The meat of pigeon squabs can be compared with that of broilers in terms of nutritive value and acceptability. Pigeon squabs have been considered profitable sources of poultry meat from Columba species as they grow more rapidly with minimum input(Reference Bhuyan, Nath and Hazarika1). Squabs have a high growth rate, which is attributed to special crop milk feeding. Several fatty acids available in crop milk are necessary for normal growth(Reference Desmeth2). Therein, linoleic acid (LA) is predominantly essential for poultry and its higher derivative arachidonate is the most important(Reference Reiser3–Reference Watkins5).
The symptoms of LA deficiency in poultry manifest in various aspects, such as retarded growth, increased water consumption, enlarged liver with increased lipid content and reduced resistance to disease(Reference Watkins5). In addition, a study stated that the LA-enriched diet enhanced growth in hens immunised with Mycobacterium butyricum after immunisation(Reference Sijben, De-Groot and Nieuwland6). The maternal LA isomer regulated hepatic lipid metabolism via the adenosine 5’-monophosphate-activated protein kinase signalling pathway in chick embryos(Reference Fu, Zhang and Yao7). Additionally, our group previously found that parental dietary LA supplementation altered lipid metabolism and antioxidant status in pigeon squabs in a dose-dependent manner(Reference Xu, Ma and Dong8). Because of its high concentration in crop milk, LA is considered to be an important active substrate to maintain pigeon squab growth and health. However, using the pigeon squab model, we previously reported that supplemental LA had negligible effects on growth and development in pigeon squabs, but parental dietary LA at a concentration of 1 % could have beneficial effects on maintaining squab health, as reflected by the improved antioxidant capacity and lipid metabolism(Reference Xu, Ma and Dong8). The present study continued to characterise the effects of LA on maintaining squab health from an intestinal perspective.
Studies in vivo and in vitro have focused on the effects of LA on intestinal health and immune function over the years(Reference Li, Xu and Liu9–Reference Rodrigues, Vinolo and Magdalon13). Li et al.(Reference Li, Xu and Liu9) indicated that in Caco2 cells, LA up-regulated both the mRNA and protein levels of ApoA-IV which is produced predominantly in the intestine and functions in anti-inflammation(Reference Vowinkel, Mori and Krieglstein14). In Ht-29 cells, the LA isomer inhibits cell proliferation and stimulates apoptosis(Reference Park, Cho and Kim10). Moreover, in humans, duodenal ulcers were associated with a deficiency of dietary LA intake(Reference Grant, Palmer and Riermesma11). In IL-10-deficient mice, Su1947-Hya, an LA metabolite, ameliorated colonic inflammation(Reference Okabe, Matsuura and Kitamoto12). A study by Rodrigues et al.(Reference Rodrigues, Vinolo and Magdalon13) also indicated that LA might affect the course of inflammation. However, research related to the effects of LA on intestinal health in poultry was scant.
The Columba pigeons typically regurgitate milk from the crop by both parents for the nourishment of the young. As squabs grow, crop milk is mixed with grains soaked in the crop and gradually totally replaced by grains(Reference Sales and Janssens15). This course is a gradual ‘weaning’ process(Reference Yang and Vohra16). Weaning is generally regarded as a stressful event that could lead to intestinal impairment(Reference Pieper, Janczyk and Zeyner17). Thus, the maintenance of intestinal function and immunity is particularly important. Considering the effects of LA on intestinal health in vitro and in mammals, we hypothesised that moderate LA could also strengthen the intestinal function and immunity of pigeon squabs. Therefore, the purpose of the present study was to explore the effects of maternal dietary LA at three levels on intestinal barrier function in squabs by determining intestinal morphology, gene expression of tight junction proteins, immune cytokines and microbial flora.
Materials and methods
All experimental protocols involving animals were approved by the Animal Care and Welfare Committee of the Animal Science College and Scientific Ethical Committee of Zhejiang University (no. ZJU2013105002) (Hangzhou, China).
Animals, diets and experimental design
A total of 240 paired parent White King pigeons, 60 weeks of age, were obtained from a commercial pigeon farm (Wenzhou, China). An artificial aviary with a perch and a nest was provided for each pair. Parent pigeons were randomly allocated to four treatments, which consisted of six replications of ten pairs of pigeons each. The four treatments were as follows: control group, 1 % LA supplementation group (LA1 %), 2 % LA supplementation group (LA2 %) and 4 % LA supplementation group (LA4 %). LA (> 99·0 % purity) was obtained from a commercial supplier (Hebei Bawei Biotechnology Co., Ltd). All experimental diets were isonitrogenous and isoenergetic. The LA source was substituted for colleseed oil of the same weight to equalise the total fat level among diets(Reference Qi, Wu and Zhang18). The ingredients, nutrient levels and analysed LA content of the experimental diets for parent pigeons are shown in Table 1. The fatty acid composition of each diet analysed according to Xie et al.(Reference Xie, Wang and Wang19) is shown in online Supplementary Table S1. The fatty acid contents of crop milk are shown in online Supplementary Table S2 in which the LA levels (% total fatty acids) in crop milk from the four treatment groups were 18·20, 23·56, 28·62 and 31·52 %, respectively. The pigeons were given water ad libitum and were fed twice daily (07.00 and 15.00 hours) throughout the experiment.
Table 1. Ingredient compositions and nutrient levels of experimental diets for parental pigeons* (on as-fed basis)
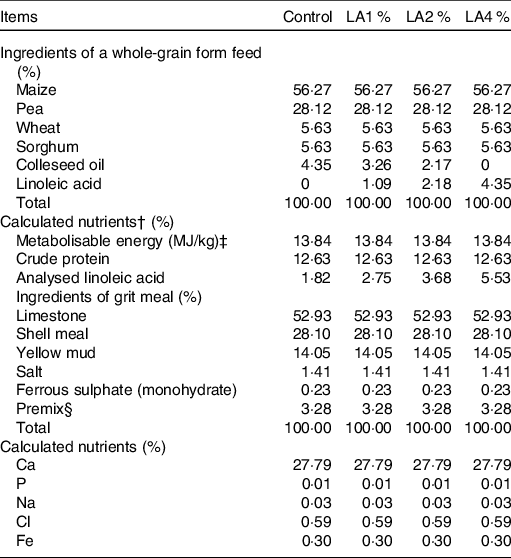
Control, control group; LA1 %, 1 % linoleic acid addition group; LA2 %, 2 % linoleic acid addition group; LA4 %, 4 % linoleic acid addition group.
* All feeds were fed in a whole-grain form at 07.00 and 15.00 hours each day, and grit meal was offered to the birds on a continuous basis.
† Nutrient values were calculated from tables of feed composition and nutritive values in China (twenty-eighth edition, 2017).
‡ Metabolisable energy values determined in pigeons were calculated from those reported for chickens in accordance with a previous study (Hullar et al. (Reference Hullar, Meleg and Fekets68)), which observed that the metabolisable energy values of feed in pigeons did not differ significantly from those in chickens.
§ The premix provided the following per kg of diet: vitamin A 5000 IU, vitamin E 50 IU, vitamin D3 2000 IU, copper sulphate 15 mg, manganese sulphate 45 mg, zinc sulphate 90 mg.
Each pair of parent pigeons laid two eggs in a nest. Eggs were picked out by hand, and fake eggs were put into nests to meet parents’ broodiness. The picked eggs were transferred to an incubator for an 18-d artificial incubation. On the day of hatching, 480 artificially hatched pigeon squabs with similar body weight were selected from this pigeon farm. These squabs were randomly pair-matched and assigned to the nests of the selected parent pigeons to replace the fake eggs. Each parent pair adopted a pair of squabs. Parent pigeons were fed experimental diets continually for the next 21 d. Squabs were fed crop milk that was secreted by parent pigeons. The ambient temperature was 18–26°C. The relative humidity was 60–70 %. The photoperiod was 12 h light–12 h dark throughout the entire experimental period.
Sample collection
On day 21 post-hatch, six squabs per treatment (one for each replication) were selected for sampling. The squabs were fasted for 12 h before weighing and slaughter. In addition, they were sedated before cervical dislocation. Ileal contents were sampled into sterile plastic tubes with a sterile spatula, immediately frozen in liquid N2 and then stored at −80°C for subsequent analyses. Three segments of the small intestine (duodenum, jejunum and ileum) were sampled, flushed with 0·9 % saline to remove all the contents and processed for morphological examination. The collected segments were the loop of the duodenum (duodenum), the tract before Meckel’s diverticulum (jejunum) and the final segment of the small intestine (ileum). The mucosa of each intestinal segment was carefully scraped off with a glass slide, frozen in liquid N2 rapidly and then stored at −80°C for subsequent analyses.
Secretory IgA and cytokine analysis
The homogenates of intestinal mucosa were prepared with PBS for secretory IgA (sIgA) and cytokine analyses. The sIgA concentration and the concentrations of TNF-α, IL-1β, IL-6, IL-10 and IL-4 were determined by absorbance changes at a wavelength of 450 nm with commercial ELISA kits (Shanghai Mlbio Institute) according to the manufacturer’s protocol. The final sIgA and cytokine concentrations were expressed as units per mg of protein.
RNA extraction and quantitative PCR analysis
Total RNA of the intestinal mucosa was extracted using the TRIzol procedure (Invitrogen) according to the instructions of the manufacturer. The extracted RNA was quantified by a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies Inc.). The RNA integrity was verified by native RNA electrophoresis on a 1·0 % agarose gel. Complementary DNA was synthesised from 2 μg of total RNA by M-MLV RT (Takara) with oligo dT-adaptor primers at 42°C for 60 min following the protocol of the manufacturer. The abundance of mRNA was assayed on a StepOne Plus Real-time PCR system (ABI 7500, Applied Biosystems). The specific primers used are indicated in Table 2. SYBR Green Real-time PCR Master Mix (Toyobo Co., Ltd) was used for PCR, consisting of an initial DNA denaturation of 95°C for 60 s, followed by forty cycles of 95°C for 15 s and 60°C for 60 s. Glyceraldehyde-3-phosphate dehydrogenase was considered an appropriate endogenous reference. The average gene expression relative to the endogenous reference for each sample was calculated according to the 2−ΔΔCt method(Reference Schmittgen20). The calibrator for each gene in the study was the average ΔCt value of the control group.
Table 2. Primers used for quantitative real-time PCR analysis of gene expression in domestic pigeons
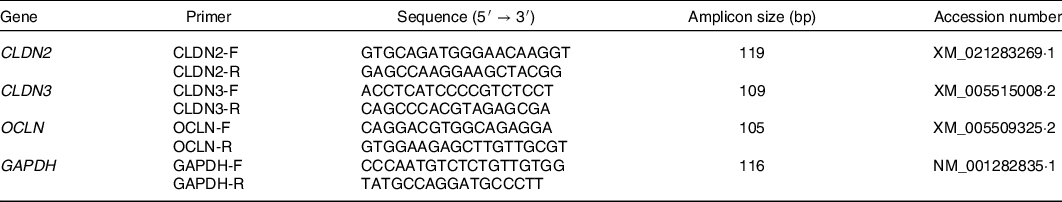
CLDN2, claudin-2; F, forward; R, reverse; CLDN3, claudin-3; OCLN, occludin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Intestinal morphological examination
Approximately 1-cm intestinal samples of each segment were collected and fixed in 10 % neutral-buffered formalin solution. Each sample was dehydrated, cleared and embedded in paraffin. Serial sections (5 μm) were placed on glass slides and submitted to two different stainings. Haematoxylin and eosin staining was used to identify villus height, villus area, crypt depth and enterocytes. Periodic acid–Schiff staining was used for the identification of goblet cells (GC). The examination was performed with light microscopy (Nikon Corp.) using Image-Pro Plus 6.0 (Media Cybernetics, Inc.). The density of enterocytes was expressed as the number per mm of epithelial layer length. The density of GC was expressed as the number per 100 enterocytes according to Tamura et al.(Reference Tamura, Soga and Yaguchi21).
16S rDNA high-throughput sequencing
Total microbial genomic DNA was extracted from the ileal contents of squabs from each treatment using the E.Z.N.A.® Stool DNA Kit (Omega Bio-Tek). The concentration and quality of the extracted DNA were assessed with 2 % agarose gel electrophoresis and a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies Inc.). The V3–V4 hypervariable region of the 16S rRNA genes was amplified by specific degenerate primers (338 F: ACTCCTACGGGAGGCAGCAG, 806 R: GGACTACHVGGGTWTCTAAT). The PCR programme was conducted with the following conditions: initial denaturation step, 98°C, 30 s; denaturation, 35 cycles, 98°C, 10 s; annealing, 54°C, 30 s; elongation, 72°C, 45 s and final extension, 72°C, 10 min. The PCR amplification products were detected by 2 % agarose gel electrophoresis. The target fragments were recovered using AMPure XT Beads (Beckman Coulter Genomics). Qubit was used to quantify the purified PCR products, and the qualified library concentration was more than 2 nm. Sequencing was performed with V3 chemistry, and 2 × 300 bp double-end reads were generated using MiSeq Reagent Kit V3 with the MiSeq Illumina platform (Illumina) at Hangzhou Personal Biotechnology Co., Ltd.
Statistical analyses
The data obtained from sIgA and cytokine analysis, quantitative PCR analyses and intestinal morphological examination were subjected to one-way ANOVA using SPSS 24.0 (SPSS Inc.) for Windows. Treatment means were separated by the Tukey least significant difference post hoc test at the P < 0·05 statistical level. When significant differences were found, orthogonal polynomial contrasts were further used to examine the linear and quadratic effects of different inclusion levels of dietary LA. Plotting was performed with GraphPad Prism 7.0 (GraphPad Software Inc.).
The raw sequence data obtained from the Illumina MiSeq platform were quality-filtered and demultiplexed using Quantitative Insights into Microbial Ecology, version 1.8.0-dev (http://qiime.org/index.html). After trimming and assembling, the final clean data were obtained and assigned into bacterial operational taxonomic units using the UCLUST function in Quantitative Insights into Microbial Ecology (http://qiime.org/scripts/pick_otus) according to a 97 % similarity threshold. The data were categorised with the Ribosomal Database Project, version 2.2 (http://sourceforge.net/projects/rdp-classifier/). The α-diversity measures, including the observed species, Chao 1 and Shannon indices, were calculated using MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/MicrobiomeAnalyst/faces/home.xhtml). A Venn diagram was generated in Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html). Principal coordinate analysis was conducted using Galaxy (http://huttenhower.sph.harvard.edu/galaxy/) according to unweighted Unifra representing β-diversity values. The Kolmogorov–Smirnov programme was used to verify the normality of the data. Data with a normal distribution were counted by one-way ANOVA, and multiple comparisons were conducted by Tukey post hoc tests. Non-normally distributed data were analysed by the Kruskal–Wallis test and Duncan’s multiple range test. P < 0·05 indicates a significant difference.
Results
Intestinal morphometric traits
Haematoxylin and eosin staining of the intestines (duodenum, jejunum and ileum) of the four groups showed the effects of maternal dietary LA on the intestinal morphometric traits in squabs (Fig. 1). In the duodenum, the villus height, villus area and villus height to crypt depth ratio (VCR) responded to increasing supplementation of LA in a linear (P < 0·001, P < 0·001 and P = 0·01, respectively) and quadratic (all P < 0·001) manner; their maximal response was observed in LA1 %. In the jejunum, the villus height and villus area emerged as linear (P = 0·001 and 0·01, respectively) and quadratic (both P < 0·001) trends with their maximum observed in LA1 % as LA supplementation was increased. The crypt depth showed a linear (P < 0·001) increase as the level of LA supplementation increased. In the ileum, villus height and the VCR responded to increasing supplementation with LA in a linear (P = 0·001 and 0·002) and quadratic (both P < 0·001) manner, and their maximum values were observed in LA1 %. The villus area displayed a quadratic (P < 0·001) trend with the maximum in LA1 % as LA supplementation was increased. In addition, the VCR in the jejunum and crypt depth in the duodenum and ileum were not significantly different among the four groups. There was no significant difference (P > 0·05) in enterocyte density among the four experimental groups.
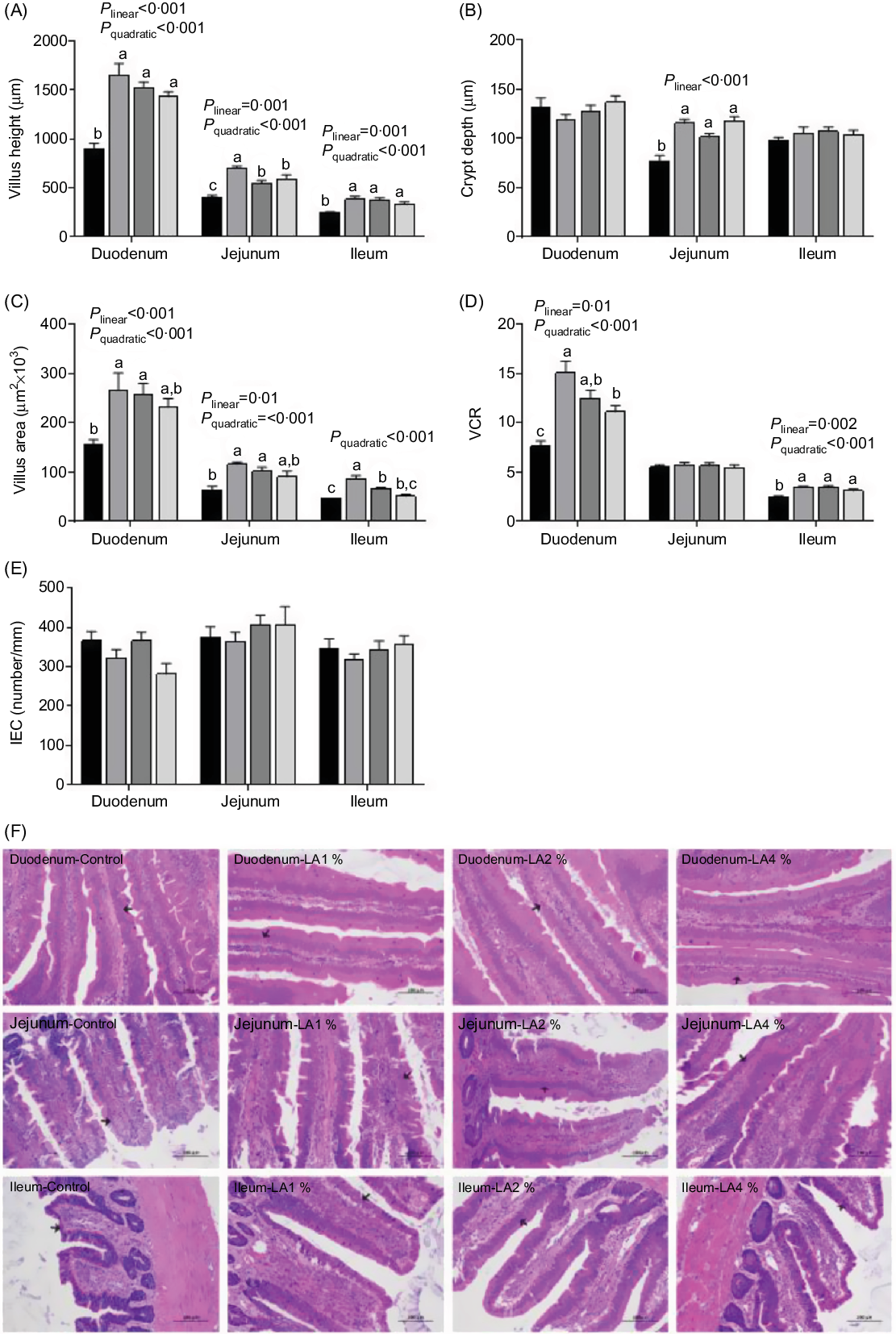
Fig. 1. Effects of maternal dietary linoleic acid (LA) on intestinal morphometric trait in domestic pigeon squabs. (A) Villus height, (B) crypt depth, (C) villus area, (D) ratio of villus height to crypt depth (VCR), (E) density of enterocytes (intestinal epithelial cells; IEC), (F) haematoxylin and eosin staining of intestine (duodenum, jejunum and ileum) in the four treatment groups. The arrow points to enterocyte. Bar = 100 μm. Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Values are means with their standard errors of six squabs, n 6. a,b,c Means with unlike letters are significantly different (Tukey test, P < 0·05). (A–E) ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %.
, LA4 %.
Tight junction protein gene expression
Tight junction proteins include the claudin family and occludin(Reference Khatlab, Del Vesco and de Oliveira Neto22). Recently, claudin-1, claudin-2, claudin-3, claudin-5, claudin-16 and occludin have been reported in poultry(Reference Ozden, Black and Ashwell23–Reference Pham, Kan and Huang25). Among these genes, the mRNA expression of claudin-2 (CLDN2), claudin-3 (CLDN3) and occludin (OCLN) is usually used to evaluate tight junction functions and barrier permeability(Reference Rajput, Li and Xin26). In the present study, the effects of maternal dietary LA on the mRNA expression of these three tight junction proteins are shown in Fig. 2. In the duodenum, the mRNA expression of CLDN3 exhibited a quadratic (P = 0·03) trend, with the maximum observed in LA1 % as LA supplementation was increased. CLDN2 mRNA expression responded to increasing supplementation with LA in a linear (P = 0·02) and quadratic (P < 0·001) manner, with the minimum in LA1 %. Although maternal dietary LA had no significant effect (P > 0·05) on the mRNA expression of OCLN, a quadratic (P = 0·01) trend was observed, with the maximum response in LA2 %. In the jejunum, the mRNA expression of CLDN2 exhibited a quadratic (P < 0·001) trend, with the minimum in LA1 % as the supplementation of LA was increased. However, CLDN3 and OCLN in the jejunum had no significant differences (P > 0·05) among the four treatments. In the ileum, CLDN2 mRNA expression displayed linear (P = 0·001) and quadratic (P = 0·004) trends, with the maximum observed in LA4 % as LA supplementation was increased. Moreover, CLDN3 and OCLN mRNA expression responded to increasing supplementation of LA in a linear (both P = 0·003) and quadratic (P = 0·003; P < 0·001) manner; their maximal responses were observed in LA1 %.

Fig. 2. Effects of maternal dietary linoleic acid on mRNA expression of tight junction proteins in domestic pigeon squabs. (A) mRNA expression of claudin-2 (CLDN2), (B) mRNA expression of claudin-3 (CLDN3), (C) mRNA expression of occludin (OCLN). Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Values are means with their standard errors of six squabs, n 6. a,b,c Means with unlike letters are significantly different (Tukey test, P < 0·05). (A–C) ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %.
, LA4 %.
Goblet cell density
Periodic acid–Schiff staining of the intestines (duodenum, jejunum and ileum) of the four groups showed the effects of maternal dietary LA on the density of GC (Fig. 3). The GC density in the duodenum and jejunum responded linearly to increasing supplementation with LA (P = 0·006; P < 0·001), with the maximum observed in the LA1 % and control group, respectively. The GC density in the ileum displayed linear (P = 0·02) and quadratic (P = 0·009) trends, with the maximum density in LA1 %.

Fig. 3. Effects of maternal dietary linoleic acid on the density of intestinal goblet cells (GC) in domestic pigeon squabs. (A) Density of GC (per 100 intestinal epithelial cells; IEC), (B) periodic acid–Schiff staining of intestine (duodenum, jejunum and ileum) in the four treatment groups. The arrow points to GC. Bar = 50 μm. Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Values are means with their standard errors of six squabs, n 6. a,b Means with unlike letters are significantly different (Tukey test, P < 0·05). (A) ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %.
, LA4 %.
Secretory IgA and cytokine analysis
The effects of maternal dietary LA on the concentrations of sIgA and cytokines are shown in Fig. 4. In the duodenum, sIgA and IL-10 concentrations responded quadratically to increasing LA supplementation (P = 0·009 and 0·001), with their maximum observed in LA1 %. The IL-6 concentration exhibited linear (P < 0·001) and quadratic (P = 0·05) trends, with the minimum concentration in LA1 % as LA supplementation was increased. TNF-α and IL-1β concentrations increased linearly (both P < 0·001) with increasing LA supplementation, whereas the IL-4 concentration decreased linearly (P = 0·002). In the jejunum, the TNF-α concentration exhibited linear (P < 0·001) and quadratic (P = 0·01) trends, with the minimum in LA1 % as LA supplementation was increased. The IL-10 concentration exhibited linear (P = 0·009) and quadratic (P = 0·003) trends, with the maximum in LA1 % as LA supplementation was increased. IL-6 concentrations responded quadratically to increasing LA supplementation (P = 0·005), with the minimum observed in LA1 %. The IL-4 concentration responded quadratically to increasing LA supplementation (P = 0·05), with their maximum observed in LA1 %. There was no significant difference (P > 0·05) in the sIgA concentration in the jejunum among the four experimental groups. In the ileum, TNF-α and IL-1β concentrations increased linearly (P = 0·002 and 0·04, respectively) with increasing LA supplementation, whereas the IL-10 concentration decreased linearly (P = 0·03). The IL-4 concentration responded to increasing LA supplementation quadratically (P = 0·004), with their maximum observed in LA1 %. The IL-6 concentration responded quadratically to increasing LA supplementation (P < 0·001), with the minimum observed in LA1 %. There was no significant difference (P > 0·05) in the sIgA concentration in the ileum among the four experimental groups.
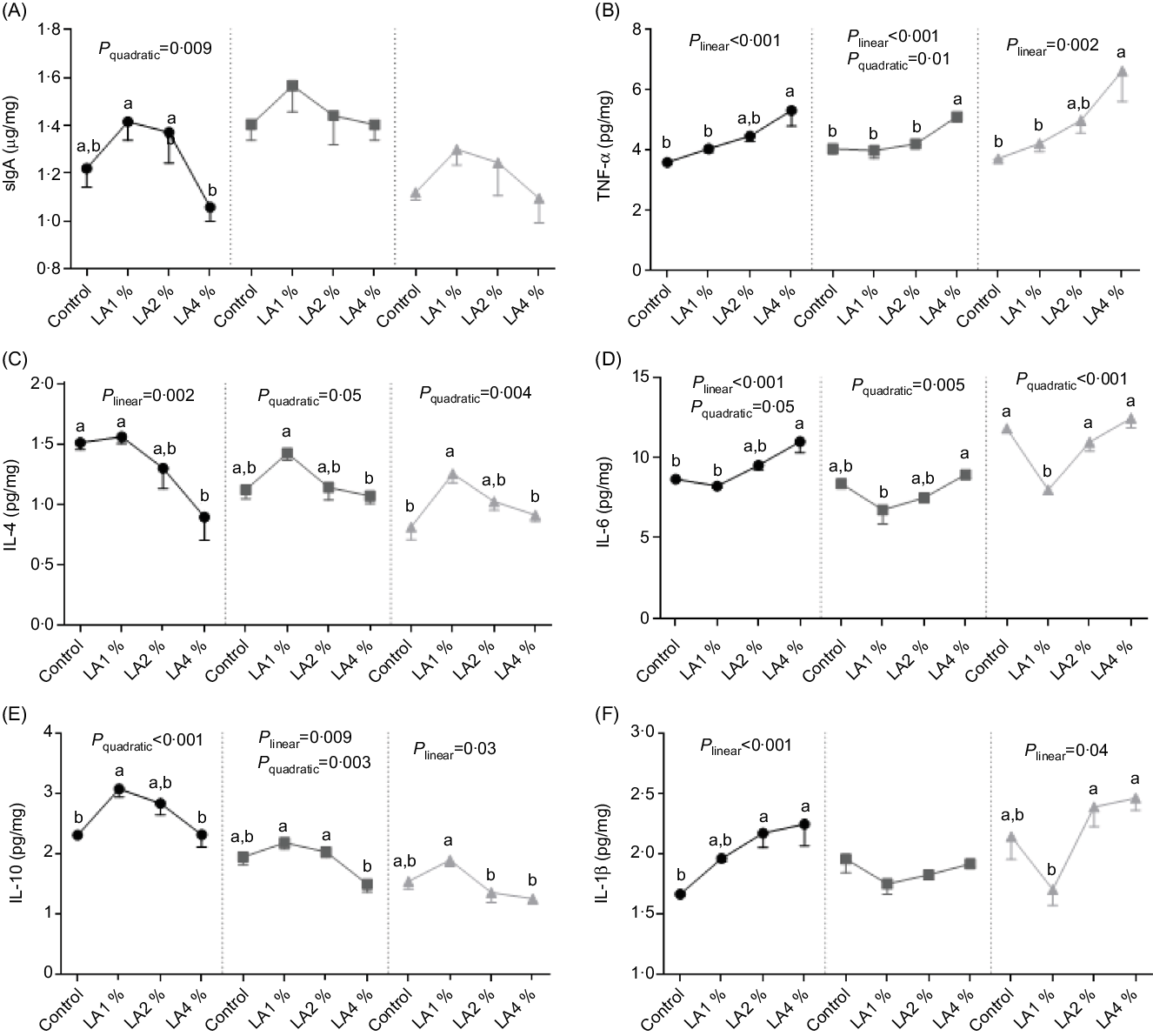
Fig. 4. Effects of maternal dietary linoleic acid on intestinal secretory IgA (sIgA) and cytokine concentrations in domestic pigeon squabs. (A) sIgA, (B) TNF-α, (C) IL-4, (D) IL-6, (E) IL-10, (F) IL-1β. Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Values are means with their standard errors of six squabs, n 6. a,b Means with unlike letters are significantly different (Tukey test, P < 0·05). (A–F) ![]() , Duodenum;
, Duodenum; ![]() , jejunum;
, jejunum; ![]() , ileum.
, ileum.
Ileal microbiota analysis
Ileal microbiota analyses of twenty-four samples were performed. An average of 33 218 raw sequences per sample was obtained. After trimming and assembling, an average of 29 573 valid sequences per sample remained. Furthermore, an average of 421 operational taxonomic units based on 97 % sequence similarity was identified from these sequences.
The effects of maternal dietary LA on the α-diversity of ileal microbiota in domestic pigeon squabs are shown in Fig. 5. Rarefaction curves for the observed species (Fig. 5(A)) approached a plateau, indicating the availability of sufficient reads to reflect each microbiome community. The total operational taxonomic unit number of ileal contents was maximum in LA1 %, as was the unique operational taxonomic unit number (Fig. 5(B)). No significant difference was observed in the Chao1 α-diversity index among the four groups (Fig. 5(C)). The Shannon α-diversity index was significantly increased in the LA1% and LA2 % compared with the control group, whereas there was no significant difference between the control group and LA4 % (Fig. 5(D)). The effects of maternal dietary LA on the β-diversity of ileal microbiota in domestic pigeon squabs are shown in Fig. 6. As shown in Fig. 6(A), the microbial communities from each treatment were mainly divided into four groups. Clustering analysis at the phylum level is shown in Fig. 6(B). Firmicutes and Proteobacteria were the predominant phyla in the ileal microbiota, followed by Actinobacteria, Cyanobacteria and Bacteroides. All samples at the phylum level were not clustered into four groups. However, clustering analysis at the genus level (Fig. 6(C)) showed that samples at the genus level were clearly clustered into four groups, although one sample in LA4 % was clustered with LA2 %. Lactobacillus was the predominant genus in most samples. A cladogram representative of the dominant microflora, which were called biomarkers identified from phyla to genera, is shown in Fig. 7. It was observed that Lactobacillus was the biomarker of the control group and Butyrivibrio was the biomarker of LA1 % at the genus level. In addition, the biomarkers of LA2 % were Aeriscardovia, Deinococcus, Streptococcus, Rhodopseudomonas, Methylobacterium, Sphingomonas, Ralstonia and Pseudomonas. The biomarkers of LA4 % were Chlorophyta, Enterococcus and Escherichia.
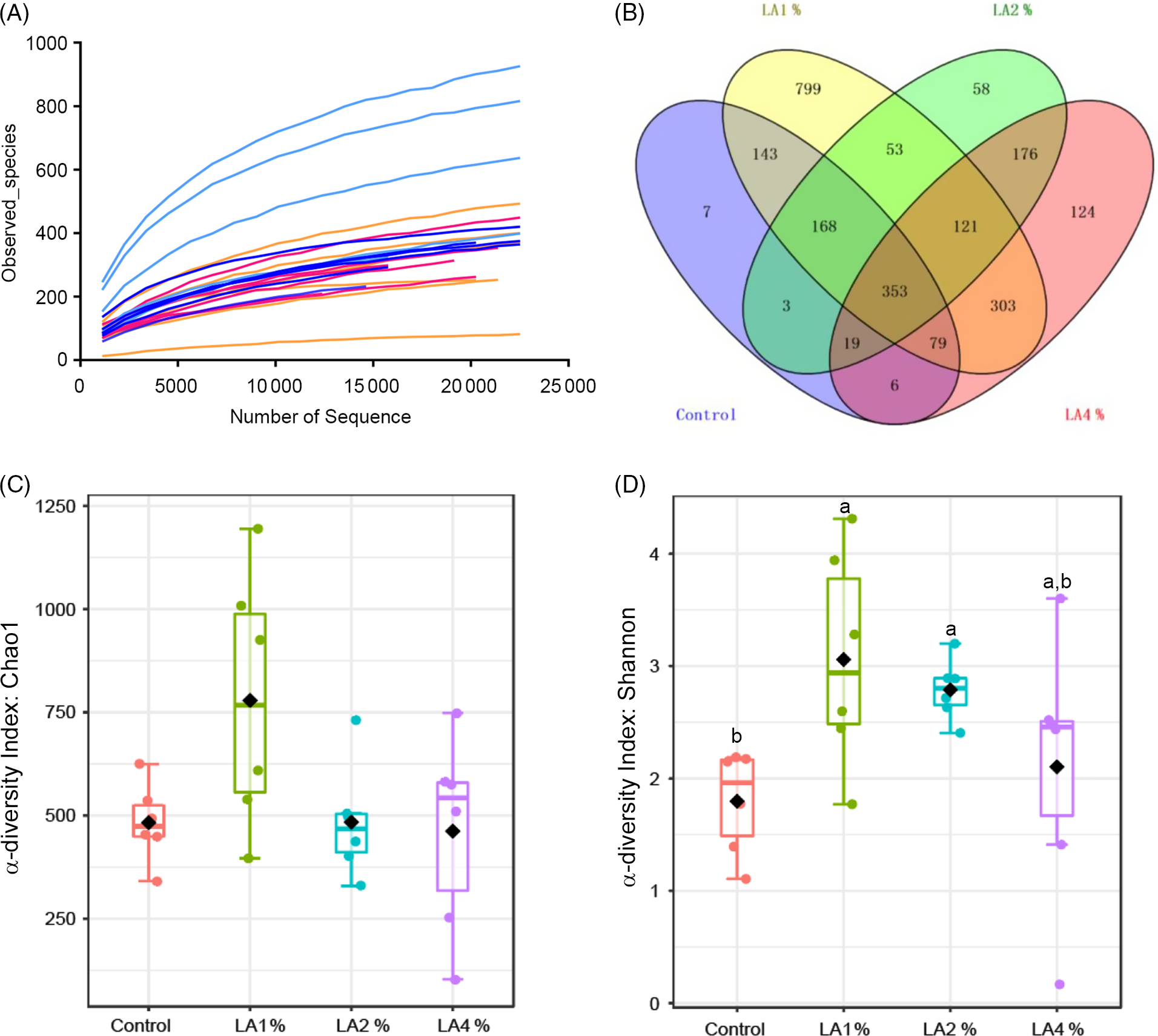
Fig. 5. Effects of maternal dietary linoleic acid on the α-diversity of ileal microbiota in domestic pigeon squabs among groups. (A) Rarefaction curves, (B) operational taxonomic unit (OTU) distribution, (C) α-diversity index: Chao1, (D) α-diversity index: Shannon. Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Values are means with their standard errors of six squabs, n 6. a,b Means with unlike letters are significantly different (Kruskal–Wallis test and Duncan’s test, P < 0·05). (A) ![]() , Control;
, Control; ![]() , LA1;
, LA1; ![]() , LA2;
, LA2; ![]() , LA4. (C and D)
, LA4. (C and D) ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %.
, LA4 %.

Fig. 6. Effects of maternal dietary linoleic acid on the β-diversity of ileal microbiota in domestic pigeon squabs among groups (n 6). (A) Principal coordinates analysis, (B) clustering analysis at phylum level, (C) clustering analysis at genus level. Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. (A) ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %. (B)
, LA4 %. (B) ![]() , p_Firmicutes;
, p_Firmicutes; ![]() , p_Proteobacteria;
, p_Proteobacteria; ![]() , p_Actinobacteria;
, p_Actinobacteria; ![]() , p_Cyanobacteria;
, p_Cyanobacteria; ![]() , p_unclassified;
, p_unclassified; ![]() , p_Acidobacteria;
, p_Acidobacteria; ![]() , p_Bacteroidetes;
, p_Bacteroidetes; ![]() , p_Deinococcus_Thermus;
, p_Deinococcus_Thermus; ![]() , p_Tenericutes;
, p_Tenericutes; ![]() , p_Verrucomicrobia. (C)
, p_Verrucomicrobia. (C) ![]() , g_Lactobacillus;
, g_Lactobacillus; ![]() , g_Ralstonia;
, g_Ralstonia; ![]() , others;
, others; ![]() , g_Streptophyta;
, g_Streptophyta; ![]() , g_Sphingomons;
, g_Sphingomons; ![]() , g_Candidatus_Arthromitus;
, g_Candidatus_Arthromitus; ![]() , g_Streptococcus;
, g_Streptococcus; ![]() , g_Escherichia;
, g_Escherichia; ![]() , g_Enterococcus;
, g_Enterococcus; ![]() , g_Acinetobacter;
, g_Acinetobacter; ![]() , g_Veillonella;
, g_Veillonella; ![]() , g_Aeriscardovia;
, g_Aeriscardovia; ![]() , g_Lachnospiraceae_unclassified;
, g_Lachnospiraceae_unclassified; ![]() , g_Ruminococcaceae_unclassified;
, g_Ruminococcaceae_unclassified; ![]() , g_Butyrivibrio;
, g_Butyrivibrio; ![]() , g_Clostridium_sensu_stricto;
, g_Clostridium_sensu_stricto; ![]() , g_Enterobacteriaceae_unclassified;
, g_Enterobacteriaceae_unclassified; ![]() , g_Lactococcus;
, g_Lactococcus; ![]() , g_Oscillibacter;
, g_Oscillibacter; ![]() , g_Porphyromonadaceae_unclassified;
, g_Porphyromonadaceae_unclassified; ![]() , g_Turicibacter.
, g_Turicibacter.
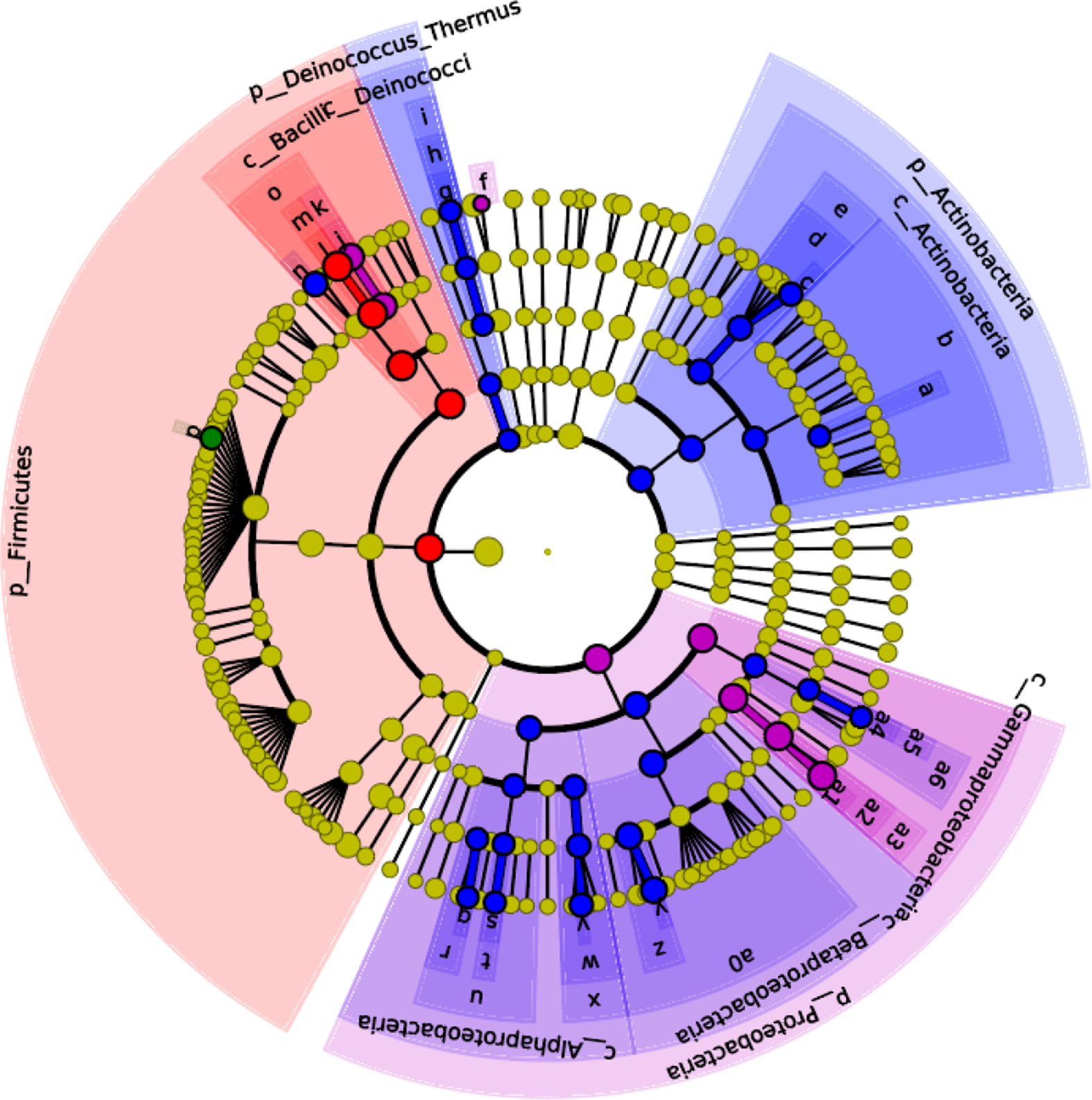
Fig. 7. Effects of maternal dietary linoleic acid on the microbial biomarkers of ileum in domestic pigeon squabs (n 6). Control = control group; LA1 % = 1 % linoleic acid supplementation group; LA2 % = 2 % linoleic acid supplementation group; LA4 % = 4 % linoleic acid supplementation group. Linear discriminant analysis effect size analysis shows differentially abundant genera as biomarkers determined using the Kruskal–Wallis test (P < 0·05) with the logarithmic linear discriminant analysis score > 2·0. ![]() , Control;
, Control; ![]() , LA1 %;
, LA1 %; ![]() , LA2 %;
, LA2 %; ![]() , LA4 %;
, LA4 %; ![]() , a: f_Microbacteriaceae;
, a: f_Microbacteriaceae; ![]() , b: o_ Actinomycetales;
, b: o_ Actinomycetales; ![]() , c: g_Aeriscardovia;
, c: g_Aeriscardovia; ![]() , d: f_Bifidobacteriaceae;
, d: f_Bifidobacteriaceae; ![]() , e: o_Bifidobacteriales;
, e: o_Bifidobacteriales; ![]() , f: g_Chlorophyta;
, f: g_Chlorophyta; ![]() , g: g_Deinococcus;
, g: g_Deinococcus; ![]() , h: f_Deinococcaceae;
, h: f_Deinococcaceae; ![]() , i: o_Deinococcales;
, i: o_Deinococcales; ![]() , j: g_Enterococcus;
, j: g_Enterococcus; ![]() , k: f_Enterococcaceae;
, k: f_Enterococcaceae; ![]() , l: g_Lactobacillus;
, l: g_Lactobacillus; ![]() , m: f_Lactobacillaceae;
, m: f_Lactobacillaceae; ![]() , n: g_Streptococcus;
, n: g_Streptococcus; ![]() , o: o_Lactobacillales;
, o: o_Lactobacillales; ![]() , p: g_Butyrivibrio;
, p: g_Butyrivibrio; ![]() , q: g_Rhodopseudomonas;
, q: g_Rhodopseudomonas; ![]() , r: f_Bradyrhizobiaceae;
, r: f_Bradyrhizobiaceae; ![]() , s: g_Methylobacterium;
, s: g_Methylobacterium; ![]() , t: f_Methylobacteriaceae;
, t: f_Methylobacteriaceae; ![]() , u: o_Rhizobiales;
, u: o_Rhizobiales; ![]() , v: g_Sphingomonas;
, v: g_Sphingomonas; ![]() , w: f_Sphingomonadaceae;
, w: f_Sphingomonadaceae; ![]() , x: o_Sphingomonadales;
, x: o_Sphingomonadales; ![]() , y: g_Ralstonia;
, y: g_Ralstonia; ![]() , z: f_Burkholderiaceae;
, z: f_Burkholderiaceae; ![]() , a0: o_Burkholderiales;
, a0: o_Burkholderiales; ![]() , a1: g_Escherichia;
, a1: g_Escherichia; ![]() , a2: f_Enterobacteriaceae;
, a2: f_Enterobacteriaceae; ![]() , a3: o_Enterobacteriales;
, a3: o_Enterobacteriales; ![]() , a4: g_Pseudomonas;
, a4: g_Pseudomonas; ![]() , a5: f_Pseudomonadaceae;
, a5: f_Pseudomonadaceae; ![]() , a6: o_Pseudomonadales.
, a6: o_Pseudomonadales.
Discussion
The intestine plays a crucial role in nutrient digestion and absorption(Reference Kim and Ho27). Intestinal functions are usually reflected by intestinal morphology, such as villus height, villus area and crypt depth(Reference Li, Yin and Wang28). In the present study, maternal dietary LA supplementation resulted in a higher villus height and villus area compared with those of the control group regardless of intestinal site. In addition, the highest was observed in LA1 %. A previous study indicated that the increased villus area allowed animals to absorb more nutrients into the body(Reference Wang, Li and Wang29). The increase in villus height might be linked with the improved health status of animals(Reference Upadhaya, Jiao and Kim30). Therefore, our results suggested that maternal dietary LA, especially at the 1 % level, could improve the intestinal morphology of squabs, indicating that their intestinal development was strengthened. A study by Thomson et al.(Reference Thomson, Keelan and Clandinin31) indicated that LA-deficient rats had significantly lower villus heights in the ileum than control rats, proving the importance of dietary LA. The higher the VCR, the higher the maturity and functional capacity of enterocytes(Reference Chee, Iji and Choct32). In the present study, the VCR in both the duodenum and ileum was significantly increased in squabs by maternal dietary LA supplementation. The VCR in the jejunum was not significantly changed by LA supplementation because both villus height and crypt depth in the jejunum were significantly increased compared with the control. Taken together, LA enhanced the development of the duodenum and ileum more obviously, and the effects of LA on the jejunum in pigeon squabs might be complicated. Because information about the relationship between LA and intestinal morphology in poultry is not available, further studies are needed to explore the mechanisms.
The intestinal epithelium serves as a front-line protective barrier between the host and the external environment against enteric pathogens, food antigens and physiochemical stresses(Reference Pinton, Tsybulskyy and Lucioli33,Reference Buzza, Netzel-Arnett and Shea-Donohue34) . Enterocytes that are closely connected with one another are the main components of the intestinal epithelium(Reference Hui, Ren and Cao35). In our study, the density of enterocytes in the epithelial layer had no significant effect in the three intestinal tracts of squabs by maternal LA supplementation, meaning that the density of enterocytes was not affected by increased villus height. This result further confirmed that LA promoted villus growth. GC in the intestine play an indispensable role in mucosal protection mainly by secreting mucus that, together with water, Fe and peptides, forms a viscous gel reticular mucus layer overlying the epithelial cell surface(Reference Calik and Ergün36). The density of GC indicates the capability of mucus secretion(Reference Kridtayopas, Rakangtong and Bunchasak37). In the present study, the maximum density of GC in the duodenum and ileum was observed in LA1 %. The maximum GC density in the jejunum was found in the control, and the GC density of the jejunum in LA4 % was significantly lower than that in the control. These results suggested that moderate maternal LA (1 %) supplementation might be beneficial to GC formation and distribution in the duodenum and ileum, thus enhancing mucosal protection, whereas excessive LA (4 %) supplementation might have the opposite effect in the jejunum. We conjectured that LA might alter the mucus layer of the intestine in pigeon squabs in a site-dependent and dose-dependent manner. Unfortunately, research regarding the influence of LA on the intestinal mucus layer was limited, and we have been unable to find any other study with which to confirm this result. Further studies are still needed to be conducted to verify this conjecture. Overall, GC density increased with distal progression along the intestine in squabs, which was similar to chickens(Reference Reynolds, Cloft and Wong38).
Tight junctions are present between individual enterocytes(Reference Karcher and Applegate39). Tight junctions create a major barrier in the intercellular space, which regulates the movement of water and solutes across epithelia(Reference Anderson40). The protein composition of tight junctions includes claudins and occludins(Reference Khatlab, Del Vesco and de Oliveira Neto22). Claudins perform different functions and can roughly be divided into two types: those involved in barrier formation and those important in channel formation(Reference Colegio, van Itallie and Mccrea41). CLDN2 expression was associated with increased tight junction permeability and decreased tightness of the epithelial barrier(Reference Suzuki, Yoshinaga and Tanabe42). In inflammatory bowel disease, CLDN2 expression correlated positively with inflammatory activity(Reference Weber, Nalle and Tretiakova43). In the present study, the lowest mRNA expression of CLDN2 was observed in LA1 % regardless of the intestinal site, whereas CLDN2 mRNA expression in the ileum in LA4 % was significantly higher than that in the control group. The results indicated that a moderate maternal dietary level of LA (1 %) could strengthen tight junctions by decreasing their permeability, while excessive LA supplementation might weaken tight junctions. A similar result was also observed in juvenile grass carp (Ctenopharyngodon idella)(Reference Zeng, Jiang and Liu44). CLDN3 is known to be a ‘tightening’ claudin(Reference Milatz, Krug and Rosenthal45). CLDN3 expression decreased in association with increased permeability in hens(Reference Ariyadi, Isobe and Yoshimura46). In the present study, the highest mRNA expression of CLDN3 in the duodenum and ileum was observed in LA1 %, whereas CLDN3 mRNA expression in the ileum in LA4 % was significantly lower than that in the control group. These results suggested that 1 % LA supplementation in maternal diets could decrease permeability in squabs, but high-dose LA supplementation had adverse effects. The indication of the results was consistent with that of CLDN2. OCLN plays an important role in the assembly and maintenance of tight junctions(Reference Gadde, Oh and Lee47). In our study, OCLN mRNA expression was up-regulated in the ileum by supplementation with three LA levels. The result was inconsistent with that of CLDN2 and CLDN3, suggesting that different tight junction proteins had different expression patterns.
The intestine is not only a nutrient digestion and absorption centre but also an important place for immune function(Reference Wittig and Zeitz48). SIgA is a vital immune effector molecule on the intestinal mucosal surface, which is the first line of defense against the adhesion of pathogenic bacteria in the intestinal mucosa(Reference Pabst49). In the present study, the sIgA in the duodenum was maximum in LA1 %, which indicated that maternal dietary LA supplementation at 1 % might enhance the ‘immune exclusion’ ability by stimulating the secretion of sIgA to some extent. Cytokines are important mediators and regulators of the host against foreign antigens, and their main function is to coordinate the functional activities of immune system cells(Reference Liu, Zhang and Han50). IL-1β, TNF-α and IL-6 generated by macrophages are proinflammatory cytokines that exert multiple roles in both physiological and pathological conditions(Reference Arranz, Arriero and Villatoro51,Reference Maloy and Powrie52) . IL-4 and IL-10 are classified as anti-inflammatory cytokines because they prevent the production of proinflammatory cytokines such as IL-2 and IL-12 by stimulated monocytes/macrophages(Reference Nathan and Denizot53). In our study, the IL-6 in the intestine was minimised by supplementation with 1 % maternal dietary LA, and the IL-4 and IL-10 were maximised in this group, suggesting that 1 % maternal dietary LA supplementation could partly reduce inflammation. The decreased IL-6 was consistent with the CLDN2 expression results since a previous study showed that IL-6 markedly induced CLDN2 expression, which is associated with increased tight junction permeability, undermining the integrity of the intestinal barrier(Reference Suzuki, Yoshinaga and Tanabe42). Overall, appropriate LA supplementation consolidated intestinal function. However, excessive (4 %) LA supplementation might lead to adverse impacts. In the present study, IL-1β and IL-6 in the duodenum as well as TNF-α in the intestine of squabs in the LA4 % were significantly higher than those in the control group, whereas IL-4 in the duodenum was lower. A previous study showed that a higher than normal ratio of proinflammatory and anti-inflammatory factors promoted pathological changes in tissues(Reference Xu, Yu and Liu54). Moreover, IL-1β, TNF-α and IL-6 are expanded in inflammatory bowel disease(Reference Maloy and Powrie52). These results suggested that 4 % maternal dietary LA supplementation might enhance intestinal pathology.
The intestine contains an enormous number of micro-organisms primarily consisting of bacteria, which play a crucial role in animal health(Reference Li, Fu and Ma55). Dietary composition not only affects the intestinal epithelial barrier but also significantly influences the microbial community(Reference Guzman, Conlin and Jobin56). In our study, maternal dietary LA at 1 and 2 % increased the diversity of intestinal microbiota in squabs. It is widely believed that the higher the diversity, the better the intestine health, because diversity is one of the key determinants of colonisation resistance against invading pathogens(Reference Dong, Azzam and Zou57). The results indicated that squabs had greater resistance in LA1 and LA2 %. In addition, Butyrivibrio was the biomarker of LA1 %, which means that the relative abundance of Butyrivibrio was significantly higher than that of the other groups. The bacterial genus can produce butyrate which as a SCFA can lower the pH of the intestinal lumen and regulate the microbial composition especially to stimulate the growth of beneficial bacteria(Reference Le Leu, Brown and Hu58,Reference Louis and Flint59) . The results further revealed that the maintenance of intestinal health was strengthened in squabs by supplementing maternal dietary LA at 1 %. However, the changes in the microbial community in LA2 % were complicated. On the one hand, the biomarkers of LA2 % included beneficial bacteria. Therein, Aeriscardovia belongs to Bifidobacteria which can protect against enteropathogenic infection through acetate production(Reference Fukuda, Toh and Hase60). Streptococcus was observed to be negatively related to inflammation in previous research(Reference Fernández, Redondo-Blanco and Gutiérrez-del-Río61). On the other hand, the biomarkers of LA2 % also included opportunistic pathogens such as Methylobacterium, Ralstonia and Pseudomonas (Reference Truant, Gulati and Giger62–Reference Hayward64). Thus, it was vital to maintain the intestinal lumen environment and the balance between these bacteria in LA2 %. Unfortunately, pathogenic Escherichia was observed in the biomarkers of LA4 %. Previous studies showed that inflammation caused by Crohn’s disease and colitis was characterised by an increased abundance of Escherichia in the intestinal microbiota(Reference Darfeuille-Michaud65–Reference Zhang, Xu and Wang67). As mentioned above, 4 % maternal dietary LA supplementation promotes inflammation. Thus, excessive (4 %) LA supplementation might result in the breakdown of homoeostasis between the mucosal immune system and the intestinal microbiota.
In conclusion, maternal dietary LA at all levels could improve intestinal morphology in squabs. Therein, appropriate dosage (1 %) supplementation might enhance mucosal protection and epithelium barriers in squabs by promoting GC formation and distribution in the intestine, and strengthening tight junctions between enterocytes. Moreover, moderate supplementation consolidated intestinal immunity and the luminal microbial environment by partially reducing inflammation and promoting the growth of beneficial bacteria. However, unfortunately, excessive (4 %) LA supplementation might lead to adverse impacts on immunity and microbiota. We conjectured that LA might alter intestinal barrier function in pigeon squabs in a dose-dependent manner. The reason might partly rely on that moderate LA satisfied squabs, whereas excessive addition impacted the balance between n-6 and n-3 PUFA, which need further study to verify.
Acknowledgements
This article has been released as a pre-print at https://www.researchsquare.com/article/rs-60857/v1 (DOI:10.21203/rs.3.rs-60857/v1).
This work was supported by the Fundamental Research Funds for the National Natural Science Foundation of China (31902173), the Central Universities (2019QNA6028) and the Six party projects of agriculture, rural areas and farmers in Zhejiang Province (2019SNLF017).
The article was mainly conceived and designed by X. D. and X. Z. Q. X. performed the experiments and analysed the data. The article was mainly written by Q. X. X. W. and J. W. revised it critically for important content. All authors have read and approved the final manuscript.
The authors declare that they have no competing interests. All authors provide consent to publish this article.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004973












