Vitamin C is an essential water-soluble micronutrient required for multiple biological functions. This vitamin is a cofactor of several enzymes, promotes Fe absorption, and has antiscorbutic and antioxidant actions(Reference Duarte and Lunec1).
Few studies have assessed the prevalence of vitamin C deficiency or blood ascorbic acid (AA) concentrations in infants or newborns. Villalpando et al. (Reference Villalpando, Montalvo-Velarde and Zambrano2) investigated the prevalence of vitamin C deficiency in Mexican infants (aged 0–2 years) using the data from the 1999 National Nutrition Survey. The prevalence of vitamin C deficiency ( ≤ 2 mg/l or ≤ 11·4 μmol/l) in this group was 30·3 % and the mean blood AA concentration was 4 mg/l (22·8 μmol/l). Studies that assessed AA concentrations in cord blood reported means of 78·6 (sd 5·2) μmol/l in newborns of low socio-economic status from USA(Reference Jain, Wise and Yanamandra3), 92·11 (95 % CI 88·94, 95·29) μmol/l in British newborns(Reference Scaife, McNeill and Campbell4) and 172·9 (sd 39·2) μmol/l in newborns from Israel(Reference Dvir, Kohelet and Arbel5).
Studies that investigated AA concentrations in cord blood pointed to significant associations with maternal blood AA concentrations(Reference Jain, Wise and Yanamandra3, Reference Scaife, McNeill and Campbell4, Reference Vobecky, Vobecky and Shapcott6–Reference Dejmek, Ginter and Solanský9), smoking(Reference Dejmek, Ginter and Solanský9–Reference Madruga de Oliveira, Rondó and Barros11), age(Reference Vobecky, Vobecky and Shapcott6), diet(Reference Scaife, McNeill and Campbell4), type of delivery(Reference Scaife, McNeill and Campbell4, Reference Woods, Cavanaugh and Norkus12), duration of gestation(Reference Guajardo, Beharry and Modanlou13, Reference Baydas, Karatas and Gursu14), fetal distress(Reference Dvir, Kohelet and Arbel5) and birth weight(Reference Jain, Wise and Yanamandra3, Reference Baydas, Karatas and Gursu14). According to Scaife et al. (Reference Scaife, McNeill and Campbell4) vitamin C intake in pregnancy is significantly correlated with maternal (r 0·17; P < 0·001) and cord blood (r 0·10; P = 0·01) AA concentrations at delivery. Although there is no study assessing the relationship between alcohol consumption during pregnancy and blood AA concentrations, previous studies have shown an association between alcohol and AA status in humans(Reference Bonjour15, Reference Fazio, Flint and Wahlqvist16). As far as we know, there are no studies assessing vitamin C status in Brazilian newborns. We hypothesised that cord blood AA concentrations could be influenced by maternal socio-economic, demographic, obstetric, nutritional and health factors. Therefore, the aim of the present study was to determine the relationship between cord blood AA concentrations in newborns and maternal characteristics, including alcohol consumption.
Experimental methods
The methods have been described elsewhere(Reference Madruga de Oliveira, Rondó and Mastroeni17). A total of 117 healthy parturients admitted from October to December 2002 at the Obstetric Centre of Hospital Universitário (University of São Paulo, Brazil) were included in this cross-sectional study. Women with a history of infectious diseases, metabolic disorders, pre-eclampsia/eclampsia, twin-pregnancies, with a gestational age < 37 and ≥ 42 weeks, and those who gave birth to an infant with a weight < 2500 g were excluded.
Data concerning socio-economic, demographic, obstetric, nutritional and health characteristics of the parturients, including alcohol consumption and smoking habit, were assessed by a standardised questionnaire.
The parturients were asked about the amount, frequency and type of alcoholic beverage ingested in pregnancy. Smoking was estimated by the mean number of cigarettes smoked per d throughout pregnancy.
A retrospective FFQ was used to determine the maternal consumption of twenty-two Brazilian foods rich in vitamin C. The questionnaire was adapted from a study carried out by Fornés et al. (Reference Fórnes, Martins and Herman18), but not validated for vitamin C. The method was modified using five different scores: 0, never; 0·07, one to three times per month; 0·28, once to three times per week; 0·71, four to six times per week; 1, once per d. A single vitamin C-rich food intake score was obtained adding the different scores for each food. The level and bioavailability of vitamin C in each food were not considered. Cord blood (6 ml) and maternal blood (4 ml) were centrifuged, and the plasma immediately stored in a − 80°C freezer, for a maximum of 4 d, until determination of vitamin C by HPLC, using the method of Wayner & Burton(Reference Wayner, Burton, Miguel, Quintanilha and Weber19).
The differences between mean cord blood AA, maternal blood AA concentrations, per capita income, and vitamin C-rich food intake score by type of delivery, parity, alcohol consumption and use of supplements containing vitamin C (in combination with other nutrients) were assessed by the Mann–Whitney test. The χ2 test was used to assess the association between the categorical variables smoking and alcohol consumption. Cord and maternal blood AA concentrations, per capita income, and vitamin C-rich food intake score were converted to natural logs, because they did not have normal distribution. The correlations between cord blood AA concentrations, per capita income, maternal vitamin C-rich food intake score and maternal blood AA concentrations were obtained by the Pearson's correlation coefficient. Multiple linear regression models were used to determine the relationship between cord blood AA (dependent variable) and maternal blood AA concentrations, vitamin C-rich food intake score, alcohol consumption, smoking and parity (independent variables). A forward selection process was used in which the independent variables and confounders were added to the model. Analysis of the residuals of the final model showed homogeneity and no bias. The differences were considered statistically significant when P < 0·05. Data were analysed in Stata software (Statistical Software for Professionals, release 8, 2005; StataCorp LP, College Station, TX, USA).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects and patients were approved by the Research Ethics Committees of the Faculty of Public Health and Hospital Universitário. Written informed consent was obtained from all subjects and patients.
Results
Most of the infants (88·0 %) had a gestational age between 39 and 41 weeks and 95·7 % were born with a weight of 2500–4000 g. The mean cord blood AA concentration was 92·03 (95 % CI 84·61, 99·46) μmol/l, and 62·4 % of the newborns had concentrations < 100 μmol/l. Almost 70 % of the parturients had a vaginal delivery (51·3 % normal and 18·8 % forceps). The prevalence of maternal vitamin C deficiency ( < 22·7 μmol/l) was 30·8 %, and mean maternal blood AA concentration was 33·42 (95 % CI 30·15, 36·69) μmol/l. Almost 90 % of the women had a vitamin C-rich food intake score varying from 0·5 to 6·0, and thirty (25·64 %) smoked during pregnancy. From the twenty-one parturients who consumed alcohol in pregnancy, sixteen (76 %) of them ingested beer, of which eleven of them ingested less than 300 ml a time, and five of them from 400–600 ml a time. Five (24 %) of the parturients ingested wine, from 100 to 400 ml a time. Both groups of women ingested alcoholic beverages only socially, less than seven times per month, throughout pregnancy. Thirty parturients smoked cigarettes in pregnancy, of which eleven smoked less than six cigarettes per d, eleven smoked from seven to ten cigarettes per d, and eight smoked more than ten cigarettes per d.
Approximately 48 % of the parturients were primiparous and 79·5 % of them had a low economic status, with a per capita income below one minimum Brazilian wage (US$ 187·27).
There were no significant differences between mean AA concentrations in cord blood of newborns according to type of delivery and use of supplements. However, mean AA concentration was statistically higher in cord blood of newborns of primiparous than multiparous women. Maternal alcohol consumption was also associated with cord blood AA concentrations. Newborns whose mothers consumed alcohol during gestation had significantly lower concentrations of AA in cord blood at delivery compared with newborns whose mothers denied alcohol consumption (Table 1).
Table 1 Cord blood ascorbic acid (AA) concentration, per capita income, maternal blood AA and vitamin C-rich food intake score according to type of delivery, parity, alcohol consumption and use of supplements containing vitamin C
(Mean values and standard deviations for 117 subjects)

* Given as a proportion of the Brazilian minimum wage (US$ 187·27).
† For calculation of the vitamin C-rich food intake score, see the Experimental methods section.
Cord blood AA concentration was significantly correlated with per capita income (r 0·26; P = 0·005), maternal blood AA concentration (r 0·48; P < 0·001) and maternal vitamin C-rich food intake score (r 0·36; P < 0·001). Cord blood AA and maternal AA concentrations ratios were 2·89 (sd 1·51) and 3·16 (sd 1·39) for those women who consumed, and did not consume alcohol in pregnancy, respectively.
Alcohol consumption was strongly associated with smoking habit (χ2 6·48; P = 0·01). Therefore, we included smoking in the multiple linear regression analyses as a controlling variable. Even after this adjustment, alcohol consumption was still significantly associated with cord blood AA concentration. The linear regression model including maternal AA concentration, alcohol consumption, smoking, parity, vitamin C-rich food intake score and per capita income explained 31·13 % of the variation in cord blood AA concentrations in newborns (Table 2).
Table 2 Multivariate linear regression models including ascorbic acid (AA) concentration in the cord blood of newborns as the dependent variable*
(β Coefficients with their standard errors for 117 subjects)
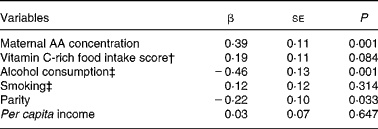
* Adjusted R 2 0·3113 (P < 0·001).
† For calculation of the vitamin C-rich food intake score, see the Experimental methods section.
‡ Yes = 1; no = 0.
Discussion
In the present study, cord blood AA concentration was not associated with type of delivery and use of supplements, but it was associated with parity, maternal blood AA concentration, per capita income, vitamin C-rich food intake score and maternal alcohol consumption.
The mean cord blood AA in Brazilian newborns (92 μmol/l) was similar to that described in British newborns (92·11μmol/l)(Reference Scaife, McNeill and Campbell4), but was lower than the mean reported by Dvir et al. (Reference Dvir, Kohelet and Arbel5) for newborns from Israel (172·9 μmol/l).
Woods et al. (Reference Woods, Cavanaugh and Norkus12) and Scaife et al. (Reference Scaife, McNeill and Campbell4) described significant differences in mean cord blood AA concentrations of newborns whose mothers laboured and did not labour at delivery. According to Woods et al. (Reference Woods, Cavanaugh and Norkus12), uterine contractile activity may generate reactive oxidative species that can lead to a significant depletion of vitamin C during labour. Maternal intake of supplements containing vitamin C during pregnancy did not influence mean cord blood AA concentration. Dejmek et al. (Reference Dejmek, Ginter and Solanský9) compared mean cord blood AA concentrations between mothers who used and did not use supplements containing vitamin C during pregnancy. Supplementation showed a beneficial effect only for smoking mothers.
Vobecky et al. (Reference Vobecky, Vobecky and Shapcott6) assessed the relationship between cord blood AA concentrations and parity, but did not describe any significant difference. In the present study, mean cord blood AA concentration was higher in primiparous than in multiparous women.
The positive correlation between maternal blood AA and cord blood AA concentrations is well described in the literature(Reference Jain, Wise and Yanamandra3, Reference Scaife, McNeill and Campbell4, Reference Vobecky, Vobecky and Shapcott6–Reference Dejmek, Ginter and Solanský9). Few studies assessed maternal dietary vitamin C intake(Reference Scaife, McNeill and Campbell4, Reference Woods, Cavanaugh and Norkus12), and only Scaife et al. (Reference Scaife, McNeill and Campbell4) described a significant correlation between vitamin C in the diet and cord blood AA concentration.
Alcohol consumption was significantly associated with cord blood AA concentration. However, mean maternal blood AA concentration was not significantly different in mothers who reported (29·5 (sd 15·8) μmol/l) and did not report alcohol consumption (34·3 (sd 18·2) μmol/l) during pregnancy (P = 0·268).
As far as we know there is no study in the literature assessing the influence of maternal alcohol consumption on cord blood AA concentrations. We hypothesise that alcohol consumption can impair vitamin C transport through the placenta and/or enhance oxidative stress to the fetus, thus, increasing antioxidant requirements.
According to Burd et al. (Reference Burd, Roberts and Olson20), prenatal alcohol exposure may play an important role in abnormalities of placental function and development. Alcohol produces placental vasoconstriction that may impair blood flow, and consequently nutrient transport. It can also induce oxidative stress in the placenta and possibly produces spasm in umbilical cord veins and arteries. Moreover, alcohol consumption can reduce placental weight, and increase villous infarction and intervillous thrombi.
Although Burd et al. (Reference Burd, Roberts and Olson20) pointed out that maternal alcohol consumption is enhanced in poor populations and with inadequate diet, we found no differences regarding per capita income or vitamin C-rich food intake score between women who consumed or did not consume alcohol during pregnancy.
We recommend the development of experimental studies assessing the placental transport of ethanol and its impact on AA uptake. It is also important to carry out further epidemiological cohort studies to evaluate, in detail, the association between alcohol consumption in pregnancy and cord blood AA concentrations in newborns, controlling for ingestion of vitamin C-rich foods, smoking and socio-economic status.
Acknowledgements
The authors would like to extend their thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development) for supporting the Master's degree scholarship of A. M. de O. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
A. M. de O. designed the study protocol, collected the data and wrote the paper. P. H. C. R. designed the study protocol, interpreted the results and wrote the paper. J. M. O. performed the statistical analysis, interpreted the results and wrote the paper.
There are no conflicts of interest.




