3 fatty acids in total PUFA, which decreased in overweight women. There was no significant difference in estimated total fat and fatty acid intake according to maternal overweight status.
Table 3 Maternal lipid and fatty acids intakes before and in the last 3 months of pregnancy
(Mean values and standard deviations)

Relationships between maternal fat and fatty acid intakes before pregnancy and sociodemographic characteristics of women
Differences in consumption were observed between centres for MUFA, PUFA and n-3 fatty acids, as well as the proportion of PUFA in total lipid intake with higher intakes in Nancy, but there was no difference for the proportion of n-3 fatty acids in total PUFA intake. Total fat and fatty acid intake (SFA, MUFA, PUFA) did not change significantly with age but n-3 fatty acid consumption increased by 0·1 g (1 %) per decade (P = 0·0002). Women with university-level education ate less fat than the others (95·1 v. 99·8 g/d; P = 0·03), in particular MUFA (34·8 v. 37·1 g/d; P = 0·004) and SFA (42·6 v. 44·5 g/d; P = 0·06). There was no significant difference for PUFA (11·5 v. 11·8 g/d; P = 0·32). No significant difference in the intake of total lipid and the different types of fatty acid was observed according to income level, marital status or smoking status during pregnancy. The same relationships were observed for intake during the last 3 months of pregnancy.
Fatty acid intakes and fetal growth
There was no relationship between the percentage of lipid in total energy intake before pregnancy and newborn anthropometric measures in the whole sample of women (Table 4). The same results were found for the proportions of SFA, MUFA and PUFA in total lipid intake, as well as the proportion of n-3 or n-6 fatty acids in total PUFA intake before and in the last 3 months of pregnancy. However, maternal overweight before pregnancy modified the relationship between fatty acid intake and most outcome parameters (for instance P < 0·04 for birth weight). Interactions were also significant for BMI as a categorical variable, so we decided to conduct the analyses separately for women < and ≥ 25 kg/m2, which separates overweight and obese women from the others.
Table 4 Associations of lipid and fatty acid intake before pregnancy with newborn anthropometric measures in the EDEN mother–child cohort, in separate regression models*
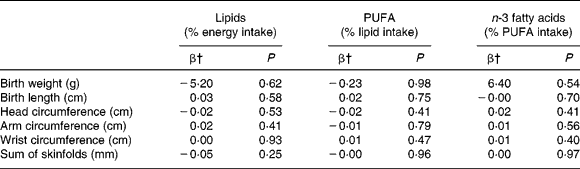
* Models adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex, delay between birth and anthropometric measures, and BMI.
† Regression coefficient: consumption considered as a continuous variable. β Corresponds to the increase of the variable for an increase of 1 sd of the intake consumed per d (1 sd = 6·4, 2·4 and 2·4 % for lipids, PUFA and n-3 fatty acids, respectively).
In overweight women (n 366), no association was found between the proportion of lipid in total energy intake and fetal growth when adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures (Table 5). A substitution of pre-pregnancy lipid intake of 6·2 % (1 sd) to other macronutrients was associated with a 25 g lighter birth weight (P = 0·27) and 7 mm smaller head circumference (P = 0·25). In models also adjusted for total energy intake, the results were unchanged (data not shown). The same results were found for lipid intake during the last trimester of pregnancy. No significant association was observed with the different fatty acid families even when models were adjusted for total lipid intake (see for PUFA/lipid in Table 5). The results were unchanged when adjusted for educational level.
Table 5 Associations of percentage of energy from lipid intakes and percentage of PUFA in total lipid intake with newborn anthropometric measures in overweight women in the EDEN mother–child cohort, in separate regression models*

* Models adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures.
† Regression coefficient: consumption considered as a continuous variable. β Corresponds to the increase of the variable for an increase of 1 sd of the intake consumed per d (1 sd = 6·2 and 6·2 % for lipids and 2·4 and 2·4 % for PUFA, before and during pregnancy, respectively).
To study the relationships between n-3 fatty acid consumption and fetal growth, we first used a regression model adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures (Table 6). A substitution of 0·3 % (1 sd) of the proportion of n-3 fatty acids in total lipid intake to other macronutrients before pregnancy was related to an average increase in birth weight of 87 g (P = 0·002), length of 28 mm (P = 0·02), head circumference of 18 mm (P = 0·02), arm and wrist circumferences of 16 and 13 mm respectively (P < 0·007) and sum of skinfolds of 0·3 mm (P = 0·01) (model 1). Further adjustment on total lipid intake shows that fetal growth was strongly associated with a greater contribution of n-3 fatty acids to total lipid intake (model 2).
Table 6 Associations of percentage n-3 fatty acids in total lipid or PUFA intakes, before and during the last 3 months of pregnancy, with newborn anthropometric measures in overweight women in the EDEN mother–child cohort, in separate regression models*

* Model 1, n-3 fatty acids (% lipid intake), adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures. Model 2 = model 1+adjusted for total lipid intake. Model 3, n-3 fatty acids (% PUFA intake), adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures. Model 4 = model 3+adjusted for total PUFA intake. Model 5 = model 4+adjusted for educational level.
† Regression coefficient: n-3 fatty acids (% total lipid or PUFA consumed daily) considered as a continuous variable. β Corresponds to the increase of the variable for an increase of 1 sd of the percentage (1 sd = 0·3 and 0·6 for n-3 fatty acids (% lipid intake); 2·4 and 2·7 for n-3 fatty acids (% PUFA intake), before and during pregnancy respectively).
Similar results were found for the proportion of n-3 fatty acids in total PUFA intake. A substitution of 2·4 % (1 sd) of this intake to other types of PUFA before pregnancy was related to an average increase in birth weight of 60 g (P = 0·01), head circumference of 13 mm (P = 0·04), arm and wrist circumferences of 14 and 9 mm respectively (P < 0·006) and sum of skinfolds of 0·2 mm (P = 0·03). Further adjustment on total PUFA intake shows that fetal growth was more strongly associated with a higher contribution of n-3 fatty acids to total PUFA intake (model 4). Results were unchanged when adjusted for educational level (model 5). Relationships with n-3 fatty acid intake during the last trimester of pregnancy were weaker and not significant (Table 6).
After further adjustment for systolic or diastolic arterial pressure, fasting glucose and TAG (at 6th month of pregnancy), these associations remained unchanged. Before pregnancy, a substitution of 2·4 % of n-3 fatty acids to other PUFA was related to an increase in birth weight of 41–51 g, depending on the adjustments (0·03 ≤ P ≤ 0·09). For n-3 fatty acid intake during pregnancy, the range of variation was 26–36 g and was not significant (data not shown).
The type of fat used for cooking or seasoning was not associated with newborn anthropometric measures (data not shown). Moreover, previous associations found between total n-3 fatty acid intake estimated by the FFQ and the newborn anthropometric measures were unchanged when we took into account the type of fat used for cooking or seasoning (data not shown).
Correlation coefficients between the number of seafood consumed per week and PUFA intake (% PUFA/energy intake) was 0·12 for intake before pregnancy and 0·06 for intake in the last 3 months of pregnancy (P < 0·04). Correlation was very strong for n-3 fatty acids/PUFA (r 0·38 and r 0·35 for intakes before and during pregnancy, respectively; P < 0·0001). Moreover, seafood consumption explained more than 32 % of the variability in n-3 fatty acid intake. When average monthly seafood consumption before pregnancy was added to the model, the relationship with n-3 fatty acids in total PUFA intake was reduced to a non-significant level. The relationship with seafood consumption remained significantly associated with fetal growth.
There were too few obese women to be studied separately, but excluding obese women from the analyses did not change the associations found in women with a BMI ≥ 25 kg/m2.
No statistically significant associations were found with placental weight and length of gestation.
No statistically significant associations were found in non-overweight women, as shown in Fig. 1 for the association between birth weight and the proportion of n-3 fatty acids in total PUFA intake divided into tertiles.

Fig. 1 Adjusted birth weight and percentage of n-3 fatty acid (FA) intake in total PUFA intake before pregnancy according to maternal overweight in the EDEN mother–child cohort. Values are means adjusted for centre, mother's age and height, smoking habits, parity, gestational age, newborn's sex and delay between birth and anthropometric measures, with standard errors represented by vertical bars. Tertile 1, 5·58–9·65 %; tertile 2, 9·65–11·38 %; tertile 3, 11·38–24·97 %. For BMI < 25 kg/m2, P for trend = 0·57; for BMI ≥ 25 kg/m2, P for trend = 0·03.
Discussion
In this French cohort, an increased proportion of n-3 fatty acids in total PUFA intake before pregnancy, and to a lesser extent of intake during the last 3 months of pregnancy, was not associated with fetal growth in the total sample of women. However, in overweight women, it was associated with increased fetal growth, indicated by birth weight, head, arm and wrist circumferences and skinfolds. Several epidemiological studies conducted in Northern countries with usual high mean seafood intake as well as marine n-3 fatty acids by pregnant women found an association with an increased birth weight either due to an increase in length of gestation or an increase in fetal growth rate but without considering the maternal BMI status(Reference Olsen5, Reference Hornstra30–Reference Facchinetti, Fazzio and Venturini32).
Although pre-pregnancy BMI may be affected by recall bias, we did not consider BMI estimated using measured weight during pregnancy as an accurate measure of maternal nutritional status. It is affected by plasma volume expansion related to pregnancy and fetal and placental weight. However, for 1042 women who had a first visit before 15 weeks of amenorrhoea, we found similar results when using measured BMI, i.e. significant interaction with maternal overweight and positive association with maternal n-3 fatty acids/PUFA intake and fetal growth.
In the present study, the women excluded from the dietary analysis were different from included women for some characteristics such as educational level. This may be related to a greater ability of educated women to average intake frequency and to concentrate on a long series of questions. Nevertheless, adjustment for educational level had no effect on the present results. Even if it is not possible to exclude possible recall bias in the report of consumption before pregnancy, a FFQ appears to be the only way to evaluate nutrient intakes before pregnancy in the present study.
The percentage of overweight and obese could appear low but was similar to the general population of French women. In France, although the prevalence is notably lower than in the USA, the frequency of obesity has nearly doubled between 1997 and 2006. In women aged 20–39 years, it increased from 5·2 % to 11 % during this period(Reference Charles, Eschwege and Basdevant33).
Furthermore, the evaluation of nutrient intakes with a FFQ has some limits. This may have altered our observed differences in food intake and therefore our ability to detect differences between groups. For instance, the estimation of PUFA intake with the FFQ did not include the type of fat and oils used for cooking or seasoning. In order to take into account differences in the type of fat used for cooking or seasoning, we studied whether using predominantly (or not) SFA or fat rich in n-6 or n-9 fatty acids was associated with fetal growth and found no specific association.
Several previously published studies have demonstrated a heavier placenta and a longer gestation among women who consumed more n-3 fatty acids(Reference Jensen34) but we did not observe these associations in the present study. Because of the imprecision in the gestational age assessment, however, the effect of fatty acid consumption on the prolongation of the intra-uterine growth period may be difficult to detect. Another explanation would be that this effect requires higher n-3 fatty acid intake than that observed in the present study. In an intervention study by Olsen et al. (Reference Olsen, Olsen and Frische4, Reference Olsen, Secher, Tabor, Weber, Walker and Gluud13), the level of n-3 fatty acid intake was higher than in the present study (6·1 g/d for the intervention group v. 2·7 g/d in the control group, whereas in the present study the estimated mean intake was 1·2 g/d).
There was no significant association between birth anthropometry and fatty acid intakes in non-overweight women. Associations found in subgroups need to be considered with caution because of likely more false-positive results. However, there may be some rationale for such a selective effect in overweight women.
Overweight women have a higher fat mass. Differences in fatty acid intake are associated with variations in the composition of fatty acids stored in the adipose tissue. In fact, several studies showed that the fatty acid composition of the diet could influence the fatty acid composition of the adipose tissue. Arterburn et al. (Reference Arterburn, Hall and Oken35) showed that tissue contents of EPA and DHA increase in response to supplementation with these fatty acids, which means n-3 fatty acids in tissue increase with their presence in the diet. Katan et al. (Reference Katan, Deslypere, van Birgelen, Penders and Zegwaard36) estimated that EPA levels in adipose tissue reflected intake over a period of months and even years for DHA. Correlations between percentage n-3 fatty acids in total fat intake estimated by FFQ and percentage n-3 fatty acids in total fat measured in adipose tissue ranked from 0·38 to 0·42 in another study(Reference Andersen, Solvoll, Johansson, Salminen, Aro and Drevon37). Therefore, overweight women may have an enhanced ability to release fatty acids from adipose tissue to sustain fetal growth. We previously suggested that the specific relationship between seafood or n-3 fatty acid intake and fetal growth in overweight women illustrates a special role of stored fatty acids in the adipose tissue. This is reinforced by the fact that seafood as well as n-3 fatty acid consumption before pregnancy is more strongly associated with fetal growth than consumption during the last 3 months of pregnancy, as also observed by Olsen et al. (Reference Olsen, Hansen, Secher, Jensen and Sandström16). The storage of long-chain PUFA and the balance of the n-3 and n-6 families in maternal adipose tissue are of great importance, since they represent a pool of fatty acids that can be used via placental transfer to supply the developing fetus(Reference Dutta-Roy38, Reference Innis39). There is evidence that the placenta itself may play a role in initiating the mobilisation of fatty acids from the maternal adipose tissue in response to fetal needs(Reference Haggarty40).
In the present study, significant relationships were observed between n-3 fatty acid intake and fetal growth when adjusted for PUFA intake. This result shows the importance of the n-3 : n-6 balance which seems more essential than the absolute intake of n-3 fatty acids. There is a competition between the two fatty acid families for entry and release from cellular phospholipids, as well as for the enzymes that catalyse their conversion to produce, for instance, arachidonic acid-derived eicosanoids, such as the prostaglandins (PGF2α and PGE2)(Reference Hansen and Olsen10, Reference Rubin and Laposata41). The balance plays a major role in the availability of n-3 fatty acids for the developing fetus. The concomitant intake of n-6 fatty acids may explain part of the discrepancies in the literature about fish or n-3 fatty acid consumption and fetal growth. All of the n-6 and n-3 fatty acids accumulated by the fetus are derived by transfer across the placenta, which is provided with a specific system to ensure this function. The substrate of the placenta is provided by the maternal diet and the high rate of mobilisation from maternal adipose stores and the mother adapts her metabolism to support the continuous draining of substrates by the fetus(Reference Herrera42–Reference Herrera, Amusquivar, Lopez-Soldado and Ortega44).
Seafood intake removed the association between the contributions of n-3 fatty acids/PUFA on fetal growth in the present study whereas the type of fat used for cooking or seasoning which is another major source of PUFA was not associated with fetal growth and did not remove this association. Seafood are indeed the main source of the variation of n-3 fatty acids/PUFA in our sample of French women(Reference Drouillet, Kaminski and De Lauzon-Guillain19). These results consolidate the hypothesis of an effect of the n-3 fatty acids from seafood, in particular EPA and DHA, which are mainly present in seafood.
In conclusion, the present study finds a relationship between the ratio of maternal n-3 fatty acids:PUFA intake and fetal growth in the French population, which is specific to overweight women. We suggest that the enrichment in long-chain n-3 fatty acids in the maternal adipose tissue stored before conception is a possible mediator of this relationship. The fact that the n-3:n-6 ratio appears more strongly related to fetal growth than the absolute intake of n-3 fatty acids may explain some of the discrepancies in the literature concerning the association of seafood intake with fetal growth. However, because the present results stem from a subgroup analysis, replication is needed before firm conclusions can be made.
Acknowledgements
We are indebted to the participating families, to the midwife research assistants (L. Douhaud, S. Bedel, B. Lortholary, S. Gabriel, M. Rogeon, M. Malinbaum) for data collection and to P. Lavoine for checking, coding and data entry. We acknowledge all the funding sources for the EDEN study: Fondation pour la Recherche Médicale (FRM), French Ministry of Research: IFR program, INSERM Nutrition Research Program, French Ministry of Health Perinatality Program, French Agency for Environment Security (AFFSET), French National Institute for Population Health Surveillance (INVS), Paris–Sud University, French National Institute for Health Education (INPES), Nestlé, Mutuelle Générale de l'Education Nationale (MGEN), French Speaking Association for the Study of Diabetes and Metabolism (Alfediam) and National Agency for Research (ANR).
There is no conflict of interest. P. D. performed the study's analysis and wrote the paper. A. F. was in charge of the coordination of the data file and analysis. B. De L.-G. participated in the setting of the dietary data files. M.-A. C. coordinates the EDEN study, supervised the analysis and participated in the design of the EDEN study, with M. K., P. D., M. S. and G. M. also. V. G. and O. T. coordinate the EDEN study in Poitiers and Nancy. All co-authors reviewed the paper.









