Maternal nutrition during pregnancy may influence the risk of leukaemia in children through its role in fetal development, including the synthesis and repair of DNA, development of epigenetic processes and establishment of the child’s immune system. Although most of the studies to date have focused on the relationship between maternal folic acid intake and risk of childhood leukaemia( Reference Metayer, Milne and Dockerty 1 ), there is evidence that maternal consumption of specific food groups may influence childhood leukaemia risk. Previous studies, including findings from the California Childhood Leukaemia Study (CCLS), which is the basis for this analysis, have suggested that higher maternal consumption of fruits and vegetables may be associated with a reduced risk of childhood acute lymphoblastic leukaemia (ALL)( Reference Jensen, Block and Buffler 2 – Reference Kwan, Jensen and Block 4 ) and possibly infant leukaemia (i.e. acute leukaemia diagnosed under 1 year of age)( Reference Spector, Xie and Robison 5 ). Previous studies have also found that other food groups, specifically protein sources such as fish and seafood( Reference Petridou, Ntouvelis and Dessypris 3 ) and beans and beef( Reference Jensen, Block and Buffler 2 , Reference Kwan, Jensen and Block 4 ), may reduce the risk of ALL. In addition, there is some evidence that maternal consumption of certain foods such as sugars or syrups may increase the risk of ALL( Reference Petridou, Ntouvelis and Dessypris 3 , Reference Bonaventure, Rudant and Goujon-Bellec 6 ).
Studies examining maternal diet and childhood leukaemia risk have mostly been limited to specific nutrients( Reference Metayer, Milne and Dockerty 1 , Reference Kwan, Metayer and Crouse 7 ) or specific food components such as processed meats( Reference Peters, Preston-Martin and London 8 ), coffee and alcohol( Reference Milne, Royle and Bennett 9 , Reference Menegaux, Ripert and Hemon 10 ) and dietary inhibitors of the nuclear enzyme topoisomerase II( Reference Spector, Xie and Robison 5 , Reference Ross, Potter and Reaman 11 , Reference Strick, Strissel and Borgers 12 ). Measures of overall diet quality may better represent nutritional status and the complex biological interaction of multiple nutrients( Reference Hu 13 ). High-quality diets characterised by diet quality indices are often positively correlated with biological markers of micronutrient intake and have been associated with reduced risk of all-cause mortality, cancer risk and CVD( Reference Kant 14 – Reference Reedy, Krebs-Smith and Miller 18 ). Maternal dietary patterns and quality have also been associated with birth outcomes such as neural tube and congenital heart defects( Reference Sotres-Alvarez, Siega-Riz and Herring 19 , Reference Carmichael, Yang and Feldkamp 20 ). The objective of this study was to examine the association between maternal diet quality, as assessed by a diet quality index, and risk of childhood ALL and acute myeloid leukaemia (AML) in a case–control study in California.
Methods
Study population
The CCLS is a case–control study conducted in up to thirty-five counties in the San Francisco Bay Area and the California Central Valley( Reference Ma, Buffler and Layefsky 21 ). Incident cases of newly diagnosed childhood leukaemia in children aged 0–14 years were ascertained from major paediatric clinical centres from 1995 to 2008. Cases were matched for date of birth, sex, Hispanic ethnicity (based on a parent self-reporting as Hispanic) and maternal race (white, black or other) with controls (ratio 1:1 or 1:2) randomly selected from California birth certificates through the Office of Vital Records at the California Department of Public Health. Control selection procedures and eligibility criteria have been described elsewhere( Reference Ma, Buffler and Layefsky 21 , Reference Bartley, Metayer and Selvin 22 ). Participation of ascertained and eligible cases and controls in the main questionnaire was approximately 86 %( Reference Bartley, Metayer and Selvin 22 ), and dietary information in the year before pregnancy was provided by 98 % of all respondents (970 cases and 1187 controls). This analysis included ALL and AML case and control participants recruited between 1995 and 2008 whose mothers reported dietary information. This study was conducted in accordance with the Declaration of Helsinki. The University of California, Berkeley Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects and the Institutional Review Boards of all participating hospitals provided approval for this study. Before interview, written informed consent was obtained from the responding parent of each participating child, and assent was obtained from children aged 7 years and older.
Data collection
Data were collected by in-person interviews in either English or Spanish and from birth certificates. Details on dietary data collection have been described elsewhere( Reference Jensen, Block and Buffler 2 , Reference Kwan, Jensen and Block 4 ). In brief, a modified version of the Block FFQ was administered during an in-person interview with the biological mother to assess her dietary intake and vitamin supplement use during the 12 months before the index pregnancy. Macronutrient and micronutrient estimates from the original Block FFQ and various adaptations have been validated in many populations, including men and women of diverse racial groups and ages( Reference Block, Hartman and Naughton 23 – Reference Block, Woods and Potosky 25 ). We assessed dietary intake in the year before pregnancy in order to examine nutritional adequacy at the time of conception and early pregnancy. The FFQ contained seventy-six food items and questions on vitamin supplement use before pregnancy. Food items were selected for inclusion in the FFQ by analysing data from the third National Health and Nutrition Examination Survey (NHANES III) and the Hispanic Health and Nutrition Examination Survey to identify foods that were the top population contributors of each nutrient among white, African-American and Hispanic populations. The FFQ also included five questions about whether the mother consumed more, the same amount, or less fruits, vegetables, tofu or soya, tea and water during the pregnancy with the child. Spanish-speaking respondents were administered a Spanish version of the FFQ by bilingual interviewers. The Spanish FFQ included seven additional items common in the diets of the Latino population: evaporated or condensed milk, cooked green peppers, avocado or guacamole, chile peppers or chile sauce, sauces such as mole or sofrito, corn tortillas and flour tortillas. Frequency of consumption of food groups was calculated by summing the reported frequency for all foods in a given food group; component foods of the food groups are reported elsewhere( Reference Kwan, Jensen and Block 4 ). BlockSys and NutritionQuest computer programmes (NutritionQuest) were used to calculate dietary nutrients from food by multiplying frequency of consumption of each food by its nutrient content and reported portion size, and then summing over all foods. Dietary folate intake, calculated in units of dietary folate equivalents( Reference Suitor and Bailey 26 ), accounted for the different amounts of folic acid in food before and after US national fortification of grain products with folic acid in 1998. Nutrients obtained from vitamin supplements were estimated by multiplying the frequency of consumption of each type of supplement (multiple vitamins and specific single vitamins) with the amount of the nutrient in standard compositions of each type.
Diet quality index
Food frequency data were used to calculate scores for a modified version of the 2010 healthy eating index (HEI-2010). The HEI-2010 is a measure of diet quality that assesses conformance to federal dietary guidance and was updated in 2010 to reflect the 2010 Dietary Guidelines for Americans, the basis for all US government nutrition recommendations and policies( Reference Guenther, Casavale and Reedy 27 ). The HEI-2010 is considered an appropriate measure of diet quality for women who are pregnant or lactating( Reference Guenther, Casavale and Reedy 27 ). The HEI-2010 comprises twelve nutritional components: nine ‘adequacy’ components (total fruit, whole fruit (excluding fruit juice), total vegetables, greens and beans, whole grains, dairy products, total protein foods, seafood and plant proteins, fatty acids) and three ‘moderation’ components (refined grains, Na, empty calories)( Reference Guenther, Casavale and Reedy 27 ). We did not have access to the raw data to permit computation of all HEI-2010 components; consequently, the index used in these analyses does not include separate components for whole fruit and seafood and plant proteins, although foods in these categories are incorporated into other components. As we could not distinguish between whole and refined grains in our data, these components were excluded. In addition, our modified index uses dietary fibre from beans for the greens and beans category, and uses percentage of energy content from sweets and grams of dietary trans-fat per day to represent empty calories. We added Fe and folate from food as components because of their inclusion in dietary quality indices for pregnancy( Reference Rifas-Shiman, Rich-Edwards and Kleinman 28 , Reference Bodnar and Siega-Riz 29 ) and because of previous studies indicating that higher maternal Fe and folate intake is associated with reduced risk of ALL( Reference Kwan, Metayer and Crouse 7 , Reference Thompson, Gerald and Willoughby 30 – Reference Bailey, Miller and Langridge 32 ). Given that the CCLS data were based on a semi-quantitative FFQ, components were scored by quartiles (based on the distribution in controls) instead of at the level of the nutritional standard: for adequacy components, 0 points were assigned to those in the lowest quartile of intake for a given food group or nutrient; 1, 2 and 3 points were assigned to those in the second, third and fourth quartiles of intake, respectively; and vice versa for moderation components (i.e. Na, trans-fat and percentage of energy content from sweets), for which participants in the lowest quartile of intake received the maximum score of 3 points( Reference Carmichael, Yang and Feldkamp 20 ). All components except for the fatty acids ratio and percentage of energy content from sweets were scored on a density basis (i.e. per 4184 kJ (1000 kcal)) to account for the diverse energy consumption of the respondents. All component scores were summed to obtain a total diet quality score with a possible range of 0 (worst) to 33 (best).
Statistical analysis
After excluding mothers of cases and controls with Down’s syndrome (n 36), because of the distinct genetic risk of leukaemia among these children, and excluding respondents reporting daily energy consumption of <2092 or >25 104 kJ (<500 or >6000 cal) (n 21), 681 ALL cases and 931 matched ALL controls and 103 AML cases and 145 matched AML controls were available for the analysis. The associations between diet quality score and select covariates were examined through t tests and ANOVA among controls. Pearson’s correlation coefficients were calculated to examine the relationship between index components and overall diet quality score among controls. Conditional logistic regression was used to estimate OR and 95 % CI for the association of ALL and AML with diet quality score, as well as the association with each of the index components. We also examined the association of ALL with greater fruit consumption (yes/no), greater vegetable consumption (yes/no) and greater soya consumption (yes/no) during pregnancy (relative to before pregnancy), and we assessed whether or not adding these variables to the diet quality score model substantially changed the OR (>10 %). Separate analyses were conducted for ALL and AML. Diet quality score was examined as both a continuous variable and in quartiles. Models were adjusted for the following covariates, which were selected a priori on the basis of known or hypothesised associations with maternal diet and childhood leukaemia: mother’s Hispanic ethnicity, annual household income, father’s education, mother’s education, maternal age category and vitamin supplement use in the year before pregnancy. Maternal BMI before pregnancy was not included as a covariate because there was a substantial number of missing values (26·1 %) and because it did not change the point estimate or improve the accuracy of the model for diet quality and ALL, as assessed through likelihood ratio tests (data not shown); its influence on the model for AML was not evaluated because of the large reduction in sample size due to missing values. As most women in our study reported modifying their alcohol consumption during pregnancy, with 94·6 % of the respondents indicating they drank much less or no alcohol at all during pregnancy, we did not consider alcohol consumption to be a likely confounder. Similarly, a small percentage of women reported smoking during the 3 months before pregnancy (12·0 %) or during pregnancy (8·1 %). As many studies have found that maternal smoking is not associated with an increased risk of childhood leukaemia( Reference Menegaux, Ripert and Hemon 10 , Reference Chang 33 – Reference Mucci, Granath and Cnattingius 35 ), we did not consider maternal smoking to be a confounder.
The potential modifying influence of maternal Hispanic ethnicity (Hispanic v. non-Hispanic white or other), maternal vitamin supplement use (yes/no) and child’s age at diagnosis (< or ≥5 years) on the association between diet quality score and ALL was assessed through the addition of interaction terms to the statistical models; interaction terms with a P value <0·2 were considered a statistically significant indication of lack of additivity. We also stratified results for ALL by vitamin supplement use. Models for AML had an insufficient sample size for stratification or test of interaction. All results were considered statistically significant if the 95 % CI excluded 1·0. Statistical analyses were carried out using STATA version 12.
Results
Compared with ALL cases, controls had parents with higher household income and education, and mothers were older at the time of the child’s birth and more likely to report vitamin supplement use in the year before pregnancy (Table 1). Controls matched to AML cases had parents with higher household income, and mothers were older at the time of the index child’s birth. Among controls, the proportion of women meeting the minimum recommended number of servings of vegetables (≥3 servings) and fruits (≥2 servings) per day according to the US Department of Agriculture Food Guide Pyramid ( 36 ) was 39·6 and 19·6 %, respectively. The proportion of women consuming the recommended Food Guide Pyramid servings per day for a given food group was 46·4 % for meat (two to three servings), 31·0 % for dairy products (two to three servings) and 31·7 % for grains (six to eleven servings). However, these results should be interpreted with caution, given that FFQ are more suited to ranking intakes than providing estimates of absolute intakes for entire food groups or nutrients.
Table 1 Select characteristics of matched case and control children, by leukaemia subtype: the California Childhood Leukemia StudyFootnote *(Numbers and percentages; mean values and standard deviations)

ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; USD, US dollars.
* Cases and controls were matched 1:1 or 1:2 on date of birth, sex, Hispanic ethnicity (based on a parent self-reporting as Hispanic) and maternal race (white, black or other). ALL cases and controls differed by income (P<0·01), mother’s education (P<0·01), father’s education (P=0·04), maternal age (P<0·01) and vitamin supplement use in the year before pregnancy (P=0·01). AML cases and controls differed by income (P=0·03) and maternal age (P=0·01).
† Missing values for income (n 58) were assigned to the median income level (45 000–59 999 USD).
‡ Any use of single or multiple vitamins.
Among all cases and controls, diet quality scores ranged from 2 to 30. The mean score was 15·6 (sd 5·1) and 16·5 (sd 5·2) among cases and controls, respectively. The 25th, 50th and 75th percentiles were 12, 16 and 19 among cases and 13, 16 and 20 among controls, respectively. Among controls, the mean diet quality score was significantly higher in mothers who did not smoke during the 3 months before pregnancy (P<0·001), who used vitamin supplements during the year before pregnancy (P<0·001) and who were older at the time of the index pregnancy (P<0·001). The mean diet quality score was not significantly different in obese v. non-obese controls (16·2 v. 16·8, respectively, P=0·35). Hispanic women had a higher mean diet quality score than non-Hispanic white women or women of other races/ethnicities (P<0·001). Consequently, the mean diet quality score among controls was the highest in the lowest education group, comprised of 98 % Hispanic women, followed by the highest education group (65 % non-Hispanic white, 12 % Hispanic and 23 % non-Hispanic other race/ethnicity). Correlations of diet quality score with the index components ranged from −0·70 to 0·64 among controls and were in the expected directions, with adequacy components positively correlated and moderation components negatively correlated with overall diet quality score, respectively (Table 2).
Table 2 Descriptive information about the components of the modified healthy eating index (HEI) 2010 among controls(Medians and 25th–75th percentiles)

DFE, dietary folate equivalents.
* The correlations of each index component with the overall HEI score among controls were in the expected directions (i.e. adequacy components were positively correlated with the HEI score, and moderation components were negatively correlated with the HEI score).
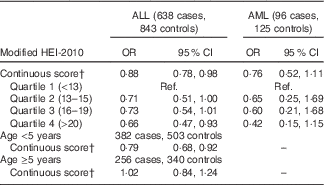
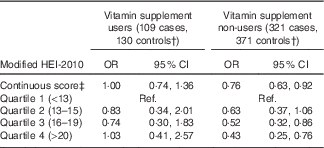
Higher maternal diet quality score was associated with a reduced risk of childhood ALL (OR 0·88; 95 % CI 0·78, 0·98 for each five-point increase) (Table 3). When examined by quartiles, the reduction in risk was most pronounced among those in the highest quartile of diet quality score (OR 0·66; 95 % CI 0·47, 0·93 for highest v. lowest quartile). Maternal Hispanic ethnicity did not modify these associations (P=0·62for interaction term). Although interaction by vitamin supplement use was not statistically significant (P=0·65), the reduction in ALL risk associated with higher diet quality score was greater among non-users of vitamin supplements (Table 4). There was a negative association of diet quality score with ALL among children younger than 5 years at diagnosis (OR 0·79; 95 % CI 0·68, 0·92 for a five-point increase in diet quality score among 382 cases and 503 controls) and no association among children diagnosed at 5 years of age or older (OR 1·02; 95 % CI 0·84, 1·24 for a five-point increase among 256 cases and 340 controls; P=0·08for interaction term).
Table 3 Association between modified healthy eating index (HEI) 2010 and risk of childhood acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML), overall and by child’s age at diagnosis/reference dateFootnote *(Odds ratios and 95 % confidence intervals)
Ref., referent values.
* Models adjusted for mother’s Hispanic ethnicity, father’s education, mother’s education, household income, maternal age at child’s birth and vitamin supplement use before pregnancy. The categorisation of diet quality score by quartiles was based on the distribution in controls.
† OR for a five-point increase in the HEI-2010 score.
Table 4 Association between modified healthy eating index (HEI) 2010 and risk of childhood acute lymphoblastic leukaemia among vitamin supplement users and non-users before pregnancyFootnote *(Odds ratios and 95 % confidence intervals)
Ref., referent values.
* Models adjusted for mother’s Hispanic ethnicity, father’s education, mother’s education, household income and maternal age at child’s birth. The categorisation of diet quality score by quartiles was based on the distribution in controls.
† By stratifying the conditional logistic regression analyses on a non-matching variable, case–control matched sets discordant on vitamin supplement use were excluded from analyses.
‡ OR for a five-point increase in the HEI-2010 score.
The negative association of higher maternal diet quality score with risk of AML was of similar magnitude to that observed for ALL, although the 95 % CI included one (OR 0·76; 95 % CI 0·52, 1·11) (Table 3). There was a similar trend of decreasing AML risk with higher diet quality score when examined by quartiles (OR 0·42; 95 % CI 0·15, 1·15 for highest v. lowest quartile).
When components of the diet quality index were examined separately, a reduction in risk of ALL and AML was observed for higher maternal consumption of fruits (Table 5). Other index components were not associated with ALL or AML. Because of the strong association between daily fruit servings and ALL and AML, we calculated a limited diet quality score without fruit consumption as a component and found that its associations with ALL and AML did not substantially change (i.e. OR 0·89; 95 % CI 0·79, 1·01 and OR 0·79; 95 % CI 0·53, 1·17, respectively, for a five-unit change in score). The moderate correlation between fruit consumption and the limited score (r 0·37) suggests that the association of overall diet quality score with ALL and AML may not be entirely due to the influence of the fruit component.
Table 5 Associations between individual components of the modified healthy eating index (HEI) 2010 and risk of childhood acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML)Footnote *(Odds ratios and 95 % confidence intervals)

DFE, dietary folate equivalents.
* Separate models for each energy-adjusted food group/nutrient as continuous variables adjusted for maternal Hispanic ethnicity, household income, mother’s education, father’s education, maternal age category and vitamin supplement use. Fatty acid ratio and percentage of energy content from sweets were not energy adjusted.
Children of women who reported consuming much more or somewhat more vegetables during pregnancy had a reduced risk of ALL (OR 0·70; 95 % CI 0·56, 0·89 among 593 cases and 774 controls), as did children of women who reported consuming much more or somewhat more fruits during pregnancy, although the CI included 1·0 (OR 0·87; 95 % CI 0·69, 1·09 among 622 cases and 819 controls). There was no statistically significant association of greater soya consumption during pregnancy with risk of ALL (OR 1·21; 95 % CI 0·76, 1·93). Adding more fruit, more vegetable or more soya consumption during pregnancy to the diet quality score model as a covariate did not substantially change the OR for diet quality score (data not shown).
Discussion
Our data suggest a reduction in the risk of ALL with higher maternal diet quality. The association of AML with higher maternal diet quality was of similar magnitude to the association observed for ALL, although this finding did not reach statistical significance. No single food group or nutrient that was part of the diet quality score appeared to be driving the results, suggesting that the quality of the whole diet and the cumulative effects of many dietary components may be important in influencing childhood leukaemia risk. To our knowledge, this is the first study to examine maternal diet quality in relation to childhood leukaemia. However, our findings are consistent with previous studies, indicating that higher maternal consumption of fruits and vegetables and higher intake of micronutrients such as folic acid may be associated with a reduced risk of childhood ALL( Reference Metayer, Milne and Dockerty 1 – Reference Kwan, Jensen and Block 4 ).
Although much attention has been focused on the role of folate in children’s health outcomes, there is increasing evidence of the importance of other micronutrients for prenatal development and birth outcomes, such as Fe, vitamin D and I( Reference Allen 37 , Reference Ramakrishnan, Grant and Goldenberg 38 ). A measure of diet quality may provide a holistic representation of maternal diet, as diet quality index scores are positively associated with a wide range of beneficial nutrients (e.g. antioxidants, carotenoids) and negatively associated with intake of potentially harmful dietary components (e.g. SFA)( Reference Kant 14 ). Previous studies have found that the HEI score is strongly correlated with biomarkers of several micronutrients important for maternal and child health, including folate, vitamins C and E, and carotenoids( Reference Weinstein, Vogt and Gerrior 15 , Reference Hann, Rock and King 39 ).
Maternal diet quality may influence leukaemia risk in children through the influence of specific nutrients such as folic acid or other methyl donors on DNA synthesis and repair or epigenetic processes( Reference Locasale 40 , Reference Fenech 41 ). An alternative or complementary pathway by which maternal nutrition may influence childhood leukaemia risk is through its impact on the development of the child’s immune system both before and after birth. Immune system development begins early in gestation, with a possible period of heightened vulnerability in immune cell development thought to occur when tissues are being seeded by precursors of immune cells (i.e. 4–7 weeks for myeloid-derived cells and 8–18 weeks for lymphoid cells)( Reference Marques, O’Connor and Roth 42 ). There are at least three hypothesised pathways by which maternal malnutrition may influence the development of the fetal immune system: (1) maternal malnutrition may be a stressor, resulting in a high concentration of maternal cortisol previously shown to influence the developing fetal immune system; (2) low levels of micronutrients may interfere with organogenesis and the normal proliferation of immune cells; and (3) poor maternal nutrition could alter the quality and quantity of immune factors transferred prenatally through the placenta or postnatally through the mammary gland( Reference Marques, O’Connor and Roth 42 , Reference Palmer 43 ).
There is also interest in the role of infant birth weight in childhood leukaemia risk. Numerous studies have found an increased risk of ALL and AML with higher birth weight or among children who were large for gestational age( Reference Roman, Lightfoot and Smith 44 – Reference Caughey and Michels 49 ). A recent meta-analysis of twelve case–control studies from the Childhood Leukemia International Consortium reported a statistically significant increased risk of ALL in children who were large for gestational age relative to appropriate for gestational age (OR 1·24; 95 % CI 1·13, 1·36) among 7348 cases and 12 489 controls( Reference Milne, Greenop and Metayer 50 ). Although birth weight is known to be affected by a variety of factors( Reference Barker, Lampl and Roseboom 51 – Reference Clausen, Burski and Oyen 53 ), certain dietary aspects such as the glycaemic load of the diet during pregnancy( Reference McGowan and McAuliffe 54 ) and maternal intake of one-carbon metabolism nutrients( Reference Lewis 55 – Reference Relton, Pearce and Parker 58 ) may play a role in fetal growth and birth weight. However, we did not find a difference in maternal diet quality among women who had children with high birth weight (i.e. ≥4500 g) and women who did not (data not shown). In addition, maternal diet quality was not associated with maternal BMI. Thus, our data do not suggest that maternal obesity or infant birth weight is the mechanism by which maternal diet quality influences childhood leukaemia risk.
Further understanding of the biological mechanisms by which maternal diet quality may influence childhood leukaemia risk is needed. We found that there was only an association of maternal diet quality with risk of ALL among children diagnosed under 5 years of age, which strengthens the inference that maternal nutritional status may influence the developmental processes occurring in utero that are related to the initiation of leukaemia before birth( Reference Greaves and Wiemels 59 , Reference Wiemels, Cazzaniga and Daniotti 60 ). Furthermore, increased vegetable and possibly fruit consumption during pregnancy was associated with a reduced risk of ALL even after controlling for pre-pregnancy diet quality, suggesting that both maternal nutrition around the time of conception and throughout pregnancy may influence the risk of childhood leukaemia. This finding is consistent with previous studies, which found that the associations between maternal vitamin supplement use and childhood leukaemia did not significantly differ by period of supplementation (i.e. preconception, during pregnancy and by trimester)( Reference Metayer, Milne and Dockerty 1 ).
We used a measure of diet quality defined a priori on the basis of a validated index that measures conformance with federal dietary guidelines( Reference Guenther, Kirkpatrick and Reedy 61 ). Our index was limited in that it excluded some HEI-2010 components such as whole and refined grains and was unable to examine the role of other components of interest in fetal development, such as seafood and plant proteins. However, there was substantial overlap in our index with the HEI-2010 and other diet quality indices. Although we were unable to validate our modified index, the construct validity of this index was supported by its ability to successfully distinguish between groups with known differences in diet quality (e.g. smokers and non-smokers)( Reference Guenther, Kirkpatrick and Reedy 61 ). In our index, each component received the same weight, which we believe is appropriate given the limited evidence on the association between maternal consumption of food groups and risk of childhood leukaemia. Calculation of our score by quartiles produced a smaller range of component scores and overall score than the traditional HEI-2010, but the estimated 5 and 95 percentiles of total score (7·5 and 25, respectively) indicated that there was a wide range of scores among individuals.
Income and education are positively associated with better diet quality among adults( Reference Hiza, Casavale and Guenther 62 , Reference Kant and Graubard 63 ). However, the observation in this study that Hispanic women had higher diet quality scores than women of other ethnicities/races despite having lower income and education levels is consistent with findings from other studies( Reference Hoerr, Tsuei and Liu 64 ). A recent NHANES analysis found that Hispanics have better diet quality than whites or blacks, with greater consumption of fruits, vegetables and legumes( Reference Hiza, Casavale and Guenther 62 ). In addition, a growing body of research has suggested that the diet quality among Hispanic women in the US declines with increasing levels of acculturation( Reference Neuhouser, Thompson and Coronado 65 – Reference Batis, Hernandez-Barrera and Barquera 68 ). The addition of an interaction term to the statistical model did not indicate that there was substantial heterogeneity in the association between maternal diet quality and ALL by maternal Hispanic ethnicity.
The strengths of this study include the population-based selection of controls and the thorough assessment of maternal dietary intake in the year before pregnancy. Potential limitations include measurement errors in the estimation of maternal food and nutrient intakes occurring several years in the past, which may increase the likelihood of null findings or small effect sizes( Reference Byers 69 , Reference Freedman, Schatzkin and Midthune 70 ). Although we were able to assess reported changes in fruit, vegetable and soya consumption during pregnancy, we did not have additional data on dietary exposures during pregnancy, which is a limitation of this study. Data on vitamin supplement use during pregnancy were available for phase III respondents; however, because 94 % of women reported use of vitamin supplements during pregnancy, the limited variability in this potential covariate precluded informative analyses. Although recall bias is possible, many of the validation studies examining the extent of maternal recall bias in case–control studies of child birth outcomes have found no systematic differences in recall according to case–control status( Reference Drews, Kraus and Greenland 71 – Reference Klemetti and Saxen 78 ), and research suggests that recall bias generally occurs under specific circumstances such as when a putative association has been publicised or is believed to exist by the community under study( Reference Infante-Rivard and Jacques 79 ). We believe that recall bias is less likely to occur for a complex exposure such as diet quality, which is based on reported intake of diverse food groups and nutrients calculated from seventy-six food items and has not previously been publicised as being possibly related to leukaemia risk. Furthermore, a maternal diet reliability sub-study in the CCLS (n 85) found that the reliability of five select FFQ questions did not differ by case–control status (unpublished results). The socio-demographic characteristics and health behaviours of mothers with higher diet quality differ from mothers with low diet quality, and it is possible that we did not adjust for all relevant confounders. However, adjustment for several potential confounders including measures of socio-economic status had little influence on the associations between diet quality score and ALL and AML.
A measure of maternal diet quality attempts to better capture intakes of the myriad nutrients and bioactive components consumed from foods, in contrast to a limited focus on particular nutrients. Given the importance of multiple nutrients and food components during pregnancy and lactation, this representation of maternal diet may be better suited to capture the complex interplay of diverse nutritional factors on fetal development, birth outcomes and child health. Our finding of a strong association between maternal diet quality score and risk of childhood leukaemia suggests that maternal nutritional status during pregnancy may play a role in the development of leukaemia, and that the cumulative effects of many dietary components may be more important than the effect of a single nutrient.
Acknowledgements
The authors thank the staff of the California Childhood Leukemia Study (CCLS) for their contributions to this study. The authors are grateful to the families who participated in this study and the clinical investigators and their teams at the collaborating hospitals for their role in recruiting patients to this study: University of California, Davis, Medical Center (J. Ducore), University of California, San Francisco (M. Loh and K. Matthay), Children’s Hospital of Central California (V. Crouse), Lucile Packard Children’s Hospital (G. Dahl), Children’s Hospital Oakland (J. Feusner), Kaiser Permanente Roseville (formerly Sacramento; K. Jolly and V. Kiley), Kaiser Permanente Santa Clara (C. Russo, A. Wong and D. Taggar), Kaiser Permanente San Francisco (K. Leung) and Kaiser Permanente Oakland (D. Kronish and S. Month).
This research was supported by National Institute of Environmental Health Sciences grants R01ES009137 and P-42-ES-04705-18 (University of California, Berkeley).
A. W. S. formulated the research question and led the analysis and writing of the paper. S. L. C. and G. B. advised on nutritional epidemiological methods, interpreted the results and critically reviewed the paper. S. S. advised on the statistical methods, interpreted the results and critically reviewed the paper. C. F. advised on the interpretation of the results and critically reviewed the paper. C. M. is responsible for the overall design of the CCLS study, provided the dietary data, advised on the analysis, interpreted the results and critically reviewed the paper.
The authors declare that there are no conflicts of interest.








