Patients with advanced liver disease, such as those with hepatoma and regionally localised malignant lesions and those with loculated intrahepatic abscesses, may require surgical intervention to remove a tumour with negative margins(Reference Nguyen, Gamblin and Geller1,Reference Mezhir, Fong and Jacks2) . Furthermore, certain patients with symptomatic or mass-occupying cysts that press on the surrounding parenchyma can present with hepatic congestion and expansion of the liver capsule(Reference Pitale, Bohra and Diamond3,Reference Barbier, Ronot and Aussilhou4) . These patients may require hepatectomy procedures to reduce the cyst burden and the pathological effects on the hepatic architecture(Reference Rawla, Sunkara and Muralidharan5). In such scenarios, since hepatectomy is an intraabdominal and peritoneal procedure, the operation requires careful preoperative mapping and planning before surgical intervention(Reference Paes-Barbosa, Ferreira and Szutan6). Furthermore, during this process, the preoperative risks should be considered to accurately prognosticate patient outcomes, including an assessment of the inherent risks based on the patient’s anatomy and nutritional status.
In particular, patients with advanced liver disease or liver lesions suffer from protein-energy malnutrition that stems from disrupted cycles of gluconeogenesis, which coincides with the liver-induced rapid turnover of muscular volume and sarcopenic changes, as well as reduced oral intake and cachexia(7,Reference Cheung, Lee and Raman8) . As validated in prior literature, these nutritional deficiencies in liver patients can have negative effects on peri- and postoperative outcomes(Reference Huang, Hsieh and Kuo9).
Therefore, given the prevalence of malnutrition in hepatectomy populations, this study aims to investigate the role of malnutrition on the postoperative risks following liver resection procedures. To evaluate this research question, this study utilises a national registry of hospitalised patients to perform propensity score matching and compare the postsurgical endpoints and mortality in those undergoing hepatic resections to control cohorts.
Methods
National Inpatient Sample database
The Agency for Healthcare Research and Quality sponsors the Healthcare Cost and Utilization Project, which contains the National Inpatient Sample. This database comprises hospital inpatient stays sampled from State Inpatient Databases, filtering out hospitals that specialise in long-term acute care or rehabilitation. The data are drawn from a 20 % stratified sample of discharges from community-based hospitals annually. Because this database is drawn from all forty-seven states participating in Healthcare Cost and Utilization Project, the National Inpatient Sample covers 97 % of the US population. In 2017, there were 7·2 million patient visits to 4584 hospitals, with information on admission, hospital course, discharge, demographics, location, International Classification of Disease (ICD) diagnoses and Procedure Coding System (PCS) codes(10). This study utilises data originating from the National Inpatient Sample database 2011–2017. Within this time period, several changes were made in the organisation of this data structure. Starting in 2014, the database began sampling a fraction from all the hospitals involved in the project, as opposed to drawing all of their data from a select 1000 hospitals. In addition, in 2015, the National Inpatient Sample database transitioned from ICD-9 to ICD-10. The standard guidelines for including these revisions have been followed in this study(11,Reference Elixhauser, Heslin and Owens12) . Previous studies with the same co-authors have detailed a similar data gathering process which allowed cross-referencing between ICD-9 or ICD-10 codes while limiting heterogeneity(Reference Lee, Fan and Ahern13,Reference Lee, Fan and Hastie14) . The heterogeneity was reduced with a pre-programmed, shared database that used CM ICD-9 to 10 conversion tables for the diagnosis-related groups.(15,16) A v32 ICD-9 code database was paired with the equivalent 2017 ICD-10 CM or PCS code that was drawn from the 2017 CM and PCS General Equivalence Mappings database. This created two databases that allowed forward and backward mapping. This was then merged to create a joint code database that included code descriptions. Thus, diagnosis-related groups could be found with keywords found in ICD-9, ICD-10 or code descriptions(Reference Fung, Richesson and Smerek17).
Study cohort and study variables
The study cohort included patients with discharge diagnoses of hepatectomy procedure (including partial hepatectomy, total hepatectomy, lobectomy, other destruction of the liver). Those under 18 years of age were excluded. Using the exposure variable of malnutrition (which was a composite of protein-energy malnutrition, sarcopenia and weight loss/cachexia), the eligible study cohort was stratified into a malnutrition-present cohort and malnutrition-absent controls. The underlying diagnostic aetiologies were listed as part of the analysis work up. The primary endpoints of the study included mortality, length of stay, hospitalisation costs and disposition at discharge; the secondary endpoints included the following postoperative complications: postoperative bleeding, postoperative infection, postoperative wound complications and postoperative respiratory failure. All study variables and corresponding ICD-9/10 codes are recorded in online Supplementary Table S1.
Statistical analysis
The propensity score matching with post-match residual regression analysis was used as our standardised approach. The propensity score covariates included age, sex: female, race, diabetes, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease, coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathy, alcohol use disorder, cigarette use, obesity, elective (v. emergent) procedure, alcoholic liver disease, hepatitis B, hepatitis C, non-alcoholic fatty liver disease and cirrhosis. In post-match, the malnutrition cohort and non-malnutrition cohort were compared with univariate and multivariate statistical comparison methods, which were mean-based comparisons or categorical comparisons (χ 2 tests/Fisher’s exact tests). The multivariate analysis used Poisson’s regression or logistic analysis with the primary endpoints (mortality, length of stay, cost, disposition) as dependent variables. The multivariate models were assessed for accuracy, fit and multicollinearity with variance inflation factor analysis, Akaike information criterion and Bayesian information criterion measurements(Reference Cherkassky and Ma18,Reference Chakrabarti, Ghosh, Bandyopadhyay and Forster19) . A P-value < 0·05 was considered significant, and non-adjusted OR with CI were also used for the primary endpoints.
Additional complementary analyses were performed using substratums of the study population; for instance, the surgical type was used (total, partial – which includes partial and lobular hepatectomy, and other procedures – which include iatrogenic destruction of liver and marsupialisation), as well as lesion characteristics (malignant v. benign or primary v. secondary hepatic lesion) and the electivity of the procedure/admission. These sub-analyses are added to the online Supplementary Tables.
When evaluating the missing data, the missingness of the indexed variables was characterised using plotted representations. As the missingness was determined to be missing at random, data imputation was performed using the multiple imputation by chained equations(Reference Zhang20), which is a well-validated imputation method that is incorporated in other administrative database studies, purportedly with diminished tendency to overestimate the imputed value precision(Reference Liu, Han and Shah21).
RStudio version 1.2.5042 with R code version 3.6.3 was used for statistical analysis. This study did not need institutional or national review board approval due its usage of the national database.
Results
Patient selection
The patient selection process is detailed in Fig. 1. There were 27 028 patients in our database undergoing hepatectomy with patients under the age of 18 excluded. The patients were further divided into a general group, patients undergoing elective hepatectomies, hepatectomies for malignant liver cancer or non-malignant liver cancer, hepatectomies for primary liver cancer or secondary liver cancer, total hepatectomies, partial hepatectomies and other liver procedures, for propensity score matching and comparison of malnutrition present and absent groups. There was also a group comparing patients with malnutrition undergoing hepatectomy for primary liver cancer and patients with malnutrition undergoing hepatectomy for non-primary liver cancer.
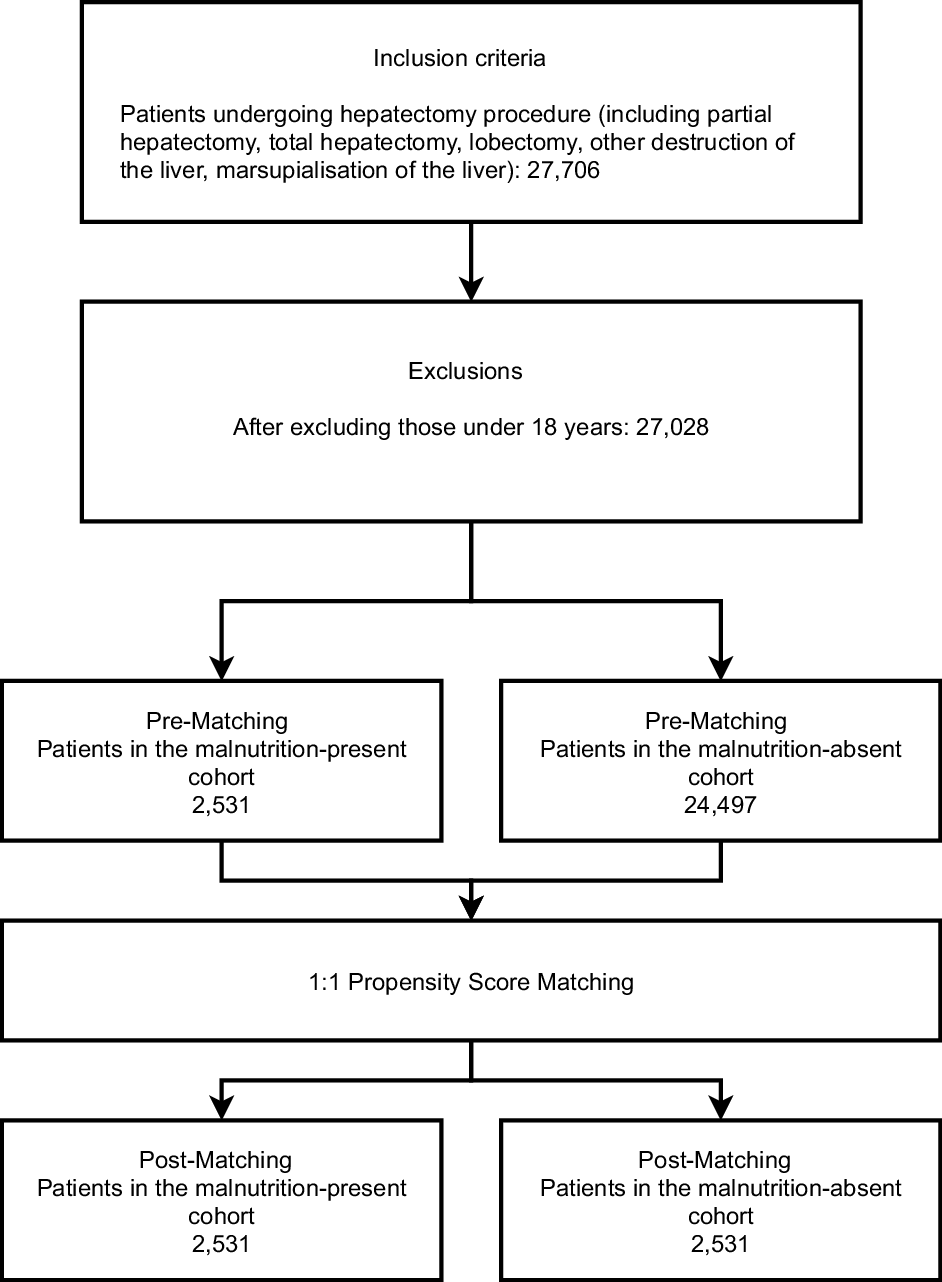
Fig. 1. The patient selection procedure of the study.
Univariate comparison of the pre- and post-match patient demographics and medical covariates
The demographics and co-morbidities of our study are described in Table 1 as well as our online Supplementary Tables. In terms of the demographics in the general group post-match, patients in the malnutrition and non-malnutrition group were around the same age (61·1 years; P = 0·750) and similar proportions of sexes (male: 49 % v. 48·8 %; P < 0·960). The patient’s identified race was also similar. The table also details the indications and aetiologies for hepatectomy, as well as the electivity of the procedure/admission and the type of hepatectomy performed. The leading indication for hepatectomy was secondary liver cancer, followed by primary liver cancer (including hepatocellular carcinoma and cholangiocarcinoma), then liver abscess. The leading procedure type was partial hepatectomy, followed by lobectomy.
Table 1. Pre- and post-propensity matching comparisons of patients with and without malnutrition; demographics and medical covariates of those who underwent hepatectomy
(Numbers and percentages)
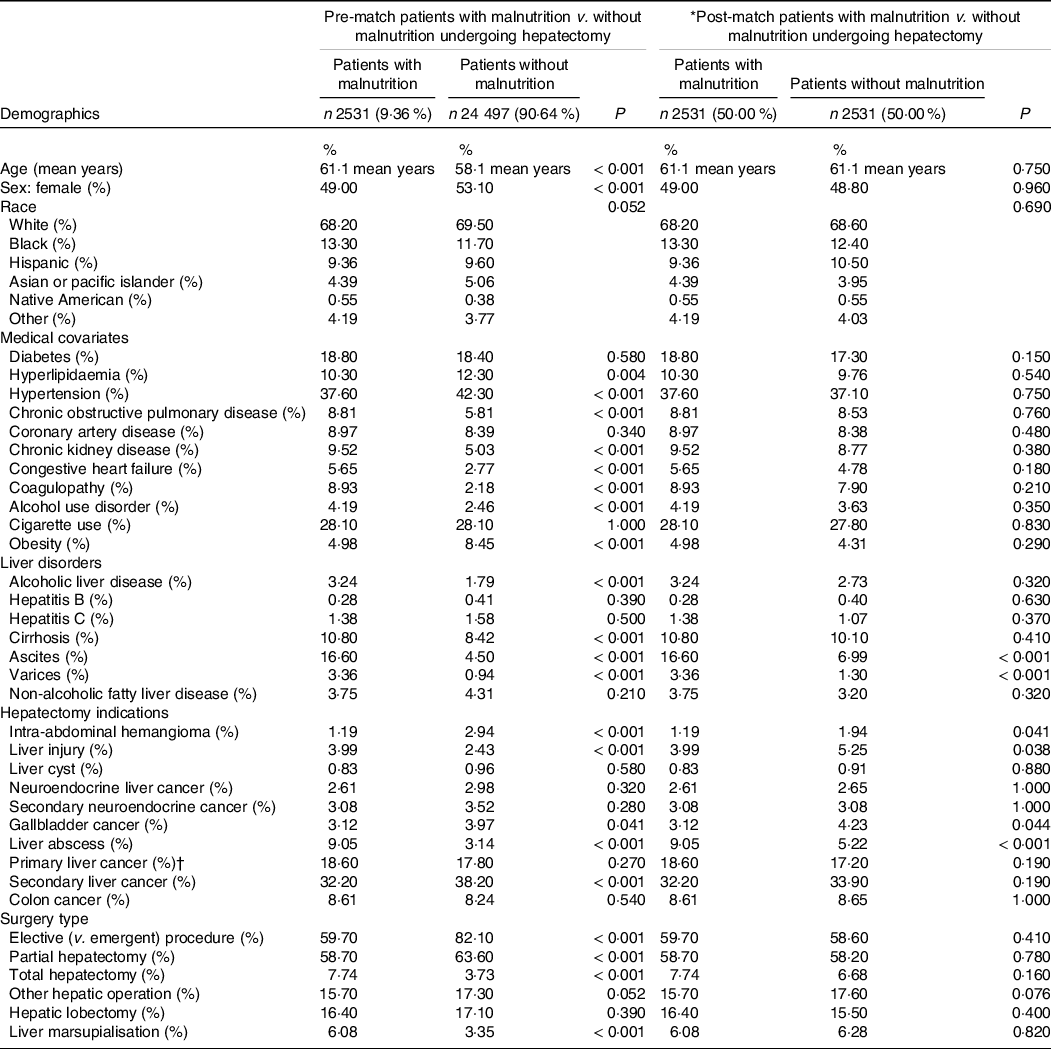
* The following variables were included in the propensity-score matching procedure: age, sex: female, race, diabetes, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease, coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathy, alcohol use disorder, cigarette use, obesity, elective (v. emergent) procedure, alcoholic liver disease, hepatitis b, hepatitis c, non-alcoholic fatty liver disease, cirrhosis.
† Includes hepatocellular carcinoma and cholangiocarcinoma.
Univariate comparison of the pre- and post-match patient socio-economic status and hospital characteristics
Table 2 shows the pre- and post-match comparison of patient socio-economic and hospital characteristics in the general hepatectomy group. In the post-match comparison, malnutrition cohorts were more likely to be under lower median household income represented by lower quartiles and were more likely to be admitted in large, urban-teaching hospitals located in the Midwest and Western US. The malnutrition cohort was more likely to be insured through Medicare or Medicaid.
Table 2. Pre- and post-propensity matching comparisons of patients with and without malnutrition; patient socio-economic and hospital characteristics of those who underwent hepatectomy
(Numbers and percentages)
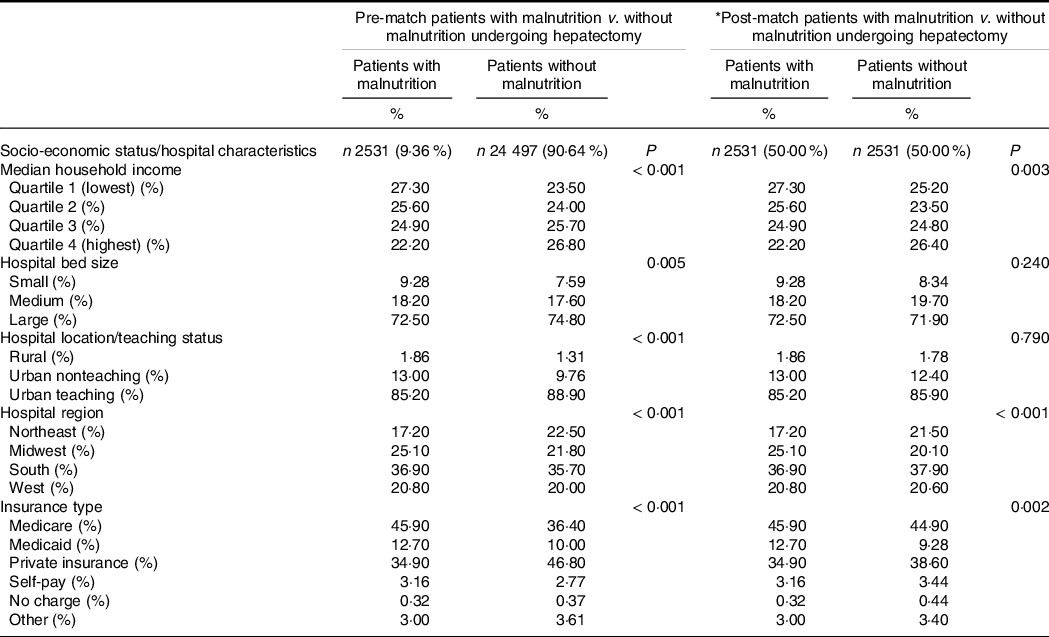
* The following variables were included in the propensity-score matching procedure: age, sex: female, race, diabetes, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease, coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathy, alcohol use disorder, cigarette use, obesity, elective (v. emergent) procedure, alcoholic liver disease, hepatitis b, hepatitis c, non-alcoholic fatty liver disease, cirrhosis.
Univariate and multivariate comparison of the pre- and post-match postoperative outcomes and clinical endpoints
Table 3 shows the pre- and post-match comparison of postoperative outcomes in patients with malnutrition v. without malnutrition in the general group of hepatectomy. In post-match, malnutrition cohort (compared with controls) was more likely to experience in-hospital death (6·60 % v. 5·25 %, P = 0·049, OR 1·3, 95 % CI 1·03, 1·65) and was more likely to have higher length of stay (18·1 d v. 9·32, d P < 0·001) and hospitalisation costs ($278 780 v. $150 812, P < 0·001). In terms of hospital discharge, those with malnutrition were more likely to be discharged to non-routine care facilities, including short-term hospital, skilled nursing facility or home with healthcare. For postoperative complications, malnutrition cohort was more likely to experience the following complications: bleeding (6·52 % v. 3·87 %, P < 0·001, OR 1·73, 95 % CI 1·34–2·24), infection (6·64 % v. 2·49 %, P < 0·001, OR 2·79, 95 % CI 2·07, 3·74), wound complications (4·50 % v. 1·38 %, P < 0·001, OR 3·36, 95 % CI 2·29, 4·93), respiratory failure (9·40 % v. 4·11 %, P < 0·001, OR 2·42, 95 % CI 1·91, 3·07). In multivariate analysis, malnutrition was associated with higher mortality (P = 0·028, adjusted OR 1·30, 95 % CI 1·03, 1·65), length of stay (P < 0·001, adjusted OR 1·95, 95 % CI 1·92, 1·98) and hospitalisation costs (P < 0·001, adjusted OR 1·87, 95 % CI 1·87, 1·87). The multivariate model using mortality as the primary endpoint with inclusion of the covariates is demonstrated as a forest plot in Fig. 2.
Table 3. Pre- and post-propensity matching comparisons of patients with and without malnutrition; clinical outcomes of those who underwent hepatectomy
(Numbers and percentages; odds ratios and 95 % confidence intervals)
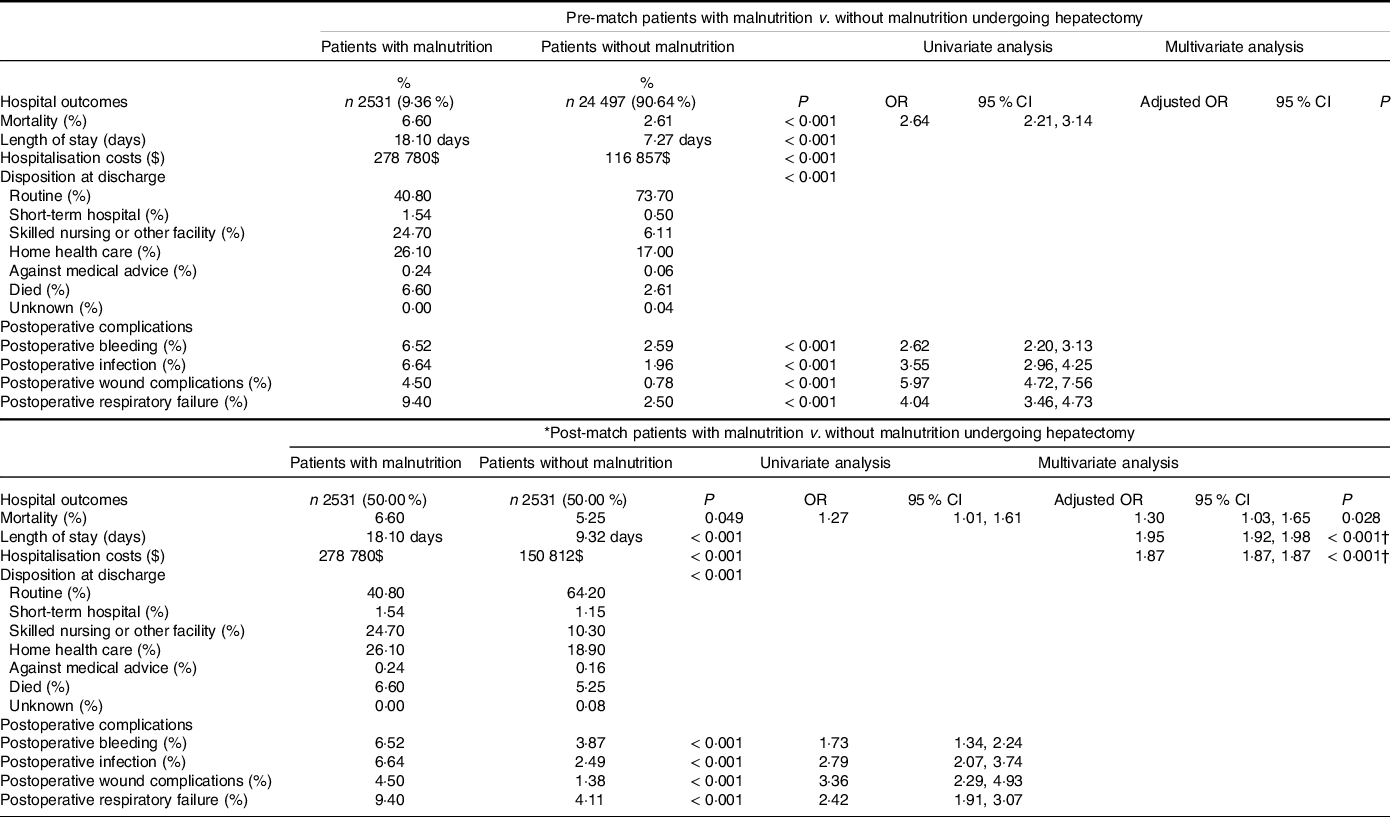
* The following variables were included in the propensity-score matching procedure: age, sex: female, race, diabetes, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease, coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathy, alcohol use disorder, cigarette use, obesity, elective (v. emergent) procedure, alcoholic liver disease, hepatitis b, hepatitis c, non-alcoholic fatty liver disease, cirrhosis.
† Used Poisson regression analysis.

Fig. 2. The multivariate forest plot that used mortality as the primary endpoint; the included covariates were hospital admission and location characteristics.
The online Supplementary Tables use stratum-specific comparisons of post-hepatectomy outcomes: in online Supplementary Table S2, the population is stratified according to the procedure type (total, partial and other hepatectomy); in online Supplementary Table S3, the populations are stratified according to malignant v. non-malignant hepatic lesions; in online Supplementary Table S4, the population is stratified using primary v. non-primary liver lesions; and in online Supplementary Table S5, electivity of the procedure/admission is used to sub-stratify the population undergoing hepatectomy. In general, the malnutrition groups, regardless of type of procedure or the indication, showed higher mortality and higher incidences of adverse postoperative outcomes.
Discussion
Based on these findings, the presence of malnutrition within our cohort of patients undergoing hepatectomy increases the incidence of mortality, hospitalisation costs and the probability of discharge to a skilled nursing or other facility. In addition, the incidence of postoperative bleeding, infection, wound complications and even respiratory failure was increased in patients with malnutrition. Furthermore, the sub-analyses using stratum-specific comparisons also show that the post-hepatectomy outcomes are independent of surgery type, indications, lesional characteristics and electivity of the procedure, but, instead, they universally demonstrated the presence of malnutrition is associated with higher mortality and complication risks following hepatectomy. All of these findings validate the prior data from cohort-based studies demonstrating malnutrition to be a potent risk factor of adverse postoperative outcomes, longer hospital stays and significant postoperative co-morbidities(Reference Huang, Hsieh and Kuo9,Reference Fukami, Saito and Arikawa22) . Our study demonstrates the negative effects of malnutrition in a larger cohort of patients using the national registry of hospitalised patients with the inclusion of propensity score matching and post-regression analysis of the postoperative endpoints. This analysis accounts for the preoperative risk co-morbidities and confounders while improving statistical power in the evaluation of malnutrition on the post-hepatectomy outcomes.
The negative postoperative outcomes in the malnutrition cohort are especially concerning due to the close relationship between malnutrition and our patient population. The indications for hepatic resections are generally primary or secondary malignancy, benign disease or trauma. Patients with hepatocellular carcinoma undergoing hepatic resection most likely have advanced liver disease, increasing the risk of malnutrition. Some studies estimate the prevalence of malnutrition in patients with advanced liver disease between 50 and 90 %(Reference Cheung, Lee and Raman8). The aetiology of malnutrition for patients with advanced liver disease is multifactorial and most notably involves anorexia, malabsorption and altered macronutrient metabolism(Reference Cheung, Lee and Raman8). Anorexia can be caused by physical symptoms such as nausea, bloating or fatigue, as well as ascites leading to early satiety. Inflammatory and appetite mediators, such as TNF-α and leptin, are elevated in patients with liver cirrhosis and can also potentiate anorexia(Reference Le Moine, Marchant and De Groote23,Reference Kalaitzakis, Bosaeus and Ohman24) . In addition, nutrient absorption is affected in liver cirrhosis because of portosystemic shunting and decreased bile production. Even when the nutrients reach the liver via the portal system, the ability to metabolise nutrients is impaired. Cirrhotic livers have decreased ability for processing of glycogen, promoting gluconeogenesis from protein and fatty acids. This nutritional compensation is further exacerbated by increased insulin resistance in peripheral tissues associated with liver cirrhosis(Reference Owen, Reichle and Mozzoli25). If the patient suffers from alcohol-related liver cirrhosis, the malnutrition could be compounded by insufficient feeding in addition to increased rates of chronic pancreatitis.
In terms of other aetiologies for malnutrition in patients undergoing hepatic resection, patients with malignancy, including primary liver cancer and metastases to the liver, can experience cachexia, driven by reduced food intake, catabolism and chronic inflammation, predisposing patients to malnutrition(Reference Baracos, Martin and Korc26). Approximately 20–55 % of patients affected by colorectal metastases to the liver suffer from malnutrition(Reference Ciuni, Biondi and Grosso27). Other indications for hepatic resection, such as trauma or hepatic abscesses, also increase energy expenditure, predisposing patients to malnutrition as well.
In general, a mismatch of intake and usage of energy content leads to loss of muscle, fat, skin, eventually affecting the body’s ability to adapt to new stressors such as surgery. This potentially affects the physiological processes involved in wound healing, coagulation, immunity and ventilation(Reference Demling28–Reference Chen, Afzal and Sohn31). The lack of sufficient protein affects the wound healing pathway, increasing wound infection rates and wound dehiscence(Reference Huang, Hsieh and Kuo9,Reference Gu, Malahias and Strigelli32) . In addition, because of the decreased production of coagulation factors, malnutrition leads to poor haemostasis and a markedly increased probability of postoperative bleeding(Reference Chen, Afzal and Sohn31). Impaired nutrition affects many immune system domains, including the complement system, opsonisation and leucocyte function, leading to increased nosocomial infections postoperatively(Reference Mainous and Deitch29,Reference França, Ishikawa and Zorzella-Pezavento33) . Nutrient deficiency also causes a decreasing neural ventilatory drive and decreases the total mass and contractility of the diaphragm(Reference Rochester and Esau30). The malfunctioning of the diaphragm can lead to mechanical ventilation issues both peri- and postoperatively, with patients potentially affected by increased respiratory complications(Reference Avolio, Gaspari and Teofili34–Reference Fischer, Shang and Butler36). All these postoperative complications can lengthen the hospital stay and cause significant morbidity and mortality.
Clinical implications
With malnutrition increasing the risk of mortality and morbidity in patients undergoing hepatic resections, special care must be utilised in treating these patients. According to our analyses, even in elective procedures, malnutrition was a strong risk factor for morbidity. To prevent this, patients should be screened for malnutrition. Preoperatively, patients can be assessed for malnutrition using serum albumin, which has been linked to negative postoperative outcomes(Reference Gibbs, Cull and Henderson37). Nutritional screening tools, such as the Subjective Global Assessment of Nutritional Status, Nutritional Risk Screening Tool 2002 or Nutrition Risk Index, can be used to further stratify patients(Reference Detsky, McLaughlin and Baker38–40), though there is a lack of head to head studies comparing accuracy in predicting postoperative outcomes. We recommend the above tools in evaluating malnutrition, though understandably clinicians may choose other assessments due to familiarity or logistical reasons. The present studies supporting preoperative nutritional support only suggest nutritional support for patients with severe malnutrition to avoid delaying surgery(Reference Jie, Jiang and Nolan41). Postoperatively, early (< 24 h) enteral feeding should be initiated if possible, and parenteral nutrition should only be initiated if oral supplementation is not possible(Reference Koretz, Avenell and Lipman42,Reference Saunders, Brian and Wright43) . Besides managing malnutrition, patients should also be followed by an interdisciplinary team to manage the complications associated with this risk factor. This team should include a respiratory therapist to assist with extubation and in the event of a respiratory failure and wound care teams to manage the poor wound healing.
Limitations
One of this study’s limitations pertains to the missing biochemical and laboratory indices and diagnostic variables relevant to formulating postoperative risk assessment via the standard risk-classification systems such as the Clavien–Dindo classification. However, this lack of biochemical and granular health data is accounted for through the use of substitutionary diagnostic codes that evaluate the common postoperative complications following the hepatectomy procedure, which is further improved in the statistical approach through pre-comparison matching with the common medical covariates present in either study population (malnutrition-present study population or the absent controls).
Conclusion
Nonetheless, our study still shows a significant association between malnutrition in hepatic resection and various postoperative complications and mortality. We recommend early and accurate screening of patients undergoing hepatic resection for nutritional status. Then, early nutritional intervention and timely postoperative management of expected complications should ultimately improve patient outcomes.
Acknowledgements
There was no funding provided for this study.
D. U. L.: conceptualisation, data curation, formal analysis, investigation, methodology, supervision, validation, writing – original draft, writing – review and editing. E. W.: writing – original draft, investigation, methodology, writing – original draft, visualisation, writing – review and editing. D. J. H.: writing – original draft, investigation. E. A. A.: writing – original draft, investigation. H. C.: writing – original draft, investigation. R. K.: supervision, writing – review and editing.
The authors of this manuscript certify they share no affiliation or involvement with any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. None declared.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521003809








