Cows’ milk allergy (CMA) is a common food allergy, affecting 2–6 % of Finnish children under the age of 3–4 years(Reference Pyrhönen, Näyh and Kaila1,Reference Saarinen, Juntunen-Backman and Järvenpää2) . It is often the first manifestation of allergic diseases. Maternal nutrition during pregnancy among other environmental factors has been implicated to play a role on the development of allergic diseases in the offspring(Reference West, D’Vaz and Prescott3). Because CMA usually manifests during infancy, these early exposures may be of importance in the development of CMA. Antioxidant nutrients have been shown to exert immunological effects, and they could potentially influence the development of allergic diseases(Reference Sardecka, Krogulska and Toporowska-Kowalska4). Two diverse hypotheses have emerged: epidemiological evidence suggests that a diet lower in antioxidants is associated with an increase in allergic diseases(Reference Miyake, Sasaki and Tanaka5), whereas the mechanistic hypothesis points towards antioxidants leading to the suppression of T helper type 1 (Th1) cytokines and thus a higher susceptibility to allergic diseases (reviewed by Allan et al.(Reference Allan, Kelly and Devereux6)). In addition to antioxidative effects, the nutrients may have other immunoregulatory pathways. For example, retinoic acid is involved in regulatory T-cell formation and thus the development of oral tolerance(Reference Hall, Grainger and Spencer7). The most recent meta-analysis on maternal nutrition during pregnancy and allergic diseases in the child states that the current evidence is in favour of protective association between vitamin E as well as for Zn and childhood wheezing, but is inconclusive against other allergic diseases. For other antioxidant nutrients, the associations were even more contradictory(Reference Beckhaus, Garcia-Marcos and Forno8).
The knowledge of maternal dietary factors affecting the risk of CMA in the offspring is limited. One study reported that maternal intake of vitamin D from the diet during pregnancy was associated with decreased risk and intake of folate with increased risk of CMA in the offspring(Reference Tuokkola, Luukkainen and Kaila9). To our knowledge, associations between antioxidant nutrient intake during pregnancy and the development of CMA in the offspring have not been reported before. The aim of this study was to investigate the associations between maternal intake of antioxidant vitamins and minerals during pregnancy both from diet and supplements and the subsequent development of CMA in the offspring.
Subjects and methods
Subjects
The Finnish Type 1 Diabetes Prediction and Prevention (DIPP) Study is a multidisciplinary prospective population-based birth cohort study(Reference Kupila, Muona and Simell10). The study is conducted in three university hospitals in Finland (Turku, Oulu and Tampere) and after parental informed consent, all newborn infants from these areas were screened for human leucocyte antigen (HLA)-conferred susceptibility to type 1 diabetes from cord blood samples. Infants who carry HLA genotypes conferring high and moderate risk for type 1 diabetes (14 % of those screened) were invited to participate in the study. The children with severe congenital abnormalities or diseases, or whose parents were of non-Caucasian origin or did not understand Finnish, Swedish or English were excluded. The study was conducted in accordance to the Declaration of Helsinki. The local Ethical Committees approved the study. All families have given their written informed consent.
The DIPP Nutrition study is a part of the main DIPP study comprising children born in the Oulu and Tampere areas. The present study comprised 6288 children born between October 1997 and September 2004. Both maternal dietary data during pregnancy and the information of child’s CMA were available for 4921 children (78·3 %) from a total of 4861 pregnancies. Complete information on background factors used in the current analyses in the pregnancy cohort was available for 4403 children (Fig. 1).
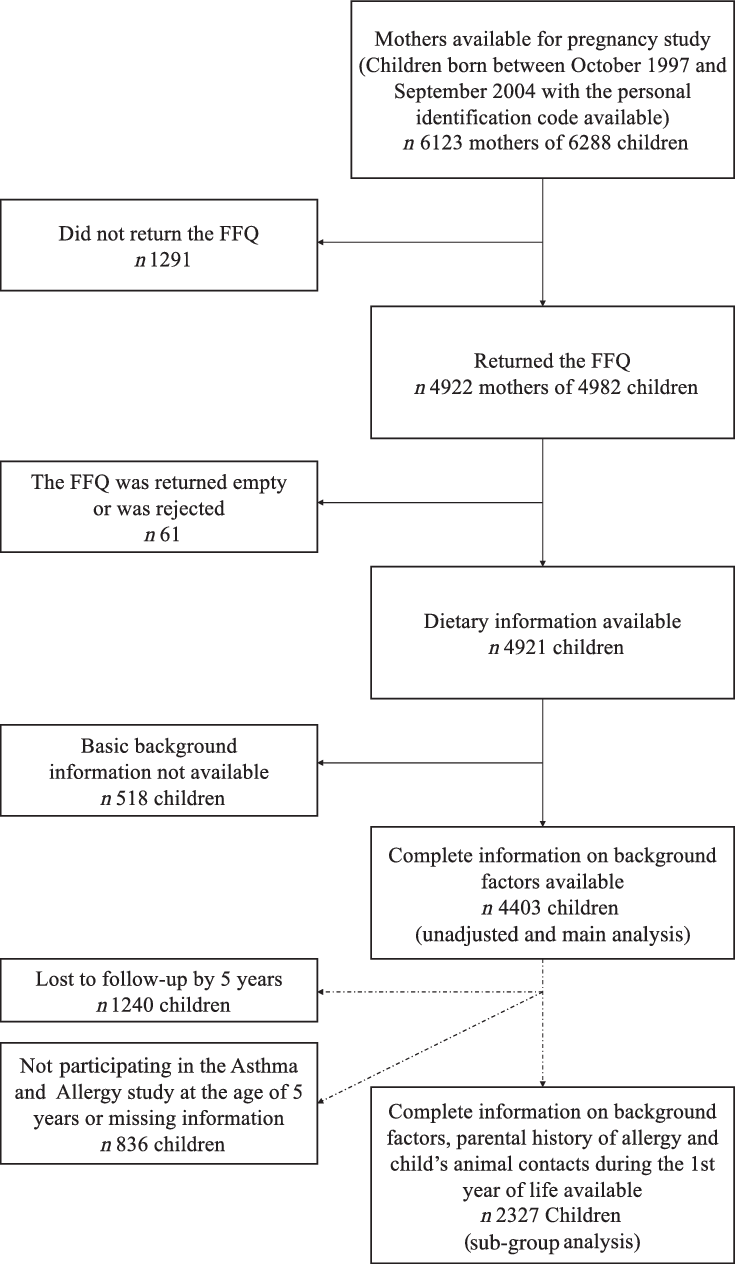
Fig. 1. Flow chart of the study cohort.
When the children were 5 years of age, an Asthma and Allergy sub study (including parental history of allergy and child’s animal contacts) was performed. All children at follow-up, also those who did not have maternal dietary data, were invited. Of the 4075 children (65 % of the 6288 children) still at follow-up at 5 years, 3781 children (93 % of those 4075 invited) participated in the Asthma and Allergy sub study. Of these children, 2327 children had information also on maternal diet, basic background factors and cows’ milk allergy, enabling a sub-group analysis with information on parental allergies included in the current study. The flow chart of the cohort with maternal dietary data and the information of child’s CMA is presented in Fig. 1.
Dietary assessment
Maternal diet during pregnancy (eighth month) was assessed by a 181-item semi-quantitative FFQ, which has been validated against food records (two times 5-d food record) in a setting that reflects the present study(Reference Erkkola, Karppinen and Javanainen11). The validation study did not take into account the nutrient intake from supplements. In the validation study, Pearson correlations with food records were 0·37 for vitamin A, 0·71 for retinol, 0·53 for β-carotene, 0·22 for vitamin E, 0·65 for vitamin C, 0·45 for Zn and 0·46 for Se(Reference Erkkola, Karppinen and Javanainen11). The FFQ was designed to represent the entire diet over the eighth pregnancy month. As in Finland mother’s pregnancy leave begins right after eighth pregnancy month, it is likely to be the most representative month of the average nutrient intake during the whole pregnancy. The mother’s received the FFQ via mail after delivery, and they returned it at the 3-month study visit.
A trained study nurse checked the FFQ when returned. The FFQ was specifically designed to reflect Finnish food consumption habits, and it assessed the consumption frequency of foods or food groups (not at all, number of times per d, week or month) as common serving sizes, such as a glass, a plateful or decilitre. The individual habits of fat used in cooking and baking were taken into account. The food consumption data were double entered. The FFQ was rejected if there were ten or more missing frequencies or the form was inadequately filled in (n 53, 1·1 %). Daily intakes of vitamins A, C and E, β-carotene, retinol, Se and Zn were calculated with the use of the Finnish food composition database, Fineli®(12), by an in-house software of the Finnish institute for health and welfare. The detailed content of the FFQ and data processing has been described elsewhere(Reference Erkkola, Karppinen and Javanainen11,Reference Prasad, Lumia and Erkkola13) . During the study time, the recipe compositions were updated in order to reflect the changes in food consumption habits and changes in the food market. That is why two versions of the database were used: the first version for the study years 1997–2002, and the second version for the years 2003–2004. The changes in recipes were mainly based on food consumption information of women aged 25–44 years from the national dietary surveys, FINDIET 1997(14) and FINDIET 2002(Reference Männisto, Ovaskainen and Valsta15). Recording of the FFQ and the accuracy of the nutrient database of the Finnish institute for health and welfare were checked at dietary analyses. The FFQ also included a question about dietary supplements, asking about the type, brand name and manufacturer’s name, as well as the amount of each supplement per d or per week, and the pregnancy weeks during which the supplements were used. The nutrient contents of the dietary supplements were obtained from the National Food Administration, manufacturers and from the Finnish pharmacopoeia for supplements registered as drugs.
Endpoints
Information on CMA, obtained from the registers of the Social Insurance Institution, complemented with parental reports, was used as the endpoint. The register-based information on CMA was based on a granted special reimbursement for the costs of special infant formulas needed in the management of diagnosed CMA (ICD-10 codes L27.2 or K52.2). The special reimbursement is entitled to all Finnish infants up to age 2 years irrespective of infants/parents socio-economic status, place of residence or place of treatment. An application and a certificate from a paediatrician, stating that the CMA diagnosis has been made according to specified medical criteria, are required. Paediatricians have agreed on the criteria for the diagnosis, which, during the study period, was usually a response to an elimination diet and an open challenge, and rarely a response to an elimination diet with a positive skin prick test or a specific IgE. Only in some rare cases, a double-blind placebo-controlled challenge was performed. In addition, CMA of the children was queried with open questions from parents at the age of 6 months and 1 and 2 years, and with a structured, validated questionnaire at the age of 3 years(Reference Tuokkola, Kaila and Pietinen16,Reference Tuokkola, Luukkainen and Kaila17) .
Background factors
Families were asked for information on maternal and paternal vocational education, age and place of residence at the recruitment. Information on pregnancy and delivery complications, gestational age, birth weight and height, earlier deliveries and maternal smoking during pregnancy was received from the medical birth records of the delivery hospitals. Information about breast-feeding was asked from the parents in the dietary questionnaires. Among those families who participated in the Asthma and Allergy study at the child’s age of 5 years, the parents were asked for their asthma and allergic rhinitis background, and child’s animal contacts during the first year of life(Reference Nwaru, Lumia and Kaila18).
Statistical methods
The differences in background factors between children with and without CMA were analysed by using the χ 2 test. Logistic regression was applied to study the associations between maternal antioxidant nutrient intakes and the risk of CMA in the offspring. The possible reliance among siblings was accounted for by using the generalised estimating equations with the sandwich estimator of variance to estimate regression coefficients in logistic regression analysis(Reference Liang and Zeger19). Selection of variables included in adjusted models was based on previous evidence(Reference Faure, Preziosi and Roussel20–Reference Botha, Basera and Facey-Thomas24) as well as their association with CMA in the present study. The nutrient intakes were adjusted for energy intake by the residual method(Reference Willett25) after logarithmic transformation. The total intake of each nutrient was calculated as the sum of intake from foods and supplements. The intake of nutrients from food alone and from food and supplements together was analysed if the nutrient intake from supplements was meaningful. Nutrient intake variables were used as continuous explanatory variables in the analyses, and first, the unadjusted analysis was conducted. The variables included in the first adjusted model were study centre, sex, birth weight of the child, maternal age and education, maternal smoking during pregnancy, duration of gestation, mode of delivery, number of older siblings, season of birth, urbanity of living environment and length of breast-feeding. These analyses were considered as main analyses. In addition, we made analysis in a sub group of children who participated in the Asthma and Allergy study at the child age of 5 years. In this analysis, the adjusted model included all those variables included in the main adjusted model as well as the information about maternal history of allergy, paternal history of allergy, visits to a stable and pet keeping during the child’s first year of life. Due to the significant drop out of children at the follow-up at 5 years of age, when the Asthma and Allergy sub study was performed, we also repeated the main analyses in this sub group to evaluate whether the results, adjusted for allergy variables, are due to the reduced number of subjects or confounding by the allergy variables. Interaction between maternal history of allergy (mother has allergic rhinitis or asthma) and nutrient intakes was tested, and analyses were done separately for mothers with history of allergy and mothers without history of allergy if the interaction was significant (P < 0·05). SAS version 9.3 (SAS Institute Inc.) was used in the analysis.
Results
The mean total and dietary intake of the antioxidant nutrients is shown in Table 1. The cumulative incidence of CMA was 9·3 % by the age of 3 years. The background factors associated with an increased risk of CMA were male sex, high parental education level, parental allergic history and maternal use of vitamin supplements containing vitamins A, C and E during pregnancy (Table 2). Maternal smoking and having pets inside the home during the child’s first year of life were, in turn, associated with a decreased risk of CMA.
Table 1. Maternal daily intake of nutrients from diet and supplements (total) and from diet during pregnancy (n 4403)
(Mean values and standard deviations)

* Retinol equivalent (RAE) = 1 µg of retinol = 12 µg of β-carotene.
Table 2. Distribution of background characteristics of all children who participated in the study and for cows’ milk-allergic children (cases)
(Numbers and percentages)
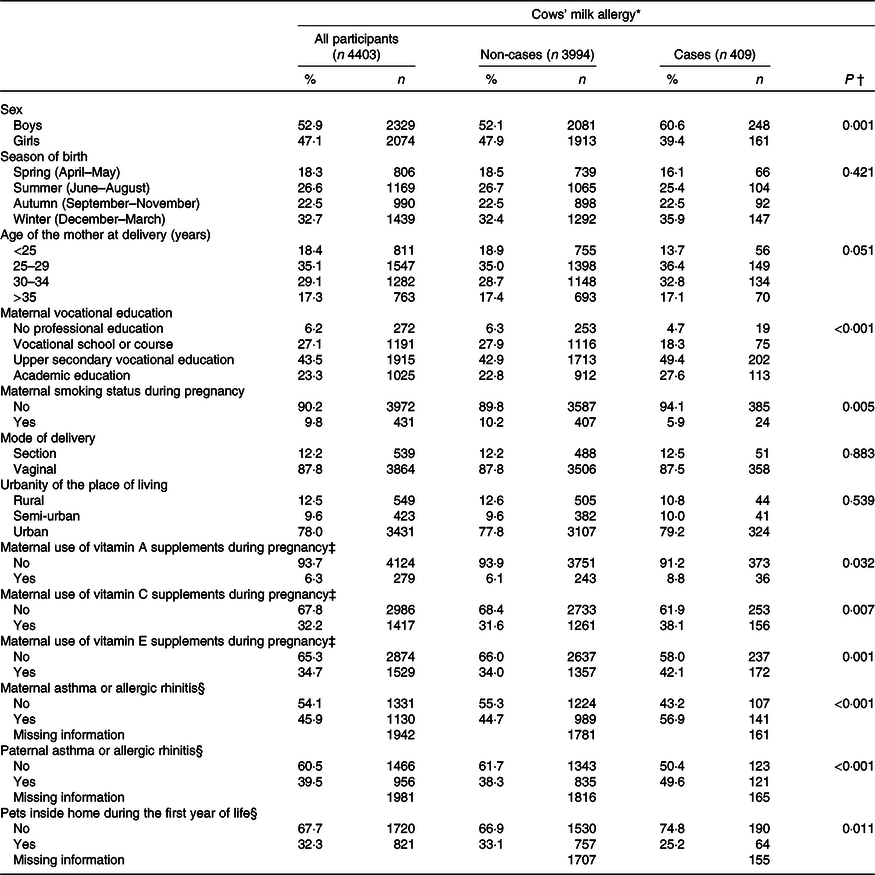
* Cumulative incidence of cows’ milk allergy by the age of 3 years.
† Comparison with the χ 2 test comparing distributions of cows’ milk allergy across the categories.
‡ Including the use of multivitamin supplements.
§ Information was collected from the Asthma and Allergy study, which was performed at the child’s age of 5 years.
Maternal total intake of β-carotene and intake of β-carotene and vitamin E from food were associated with an increased risk of CMA in the offspring in the unadjusted model (Table 3). In the main analysis, after adjusting for putative baseline confounders, the associations remained for intake of β-carotene, both total (OR 1·10; 95 % CI 1·02, 1·20) and from food (OR 1·10; 95 % CI 1·01, 1·19) (Table 3).
Table 3. Risk of cows’ milk allergy in the offspring by 3 years of age associated with maternal daily intake of energy and nutrients during pregnancy*
(Odds ratios and 95 % confidence intervals)
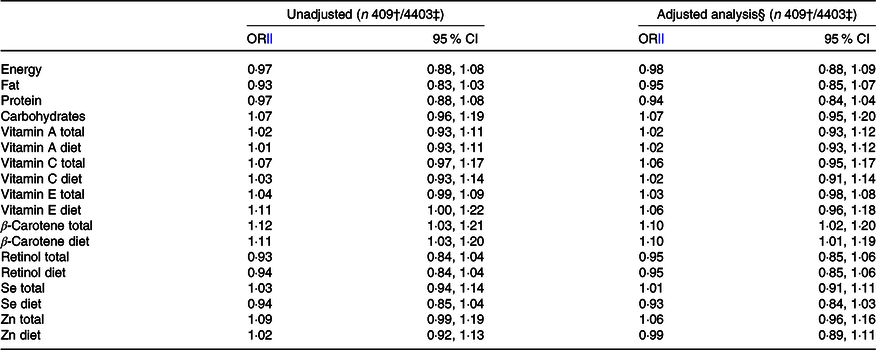
* Both total intake and intake from food sources alone are reported for those nutrients which are also derived from supplements.
† Number of children with cows’ milk allergy.
‡ Number of children in the analysis.
§ Adjusted for study centre, sex, birth weight of the child, maternal age and education, maternal smoking during pregnancy, duration of gestation, mode of delivery, number of older siblings, season of birth, urbanity of living environment and length of breast-feeding.
‖ OR are presented per 1 sd increment of the particular nutrient intake.
In the sub-group analysis, after adjusting for putative baseline confounders, parental history of allergy and child’s animal contacts during the first year of life, the intake of Se from food (OR 0·85; 95 % CI 0·74, 0·98) was associated with a decreased risk, and the total intake of Zn (OR 1·13; 95 % CI 1·00, 1·27) and β-carotene (OR 1·12; 95 % CI 1·00, 1·26) with an increased risk of CMA in the offspring. These associations were also observed when adjusted only for putative baseline confounders (online Supplementary 1).
An interaction between maternal history of allergy and the intake of antioxidant nutrients was observed for the total intake of vitamin E (P for interaction 0·013) when adjusted for all the potential confounding factors, including paternal history of allergy. The total intake of vitamin E was associated with an increased risk of CMA in mothers with a history of allergy (OR 1·61; 95 % CI 1·14, 2·28), but not in mothers without such a history (OR 0·98; 95 % CI 0·85, 1·12). For other nutrients, no interactions with maternal allergic history were observed (data not shown).
Discussion
In this large population-based birth cohort study, we observed that maternal total and dietary intake of β-carotene during pregnancy was associated with an increased risk of CMA in the offspring up to child’s age of 3 years.
A major strength of our study is that the data were collected prospectively from a large number of mothers and children. Furthermore, a major advantage is the good quality of dietary information, which was gathered by a detailed, validated FFQ. Cross-classification in quintiles by food consumption and nutrient intake was acceptable for all nutrients.
The sub-group analysis, done within children participating in the Asthma and Allergy study, enabled us to take into account the parental history of allergy and the child’s animal contacts during the first year of life as potential confounding factors. In this sub-group analysis, we did not observe difference between the results when the adjustment was made only for putative confounding factors and when further adjusted for parental history of allergy and the child’s animal contacts during the first year of life. This suggests that these factors do not have a significant confounding role in our study group. However, the difference observed between the main and the sub-group analyses suggests that the drop out had distorted the sub-group results.
A limitation of the present study is that the study subjects were selected based on HLA-conferred susceptibility to type 1 diabetes, representing about 14 % of all newborn infants in Finland. These infants may have an increased intestinal permeability(Reference Vaarala, Atkinson and Neu26), which may explain why the incidence of CMA in our study was higher than that previously reported in Finland(Reference Pyrhönen, Näyh and Kaila1). Therefore, our results may not be fully generalisable to the unselected paediatric population. Estimating the nutrient intake by the FFQ has some limitations. First, as the mothers received the FFQ after delivery and were asked to retrospectively report their diet during the eighth month of pregnancy, it is open for a recall bias. However, in the validation study, the FFQ filled after delivery was considered to be equally representative of the diet during the eighth month of pregnancy as FFQ filled during the eighth month of pregnancy; therefore, this recall bias should be minor. Second, the FFQ is reported to slightly overestimate the nutrient intake; however, the adjustment of nutrient intakes for energy intake should diminish this problem(Reference Erkkola, Karppinen and Javanainen11). Third, as nutrient intake from supplements was not calculated in the validation study, the validity of the intake from supplements in the present study remains unclear. Fourth, in the validation study, the Pearson correlation coefficient for vitamin E was low (r 0·22), and therefore, the observed associations for vitamin E might be diminished and should be interpreted cautiously. We took several possible confounders into account in order to specifically study the effects of the selected antioxidant nutrients, to avoid the bias in results from some other lifestyle factors, which are associated with the intake of these nutrients. Unfortunately, the diet of the children themselves was not controlled for. However, because we examined a large number of dietary variables in our analyses, it is possible that the significant association may only be due to chance. A further limitation is that we only had information about the age at diagnosis, and the onset of symptoms of CMA was not known, although these two should coincide.
To our knowledge, this is the first study to examine the association between antioxidant nutrient intake during pregnancy and the development of CMA in the offspring. Only one previous study has used food allergy as an outcome when studying the associations of maternal antioxidant intake with offspring allergic diseases. The authors suggested that vitamin C and Cu could be associated with a reduced risk of food allergy, but for vitamin E, β-carotene or Zn, no association was seen(Reference West, Dunstan and McCarthy27). Our observation on the association between maternal total and dietary intake of β-carotene and the increased risk of CMA in the offspring is in line with some mechanistic studies(Reference Litonjua28,Reference Gostner, Becker and Ueberall29) , but is not supported by epidemiological findings(Reference Devereux, McNeill and Newman30,Reference Shaheen, Newson and Henderson31) .
Evidence from epidemiological studies on the influences of maternal antioxidant intake during pregnancy on allergic outcomes in childhood is scarce, studies reporting mostly protective or non-significant associations. The most recent meta-analysis observed a protective association only for the maternal intake of vitamin E and Zn during pregnancy and wheezing in the offspring(Reference Beckhaus, Garcia-Marcos and Forno8). In addition, two previous studies have reported that high maternal plasma Se concentration during pregnancy(Reference Devereux, McNeill and Newman30) or in cord blood(Reference Shaheen, Newson and Henderson31) was associated with a decreased risk of wheezing in the offspring. However, wheezing is a condition different from CMA and may not always be of atopic origin. The effect of oxidative stress on the pathogenesis of pulmonary manifestations of atopic disease is likely to differ from that of CMA. On the other hand, mechanistic studies have implied a possible predisposing effect of antioxidant nutrients on allergic diseases. The anti-inflammatory benefits of the antioxidant nutrient may be lost with higher doses because of the too strong inflammatory suppression which could lead to Th1 suppression promoting Th2 responses associated with allergic diseases(Reference Litonjua28,Reference Gostner, Becker and Ueberall29) . Murr et al. have suggested that increased antioxidant intake could suppress cytokines, namely interferon-γ, leading to Th1 differentiation(Reference Murr, Schroecksnadel and Winkler32). This suppression would then, due to cross-regulation, promote the development of a Th2 phenotype. In addition, previous in vitro studies have shown that vitamin A and its derivatives may favour Th2 immune responses(Reference Ruhl33).
In Finland, the mothers who use dietary supplements receive higher amounts of antioxidant nutrients from their diet have a higher education and smoke less compared with mothers who do not use any supplements(Reference Uusitalo, Uusitalo and Ovaskainen34). This may have affected our results as those mothers with overall healthier lifestyle may seek help for their child’s symptoms more actively, which may increase the child’s likelihood of receiving a CMA diagnosis. Even though some associations prevailed after adjusting for maternal education, it is possible that our finding on maternal antioxidant nutrient intake and higher risk of CMA in the offspring is not causal.
Because allergic diseases are strongly hereditary, we examined the associations between nutrients and CMA in children separately for mothers with and without a history of allergy. We observed that maternal intake of vitamin E during pregnancy was associated with a higher risk of CMA only in the offspring of mothers with a history of allergy. However, this observation might be affected by the selection bias and therefore needs to be interpreted cautiously. Even so, it is possible that the maternal allergy influences the immunological environment in utero; therefore, the maternal allergy may modify the immunological effects of nutrients during pregnancy(Reference Cook-Mills35). Further studies are needed to explore the significance as well as the immunological mechanisms behind this finding.
In conclusion, these results are the first to link maternal antioxidant nutrient intake during pregnancy with the development of CMA in the offspring. For majority of studied antioxidant nutrients, we did not observe an association between maternal intake and CMA in the offspring. Thus, the maternal use of supplements containing antioxidant nutrients during pregnancy does not seem to have any additional benefits. A sufficient and balanced intake of antioxidant nutrients is best achieved by adhering to the dietary guidelines for pregnant women.
Acknowledgements
We are extremely grateful to all the families who took part in this study. We would also like to acknowledge the excellent collaboration of the DIPP research nurses, doctors, nutritionists and laboratory staff over the years.
The study was supported by the Academy of Finland (grants 63672,79685, 79686, 80846, 201988, 210632, 129492, 126813 and 276475), the Finnish Paediatric Research Foundation, the Juho Vainio Foundation, the Yrjö Jahnsson Foundation, the Competitive Research Funding of the Tampere University Hospital (grants 9L035, 9M029, 9P017, 9P057, 9R012, 9R055, 9S015, 9S074, 9T072, 9U016, 9U065 and 9V012), Medical Research Funds of Turku and Oulu University Hospitals, the European Foundation for the Study of Diabetes, the Juvenile Diabetes Research Foundation (grants 197032, 4-1998-274, 4-1999-731 and 4-2001-435), the Novo Nordisk Foundation and EU Biomed 2 (BMH4-CT98-3314), Doctoral Programs for Public Health, Foundation for Allergy Research, Research Foundation of Orion Corporation, Tampere Tuberculosis Foundation, Päivikki and Sakari Sohlberg foundation and the Jalmari and Rauha Ahokas Foundation. None of the funders had any role in the design, analysis or writing of this article.
S. M. V., J. Tuokkola and M. Kaila were responsible for the current study design and concept. J. I., M. Knip, J. Toppari and R. V. are members of the stearing committee of the DIPP study. S. M. V. designed the DIPP nutrition study, and within the DIPP nutrition study, the allergy study was designed by S. M. V. and M. Kaila. J. Tuokkola and S. M. V. conducted the research. H.-M. T. and H. T. were responsible for the statistical analysis. R. V. was responsible for the clinical work in Oulu, and M. Knip was responsible of the clinical work in Tampere. J. Tuokkola and A. L. wrote the first version of the article with equal contribution. J. M., M. Kaila and S. M. V. particitipated in the writing process. S. M. V. and M. Kaila. had the primary responsibilty for the final work with equal contribution. All the authors particitipated in the critical revision of the manuscript and have accepted the final version.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003633







