Pacu, Piaractus mesopotamicus, is an omnivorous characin native of rivers, floodplains, lakes and flooded forests of South America. It is considered one of the best candidates for aquaculture production in tropical areas, due to the high growth rates (it may reach more than 1 kg in 1 year), good meat quality and good consumer acceptance(Reference Urbinati, Gonçalves, Baldisserotto and Gomes1).
Pacu diets are mainly based on local ingredients, including soyabeans. However, due to soyabean costs, its replacement in diets by more economical ingredients, such as maize distillers dried grains with solubles (DDGS), may improve production profitability. DDGS is the by-product of maize grain fermentation, by the addition of enzymes and yeast, to produce ethanol. After fermentation, the remaining product goes through a set of centrifugations, separating the thin and thicker portion, that goes to drying processes. To the thicker mass, commonly known as DDG (distillers dried grains), can be included the thin portion, or soluble portion, to originate the DDGS(Reference Wyman2). Maize DDGS has moderate protein content (330 g/kg) and low starch content (<30 g/kg) and lacks anti-nutritional factors, except for high content of NSP(Reference Jacques, Lyons and Kelsall3). Besides, part of the enzymes and yeast used during fermentation remains into the soluble portion of the final product(Reference Lim and Yildirim-Aksoy4).
Fish response to DDGS inclusion in the diets varies according to DDGS composition, dietary composition regarding processing (majorly extrusion), fish species and food habits(Reference Brown, Schaeffer, Rosentrater, Liu and Rosentrater5). Recommended dietary inclusion of DDGS ranges from 200 to 330 g/kg for carnivorous fish such as olive flounder, Paralichthys olivaceus (Reference Rahman, Choi and Lee6), rainbow trout(Reference Barnes, Brown and Rosentrater7,Reference Cheng and Hardy8) , red seabream(Reference Choi, Rahman and Lee9) and hybrid striped bass(Reference Trushenski and Gause10). However, DDGS is particularly attractive for omnivorous fish as it has a protein content that is closer to their dietary protein requirement, and because omnivorous fish also tolerate better the high fibre content of DDGS(Reference Magalhães, Coutinho and Pousão-Ferreira11). Thus, dietary inclusion of DDGS usually ranges from 300 to 700 g/kg for omnivorous fish like tilapia (Oreochromis niloticus)(Reference Abo-State, Tahoun and Hamouda12–Reference Tidwell, Webster and Yancey15), channel catfish (Ictalurus punctatus)(Reference Lim, Yildirim-Aksoy and Klesius16,Reference Webster, Tidwell and Yancey17) , blunt nose black bream (Megalobrama amblycephala) and gibel carp (Carassius gibelio)(Reference He, Wu and Liu18).
To better understand the modulatory action of DDGS on feed utilisation, growth performance and health of fish, it is necessary to study its effects on intestinal tract integrity and digestive function(Reference Krogdahl, Gajardo and Kortner19–Reference Sund, Taranger and Rungruangsak-Torrisen21). However, up to now, few studies have focused on the effects of dietary DDGS on digestive enzyme activity and intestine morphology in fish. Recently, Magalhães et al.(Reference Magalhães, Coutinho and Pousão-Ferreira11) and Rahman et al.(Reference Rahman, Choi and Lee6) evaluated DDGS inclusion in diets for carnivorous fish, and both reported no differences in digestive enzyme activities. However, studies focusing on the effects of DDGS incorporation in diets at the intestinal level for omnivorous species are still lacking. Dietary ingredient composition may also affect the oxidative status of the fish intestine(Reference Bayir, Sirkecioglu and Bayir22–Reference Pérez-Jiménez, Hidalgo and Morales26). Oxidative stress is characterised by an imbalance between reactive oxygen species (ROS) formation and removal in the organism by antioxidant defences(Reference Zheng, Liu and Meng27), which lead to the oxidation of the main biomolecules that can affect cell function leading to tissue injuries, like damages in the intestine epithelium(Reference Chen, Azad and Gibson28). However, few studies focused on the oxidative stress associated with dietary DDGS inclusion. Nevertheless, the high content of PUFA(Reference Corzo, Schilling and Loar29,Reference Stein and Shurson30) and the exposure to high temperatures during processing may increase DDGS susceptibility to lipid peroxidation (LPO)(Reference Esterbauer, Schaur and Zollner31). On the other hand, other components in DDGS such as its high sulphur content(Reference Song, Chen and Wang32) have been highlighted to provide adequate antioxidant protection. Still, dietary inclusion of DDGS may affect the intestine oxidative status and intestine morphology.
Thus, the aim of this study was to evaluate the effects of dietary replacement of soyabean meal by maize DDGS on diet digestibility, digestive enzyme activity, oxidative status and morphology and cytoarchitecture of intestine in pacu juveniles.
Material and methods
The experimental protocol used in this study was approved by the Ethics Committee of Animal Welfare of the College of Agricultural and Veterinary Sciences of Sao Paulo State University, protocol number 1910/16.
Two trials evaluated the effects of dietary replacement of soyabean meal by maize DDGS in diets for pacu, P. mesopotamicus. Trial 1 evaluated the apparent nutrient digestibility of diets with increasing DDGS levels, whereas trial 2 evaluated the effects of these diets on digestive enzyme activity, intestine oxidative stress and intestine morphology.
Experimental diets
Five diets were formulated to be isoproteic (290 g/kg digestible protein) and isoenergetic (14·5 MJ/kg digestible energy) and with increasing DDGS levels (0, 100, 200, 300 and 400 g/kg) replacing soyabean meal and maize meal. The same diet formulation was used in trial 1 and trial 2; however, 5 g/kg of chromic oxide (Cr2O3) was added as an external marker to the diets used in trial 1. Composition and proximate analyses of the diets are shown in Table 1.
Table 1. Composition and proximate analysis of the experimental diets

DDGS, distillers dried grains with solubles; BHT, butyl hydroxytoluene; CP, crude protein; NDF, neutral insoluble-detergent fibre (determined using the Ankom® Filter Bag Technique and heat stable α-amylase (973·18)); DP:DE, digestible protein:digestible energy ratio.
* DDGS (CP, 336 g/kg; 20·9 MJ/kg), Libra Etanol.
† Soyabean meal (CP 448 g/kg; 17·1 MJ/kg), maize meal (CP 84 g/kg; 16·2 MJ/kg), wheat meal (CP 148 g/kg; 16·7 MJ/kg), rice bran (CP 132 g/kg; 19·4 MJ/kg), Cargill, Sao Paulo, Brazil.
‡ Maize gluten (CP 715 g/kg; 21·1 MJ/kg), fishmeal (CP 580 g/kg; 14·6 MJ/kg), InVivo, Sao Paulo, Brazil).
§ Poultry meal (CP 489 g/kg; 13·1 MJ/kg), Agromix.
|| Lysine, Anjinomoto LTDA.
¶ Vitamin and mineral premix: vitamin A – 500 000 IU; vitamin D3 – 250 000 IU; vitamin E – 5000 mg; vitamin K3 – 500 mg; vitamin B1 – 1500 mg; vitamin B2 – 1500 mg; vitamin B6 – 1500 mg; vitamin B12 – 4000 mg; folic acid – 500 mg; calcium pantothenate – 4000 mg; vitamin C – 10 000 mg; biotin – 10 mg; inositol – 1000 mg; nicotinamide – 7000 mg; choline – 10 000 mg; Co – 10 mg; Cu – 1000 mg; Fe – 5000 mg; iodine – 200 mg; Mn – 1500 mg; Se – 30 mg; Zn – 9000 mg (Agromix LTDA).
** Values calculated according to FAO (2012) and NRC (2011).
Dietary ingredients were ground (1 mm), thoroughly mixed and processed in a single screw extruder (Imbramarq, model Labor PQ-30). The extrusion temperature was kept at 124°C, screw speed at 676·5 rpm, feeding rate of about 500 g/min, and the circular matrix was set at 2 mm. Pellets were dried at 50°C for 12 h and then stocked at −20°C until used.
Fish and experimental design
Pacu (P. mesopotamicus) juveniles were obtained from a commercial fish farm and acclimatised for 30 d in a quarantine tank (5000 litres) upon arrival to the experimental facilities, at the Aquaculture Nutrition Laboratory of Food Engineer and Animal Science Faculty, São Paulo University, Pirassununga, SP, Brazil. During this period, fish were fed a commercial diet (Pira 32, Guabi, 320 g/kg of crude protein) twice daily until apparent satiation. Trials 1 and 2 were conducted simultaneously, within the same recirculation aquaculture system, with fish from the same livestock.
Trial 1
To set up the trial, 150 P. mesopotamicus juveniles (27·1 (sd 0·11) g, mean weight) were distributed to five cylindrical-conical fibreglass tanks (100 litres), at a density of thirty fish/tank. Tanks were supplied with aeration, thermoregulated to 26°C and photoperiod of 12 light–12 dark. Each tank was considered an experimental unit, and the experiment was lain out in Latin square design 5 × 5 (five treatments and five experimental periods), where each tank received each diet only for a unique time. The experimental period consisted of 11 d, the sum of the time taken for feed adaptation and faeces collection, given an entire period of 55 d.
Each period started with fish being fed for 7 d, twice daily (09.00 and 17.00 hours) with the experimental diets, until apparent satiation. Faeces were collected using a modified Guelph system as early described(Reference Pezzato, Miranda and Barros33) with modifications. One hour after the last daily feeding, the groups of fish were transferred to five collecting tanks, conic fibreglass tanks (100 litres capacity) with a valve connecting the bottom of the tank to a Falcon tube kept on ice. At 10 h later, faeces sedimented into the tubes were collected, fish were relocated to the initial tanks and the tanks used for faeces collection were cleaned and water renewed. The procedure was repeated for 4 d until enough amounts of faeces were collected. Fish were fed in an independent system to avoid contamination of faeces with feed(Reference Pezzato, Miranda and Barros33). After collection, faeces were centrifuged (1800 g , 10 min), bulk stored for each tank at −20°C and afterwards lyophilised before chemical analysis. The apparent digestibility coefficients (ADC) of DM, protein, lipids and energy of the experimental diets were measured by the indirect method(34) as follows: ADC = 100 × (1 – (% Cr2O3 diet/% Cr2O3 faeces) × (% nutrient or MJ/g energy faeces/% nutrient or MJ/g energy diet).
Trial 2
Fish (21 (sd 0·13) g, mean weight) were randomly distributed to twenty fibreglass tanks of 100 litres capacity, at a density of fifteen fish per tank. Tanks were supplied with aeration, thermoregulated to 26°C and covered with nets to prevent fish to escape. A photoperiod of 12 light–12 dark was adopted. Each tank was considered an experimental unit and arranged in a completely randomised design with five dietary treatments and four replicates. Dissolved oxygen, total ammonia, nitrate and pH were monitored daily using a multiparameter Horiba (model U – 10). Fish were fed with the experimental diets for 100 d, twice daily (09.00 and 17.00 hours), until apparent satiation.
Sampling procedure
At the end of trial 2, fish were fasted for 24 h and then eight fish from each treatment (two fish per replicate) were randomly sampled, anaesthetised with benzocaine (50 mg/l), euthanised by spinal cord section and dissected on chilled trays. The intestines were carefully removed, freed from the adjacent adipose tissue and divided into three portions (anterior, mid and distal intestine). The distal intestine was distinguished from the medium intestine by its increased diameter, darker mucosa and annular rings. The anterior and mid potions were obtained by division of the remaining intestine in two identical portions. Each intestine portion was individually stored in Eppendorf tubes, immediately frozen in liquid N2 and then stored at −80°C until enzymatic analyses.
Mid intestine samples were also collected from two other fish per tank, gently washed with saline water (0·9%) to remove biological fragments and fixed in Bouin’s solution for 12 h. Bouin’s solution was made following the proportions: 75 ml of picric acid (2·1 %), 25 ml of formaldehyde (40 %) and 5 ml of glacial acetic acid.
Enzyme activity
Each intestine portion was weighted, and the pH measured in situ using a pH metre (pH Eutech Instrument). Samples were homogenised (1:5 dilution, w/v), on ice-cold buffer (100 mm Tris–HCl, 0·1 mm EDTA and 0·1 % Triton X-100 (v/v), pH 7·8). Then, samples were centrifuged at 3300 g for 30 min at 4°C, and the supernatant was collected and stored at −80°C until analyses. All enzyme activities were measured at 37°C using a PowerWavex microplate scanning spectrophotometer (Bio-Tek Instruments). For oxidative status, distal intestine samples were used as it is the intestine portion where most of the oxidative damages are usually observed(Reference Burrells, Williams and Southgate35–Reference van den Ingh, Krogdahl and Olli37).
Digestive enzyme activity
Total proteases activity was measured by the casein hydrolysis method according to Hidalgo et al.(Reference Hidalgo, Urea and Sanz38), with 0·1m Tris–HCl buffer (pH 7·8). The reaction mixture containing casein (1 % w/v; 0·125 ml), buffer (0·125 ml) and homogenate supernatant was incubated for 1 h at 37°C and stopped by adding 0·6 ml trichloroacetic acid (8 %, w/v) solution. After being kept for 1 h at 2°C, samples were centrifuged at 1800 g for 10 min and the absorbance read at 280 nm against blanks. A blank for each sample was prepared by adding the homogenate supernatant after incubation. Chymotrypsin activity was determined following Hummel(Reference Hummel39), with Rao & Lombardi(Reference Rao and Lombardi40) modifications, using N-benzoyl-l-tyrosine ethyl ester as a substrate in Tris-HCl 41·4 mm and CaCl2 10·4 mm (pH 8·1) and the absorbance read at 256 nm. Trypsin activity was measured according to Bergmeyer et al.(Reference Bergmeyer, Gawehn and Grassl41) using 10 nm Nα-p-toluenesulfonyl-l-arginine methyl ester as a substrate in Tris-HCl 41·4 mm and CaCl2 10·4 mm (pH 8·1) and the absorbance read at 247 nm. Amylase (EC 3.2.1.1) and lipase (EC 3.1.1.3) activities were measured with kits (Spinreact reference 41201 and reference 1001274, respectively), adapted for fish intestine samples. The substrate 2-chloro-4-nitrophenyl-α-d-maltotrioside was used for amylase and absorbance read at 405 nm. Lipase was determined using 1–2-O-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin)-ester as substrates and absorbance read at 580 nm.
Enzyme activity was expressed as specific activity, defined as μmol of product generated per minute. Soluble protein concentration was determined according to Bradford(Reference Bradford42) with bovine serum albumin solution as standard.
Oxidative status
Superoxide dismutase (SOD) activity was measured at 550 nm by the ferricytochrome C method, using xanthine oxidase as a source of superoxide radical(Reference McCord and Fridovich43). Catalase (CAT) activity was determined according to Aebi(Reference Aebi44) by measuring the decrease in H2O2 concentration at 240 nm. Glutathione peroxidase activity was assayed as described by Flohé & Günzler(Reference Flohé and Günzler45). Glutathione reductase (GR) activity was determined at 340 nm by measuring the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH), as described by Morales et al.(Reference Morales, García-Rejón and De La Higuera46). GSSG generated by glutathione peroxidase was reduced by GR, and the NADPH consumption rate was monitored at 340 nm. Glucose-6-phosphate dehydrogenase (G6PDH) activity was measured by the reduction of NADP+ at 340 nm as described by Morales et al.(Reference Morales, García-Rejón and De La Higuera46). LPO was measured at 535 nm, as the reaction of malondialdehyde and thiobarbituric acid, as described by Buege & Aust(Reference Buege and Aust47).
Enzyme activity was expressed as units (SOD and CAT) or milliunits (glutathione peroxidase, GR and G6PDH) per mg of soluble protein. Except for SOD, one unit of enzyme activity was defined as the amount of enzyme required to transform 1 μmol of substrate per min under the assay conditions. One unit of SOD activity was defined as the amount of enzyme necessary to produce 50 % inhibition of the ferricytochrome c reduction rate. LPO was expressed as nmol of malondialdehyde per g of intestine tissue.
Histological assays and analysis
Samples were washed and stored in alcohol at 70 % for 30 d until processing by classical histological techniques and stained with haematoxylin–eosin, briefly, dehydration with ethanol, paraffin inclusion, obtention of histological sections with microtome, deparaffinisation, hydration and staining. Histological preparations were photographed using a light microscope (Carl Zeiss) for analysis of histological modifications. The following parameters were used as criteria of intestine alterations: mucosa fold height (shortening, widening and fusion of intestinal folds), supranuclear vacuolisation in the absorptive cells (enterocytes) of the intestine epithelium; increased cellularity of connective tissue and widening of lamina propria and submucosa; leucocytes infiltration in lamina propria and submucosa, number of goblet cells (GC) and nucleus position within the enterocytes(Reference Krogdahl, Bakke-McKellep and Baeverfjord48). Blinded evaluation of the histological samples was performed by disposing scores from 1 to 3, where score 1 indicates normal conditions and 3 major deformities. The overall value of enteritis degree was calculated by averaging scores of all parameters.
Statistical analyses
Data are expressed as mean values with their pooled standard errors. Normality and homogeneity of variances were tested using the Shapiro–Wilk and Levene tests, respectively. All statistical procedures were performed using the SPSS software package(49), and probability level of 0·05 was used for rejection of the null hypothesis. Statistical analysis of digestive enzymes and pH was done by two-way ANOVA, with diet and intestine portion as factors. When necessary, Tukey’s test was used to detect differences in digestive enzyme activity among intestine portions within each dietary treatment. Significant differences among dietary DDGS levels within each intestine portion were determined by orthogonal polynomial contrast to identify linear and quadratic effects. Second, data from apparent digestibility, sum and ratios (A/P, L/P and A/L) of digestive enzyme activities, oxidative stress enzymes and histology were analysed by one-way ANOVA, and in case of significance, orthogonal polynomial contrast was applied to identify linear and quadratic effects of dietary DDGS inclusion. The number of fish per group was established following Thorarensen et al.(Reference Thorarensen, Kubiriza and Imsland50) recommendations.
Results
The effect of the diets on growth performance and feed utilisation was not the object of the present study and was published elsewhere(Reference Oliveira, Segura and Oliveira51). In short, no differences in growth performance were observed due to diet composition but feed conversion and protein efficiency ratios were positively affected by dietary DDGS inclusion.
Trial 1
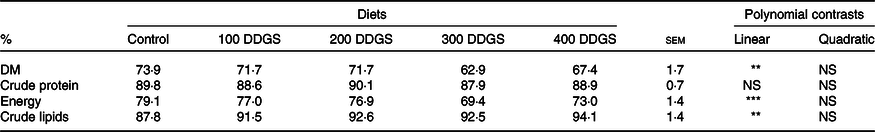
The ADC of the experimental diets are presented in Table 2. Increasing dietary DDGS levels linearly decreased the ADC of DM from 73·9 to 67·4 % and of energy from 79 to 73 %, while the ADC of lipids linearly increased from 87·8 to 94·1 % and the ADC of protein was not affected, averaging 89 %.
Table 2. Apparent digestibility coefficients (ADC) of nutrients in experimental diets containing increasing levels of distillers dried grains with solubles (DDGS) inclusion for pacu (Piaractus mesopotamicus) juveniles (n 5)†
(Mean values and pooled standard errors)
Cr2O3, chromium oxide.
** P < 0·01, *** P < 0·001.
† ADC = 100 × (1 – (% Cr2O3 diet/% Cr2O3 faeces) × (% nutrient or MJ/g energy faeces/% nutrient or MJ/g energy diet).
Trial 2
Intestinal pH
Intestine pH values were affected both by dietary treatments and intestine portion. No differences were observed on the anterior intestine pH of fish fed diets with increasing DDGS levels. On the contrary, there was a linear increase of mid intestine pH with dietary DDGS incorporation, while a quadratic effect was noticed for the distal intestine. Independently of dietary treatments, pH values were higher in the distal intestine portion (Table 3).
Table 3. pH values and digestive enzymes specific activity in different intestine portions of pacu (Piaractus mesopotamicus) fed the experimental diets (n 8)†
(Mean values and pooled standard errors)

D, diet; IP, intestine portion; A:P, amylase:total proteases ratio; L:P, lipase:total proteases ratio; A:L, amylase:lipase ratio; proteases, total proteases.
A,B,C Values sharing a common superscript letter are not significantly different at Tukey’s test within enzyme (P > 0·05).
* P < 0·05, ** P < 0·01, *** P < 0·001 (two-way ANOVA and contrasts).
† Values of proteases, lipase, amylase and chymotrypsin expressed as IU/mg protein, and trypsin as mU/mg protein.
Digestive enzyme activity
Digestive enzyme activities in the different intestine portions are presented in Table 3. Total protease activity was not affected by the intestine portion, except for higher values in the distal intestine of fish fed the control diet and in the anterior intestine of fish fed the 300 DDGS diet. On the other hand, lipase, amylase, chymotrypsin and trypsin activities decreased linearly from the anterior to the distal intestine portion, except for fish fed diet 200 DDGS where no differences on enzyme activities were observed for mid and distal intestine. Also, anterior and mid intestine portions had similar amylase activities in fish fed the 400 DDGS diet and trypsin activities in fish fed the 100, 300 and 400 DDGS diets.
With a few exceptions, there was a linear decrease of lipase, amylase and chymotrypsin activities in all intestine portions with the increase of dietary DDGS levels. However, a quadratic effect was observed for amylase on the distal intestine and chymotrypsin on the mid intestine. Trypsin activity linearly decreased in the mid intestine with dietary DDGS level.
Except for total proteases, total digestive enzyme activities, calculated as the sum activity of the three intestine portions, decreased linearly with dietary DDGS level. Amylase:total proteases (A:P), lipase:total proteases (L:P) and amylase:lipase (A:L) ratios also linearly decreased with increasing dietary DDGS level.
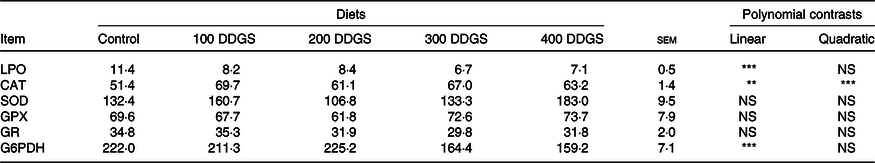
Oxidative status
There were no differences between dietary groups in glutathione peroxidase, GR and SOD activities (Table 4). There was a quadratic effect of dietary DDGS on CAT activity, while G6PDH activities and LPO linearly decreased with dietary DDGS level.
Table 4. Specific activity of antioxidant enzymes and lipid peroxidation on the distal intestine of pacu (Piaractus mesopotamicus) fed the experimental diets (n 8)†
(Mean values and pooled standard errors)
LPO, lipid peroxidase; CAT, catalase; SOD, superoxide dismutase; GPX, glutathione peroxidase; GR, glutathione reductase; G6PDH, glucose-6-phosphate dehydrogenase.
** P < 0·01, *** P < 0·001.
† Results of G6PDH, GPX and GR expressed as mU/mg protein, SOD and CAT expressed as IU/mg protein, and LPO expressed as nmol malondialdehyde per g tissue.
Histology
The scores of parameters used to assess gut morphology and the damage mean score are presented in Table 5. Dietary DDGS linearly reduced lamina propria width and cellularity, enterocytes vacuolisation and the number of GC, while it increased submucosa width and cellularity. A quadratic effect was noticed for intraepithelial leucocyte infiltration and enterocytes’ nucleus position. Intestine global damage, represented by mean score, reduced linearly with DDGS inclusion in the diets (Table 5; Fig. 1).
Table 5. Histological evaluation of pacu (Piaractus mesopotamicus) mid intestine fed the experimental diets (n 8)†
(Mean values with pooled standard errors)

FH, mucosa fold height (shortening, widening and fusion of intestinal folds); LP, increased cellularity of connective tissue and widening of lamina propria; SM, increased cellularity of connective tissue and widening of submucosa; LI, leucocytes infiltration in lamina propria and submucosa; GC, number of goblet cells; ENT, nucleus position within the enterocytes; SNV, supranuclear vacuolisation in the absorptive cells (enterocytes) of the intestine epithelium.
* P < 0·05, ** P < 0·01, *** P < 0·001.
† Score from 1 to 3, with 3 indicating major alterations. Mean scores calculated by averaging the scores of the evaluated parameters.
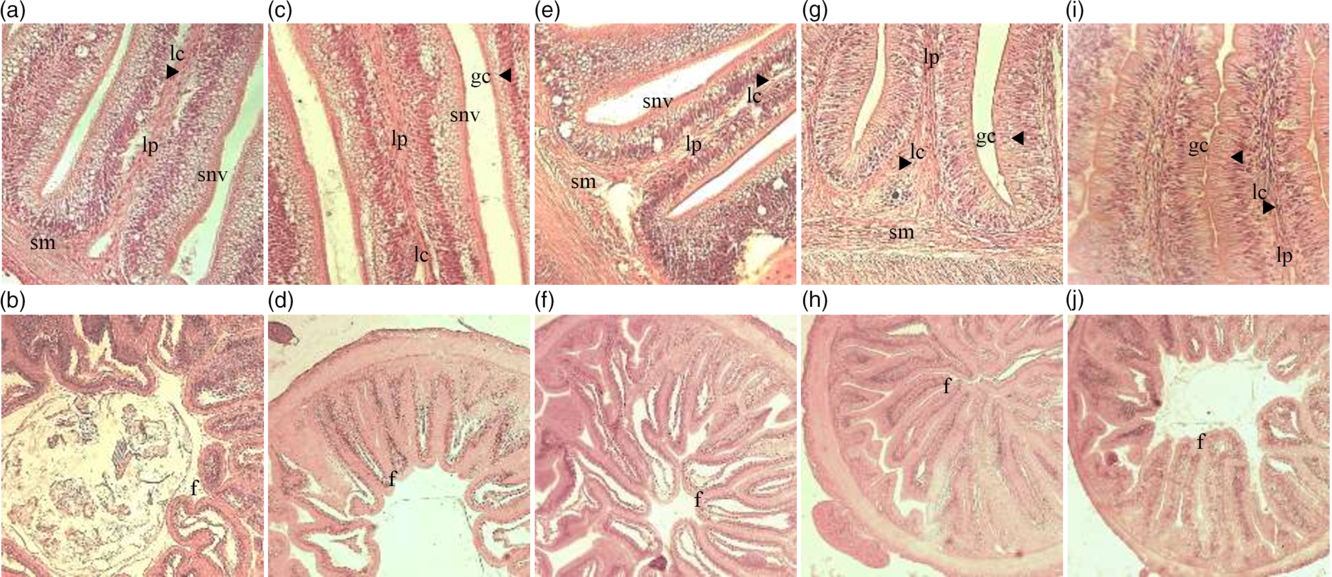
Fig. 1. Histological modifications on intestines of pacu (Piaractus mesopotamicus) juveniles fed diets with increasing levels of maize distillers dried grains with solubles inclusion and soyabean meal replacement. LP, increased cellularity of connective tissue and widening of lamina propria; SM, increased cellularity of connective tissue and widening of submucosa; GC, number of goblet cells; SNV, supranuclear vacuolisation in the absorptive cells (enterocytes) of the intestine epithelium.
Discussion
Diet digestibility
The evaluation of DM digestibility coefficients allows to get an estimative of the general digestibility of a diet or ingredient. In the present study, the observed decrease of DM digestibility with increasing DDGS levels may suggest an increment in the amount of non-digestible nutrients into the diets, also confirmed by the decrease of dietary energy digestibility. Similarly, Cheng & Hardy(Reference Cheng and Hardy8) in rainbow trout and Li et al.(Reference Li, Oberle and Lucas52) in channel catfish observed a reduction of DM and energy digestibility with increasing dietary DDGS levels. The authors correlated the results with the high amount of fibre present in DDGS.
Among factors capable of inducing changes in dietary nutrient digestibility in DDGS-rich diets, fibre is probably the most important. Indeed, Krogdahl et al.(Reference Krogdahl, Hemre and Mommsen53) mentioned as an adverse feature of DDGS use in animal feeds its high amount of cellulose and hemicellulose. High fibre content may interfere with the capacity of digestive enzymes to attach to its substrates, thus reducing the ADC of dietary nutrients(Reference Liu54) or increasing endogenous losses(Reference Bach Knudsen, Borg Jensen and Andersen55,Reference Noblet and Perez56) . Dietary fibre, specifically NSP, may interfere with feed utilisation by modulating digesta viscosity, gut emptying time and chime passage rate, changes in gut morphology, digestive physiology and microbiota(Reference Sinha, Kumar and Makkar57).
Although high dietary fibre and nitrogen-free extract content may lead to decreased protein digestibility(Reference Lech and Reigh58), in the present study, increased dietary DDGS levels, and a concomitant increase of dietary NSP, did not affect protein digestibility. Similarly, Magalhães et al.(Reference Magalhães, Coutinho and Pousão-Ferreira11) also did not find differences in protein digestibility in meagre (Argyrosomus regius) and seabass (Dicentrarchus labrax) fed diets with DDGS. The ADC of lipids increased with the dietary inclusion of DDGS, though this effect should be related to the increased dietary lipid levels in the diets with DDGS and not to the dietary DDGS inclusion. Indeed, other studies reported lower ADC of lipids in rainbow trout(Reference Cheng and Hardy8), sunshine bass(Reference Thompson, Rawles and Metts59), meagre and European seabass(Reference Magalhães, Coutinho and Pousão-Ferreira11) fed diets with DDGS. Increased ADC of lipids with the increase of dietary lipid content was also previously reported for rainbow trout(Reference Takeuchi, Watanabe and Ogino60), red tilapia(Reference De Silva, Gunasekera and Shim61), sea bass(Reference Peres and Oliva-Teles62) and rockfish, Sebastes schlegeli (Reference Lee, Jeon and Lee63).
Intestinal pH
Intestinal pH values of pacu ranged from 6·7 to 7·4, which is a smaller range than values (6·5–8·2) found in a variety of fish species(Reference Krogdahl, Gajardo and Kortner19). Though intestinal pH is expected to increase as it distances from the stomach, in this study, intestinal pH was similar between anterior and mid intestine portions and higher only in the distal intestine. Similarly, Hlophe et al.(Reference Hlophe, Moyo and Ncube64) also did not find major differences in pH of different intestinal portions in other omnivorous species, namely Tilapia rendalli, Oreochromis mossambicus and Clarias gariepinus, though pH values were slightly higher in the distal part of the intestine.
Diet composition may affect intestine pH, as dietary components may have distinct buffering capacities(Reference Maier and Tullis65,Reference Thompson and Weber66) . In this study, increasing dietary DDGS levels led to significant increase in pH in the mid and distal intestine portions. This may be related to the increased dietary fibre as it is known that dietary fibre content and source, as well as potential interactions between fibre and minerals, may affect intestinal pH(Reference Wong, Wong and Kwan67). To our knowledge, this is the first study in fish evaluating the effect of DDGS in an omnivorous intestinal pH but, similar to the present study, an alkalisation on anterior and mid intestine of turbot(Reference Diógenes, Castro and Miranda68) fed DDGS diets replacing fishmeal, and on caecum and apex in pigs fed diets with 300 g/kg DDGS(Reference Wilberts, Arruda and Kinyon69), was observed. Contrarily, Youssef et al.(Reference Youssef, Abd El-Azeem and El-Daly70) observed that increasing DDGS levels up to 150 g/kg in broilers’ diets provoked acidification of duodenum and ileum and the same was true for swine fed a diet containing 300 g/kg of maize DDGS compared with a diet with 300 g/kg of wheat middling(Reference Moran, de Lange and Ferket71). Differences in amino acid composition and digestibility capacity over the intestine may interfere with intestine pH(Reference Krogdahl, Sundby and Holm72).
Digestive enzyme activity
Overall, digestive enzyme activities decreased along the intestinal tract. This was expected, as digestive enzymes measured are produced by the exogenous pancreas and are therefore released in the first portion of the fish intestine. Thus, higher enzyme activities are expected to be found in the anterior intestine, as previously reported in different species, independent of food habits, as for the omnivorous Nile tilapia, O. niloticus L.,(Reference Hlophe, Moyo and Ncube64,Reference Klahan, Areechon and Yoonpundh73) and the carnivorous African catfish, C. gariepinus (Reference Hlophe, Moyo and Ncube64). Similarly, in rainbow trout fed either a control diet or a high protein DDGS diet, trypsin activity was much higher in the proximal than in the distal intestine(Reference Overland, Krogdahl and Shurson74). However, no difference of total protease activity was observed between intestine portions for all treatments, unless for fish fed the 300 DDGS diet that had higher total protease activity in the anterior than the mid intestine.
The negative effect of DDGS on the activity of digestive enzymes might be related to the rich content in NSP. The increase on digesta viscosity and water content in the gut, as a result of the increase in soluble NPS intake, will reduce digestive enzyme activities by reducing their contact with the substrate and/or impairing the transport of nutrients and digestive secretions, thus reducing diet digestibility(Reference Pedersen, Dalsgaard and Knudsen75), as observed. The increased water content in the gut also promoted to less extent by insoluble NSP can increase chime DM and enhance the passage of the digesta along the intestine, reducing once more the efficacy of digestive enzymes(Reference Smiths and Annison76). The negative effect of NSP on the digestive process and the digestibility of dietary ingredients has been reported for a variety of fish species such as Atlantic salmon, Salmo salar (Reference Refstie, Svihus and Shearer77), catfish(Reference Leenhouwers, ter Veld and Verreth78), Nile tilapia(Reference Haidar, Petie and Heinsbroek79,Reference Leenhouwers, Ortega and Verreth80) and rainbow trout, Oncorhynchus mykiss (Reference Glencross, Rutherford and Bourne81).
Unlikely amylase and lipase, total protease activity does not seem to be very dependent on fish nutritional habits(Reference Chan, Horn and Dickson82). Accordingly, in this study, as well as in the study of Magalhães et al.(Reference Magalhães, Coutinho and Pousão-Ferreira11), total protease activity was not affected by dietary DDGS level. This is contrary to lipase, amylase, chymotrypsin and trypsin activities, which decreased as dietary DDGS level increased. Even though trypsin and chymotrypsin activities are accounted for the total protease activity, it also includes other alkaline proteases activities, as carboxypeptidases and di- and tri-peptidases. Moreover, some NSP components may impact negatively in the activity of some proteolytic enzymes, while other enzymes may have its activity increased and consequent effect being observed on protein digestibility(Reference Glencross, Rutherford and Bourne81), as it seems to have occurred in the present study. Lack of dietary DDGS effect on trypsin, amylase and lipase activities was however reported for olive flounder juveniles (P. olivaceus) fed rice-dried distillers grain (DDG)(Reference Rahman, Choi and Lee6), suggesting that the effect of dried distillers grain on the activity of digestive enzymes may be dependent on ingredient source or processing technology. Accordingly, Magalhães et al.(Reference Magalhães, Coutinho and Pousão-Ferreira11) observed both in European sea bass and meagre that, comparatively to the control fish-based diet, one of the maize DDGS products tested promoted digestive enzyme activity, while the other DDGS product did not affect digestive enzyme activity.
Due to the differences in lipid digestibility of the experimental diets, it was expected a higher lipase activity in the intestine of fish fed diets with increasing DDGS levels. However, the opposite was observed. When detailing diet lipid composition and digestibility, it is possible to observe that the increase in lipids digestibility was far below the increase in dietary lipids due to dietary DDGS inclusion. While dietary lipids increase on over 50 % from diet control to 400 DDGS, lipids digestibility increased <7 %. Thus, the increase in apparent lipid digestibility seems to reflect the increase in dietary lipid content.
Oxidative status
Up to now, studies of dietary DDGS effects on intestine oxidative status in fish were lacking. In broilers fed diets with 150 g/kg DDGS, total antioxidant activity and SOD activity in plasma and liver appeared to decrease(Reference Min, Li and Liu83). On the contrary, Hanson et al.(Reference Hanson, Wang and Johnston84) did not find an effect of DDGS-based diets on the oxidative status of nursery pigs.
In the present study, among the antioxidant defence, enzymes assayed only CAT activity was induced in the intestine of fish fed DDGS diets, indicating that it was the preferred enzymatic pathway for eliminating the hydrogen peroxide produced. The metabolism pathway of SOD involves the supply of substrate for CAT (H2O2), and usually, those enzymes have associated activity. That was not observed in the present study as by other authors(Reference Castro, Peréz-Jiménez and Coutinho85,Reference Pérez-Jiménez, Abellán and Arizcun86) and might be related to changes on SOD isoforms. Besides the two forms present in eukaryotes organisms, Mn SOD and Cu/Zn SOD, there is evidence that isoforms can be expressed by the enzyme reaction with other components like malondialdehydes, amino acids(Reference Pedrajas, Gavilanes and López-Barea87), proteins and carbohydrates(Reference Pérez-Jiménez, Abellán and Arizcun86). The patterns and specific activity might vary depending on the isoform(Reference Pichardo, del Campo, Jos and Cameán88,Reference Campos-Shimada, Hideo Gilglioni and Fernandes Garcia89) .
Another enzyme that is related to oxidative status in animal organisms is G6PDH that converts glucose-6-phosphate into 6-phosphogluconate generating NADPH. NADPH supports the action of the enzyme GR that restores the levels of reduced glutathione in the organism(Reference Gaetani, Galiano and Canepa90). However, in the present study, reduction of G6PDH does not seem to be related to antioxidant defence but with lipid content in diets. G6PDH is one of the major rate-limiting enzymes from pentose phosphate pathway and has high relation with lipogenesis, since its product, NADPH, is very important in biosynthesis of fatty acids and cholesterol. Once lipid contents were increasing and starch decreases in experimental diets as DDGS was being included, lipogenesis was reduced due to high lipids availability, which was represented by reductions on G6PDH activity.
Nonetheless, LPO in the intestine of pacu fed DDGS diets was significantly reduced, and this seems to indicate the potential of DDGS. This may be related to the NSP and the yeast cell components in DDGS, which may directly or indirectly affect oxidation by inducing changes in fish gut microbiota(Reference Leenhouwers, Ortega and Verreth80,Reference Amirkolaie, Verreth and Schrama91,Reference Dimitroglou, Merrifield and Spring92) and intestine ecosystem(Reference Sinha, Kumar and Makkar57). For instance, it was shown that low dietary inclusion of soluble NSP (guar gum) reduced gut oxidative stress in white sea bream(Reference Enes, Pérez-Jiménez and Peres93). Also, yeast is known to have distinct protective oxidative stress responses in fish species such as rainbow trout(Reference Nakano, Kanmuri and Sato94), gilthead sea bream(Reference Reyes-Becerril, Tovar-Ramírez and Ascencio-Valle95) and European sea bass(Reference Tovar-Ramírez, Mazurais and Gatesoupe96). Not only whole yeast but also its cell wall components such as mannans and glucans have been shown to provide antioxidant benefits(Reference Jaehrig, Rohn and Krohl97,Reference Križková, Ďuračková and Šandula98) and to modulate gut microbial communities(Reference Gatesoupe99,Reference Sweetman, Torrecillas and Dimitroglou100) allowing changes in ROS production in the organism. DDGS components have been studied as prebiotic for several production animals(Reference Abudabos, Al-Atiyat and Albatshan101,Reference Monteagudo-Mera, Chatzifragkou and Kosik102) . Nevertheless, the reduction of soyabean meal in the diets cannot be completely discarded as having also contributed to the reduction of oxidative stress in the pacu intestine. Indeed, anti-nutritional factors present in soyabean were shown to be responsible for promoting the increase of free radicals, and an imbalance between ROS production and removal in mice fed raw soyabean-based diets by promoting inflammatory process(Reference Gu, Qu and Han103). Inflammation is always accompanied by lipid oxidation, due to excessive ROS production.
Due to the addition of sulphuric acid during ethanol production, the sulphur content in DDGS can be higher than that in common ingredients used in aquafeeds(Reference Kim, Zhang and Stein104). Sulphur and some sulphur-containing compounds have been reported to have antioxidant proprieties(Reference Song, Chen and Wang32,Reference Atmaca105–Reference He, Long and Han107) . Song et al.(Reference Song, Chen and Wang32) feeding nursery pig with an oxidised DDGS diet (300 g/kg) suggested that the high sulphur content of maize DDGS, as well as the high serum sulphur-containing antioxidants observed in animal fed DDGS, may be enough to prevent metabolic oxidative stress. Thus, the sulphur content of DDGS may have also contributed to the reduction of oxidative stress in pacu intestine.
Intestine morphology
The replacement of soyabean meal by maize DDGS improved pacu intestinal morphology. It is known that soyabean may induce inflammation in the distal portion of the fish intestine, particularly in salmonids(Reference Burrells, Williams and Southgate35–Reference van den Ingh, Krogdahl and Olli37). This soyabean-induced enteritis is associated with anti-nutritional factors present in soyabean, such as lectins and saponins, which were shown to induce histological alterations in gastrointestinal tract cells affecting mucosal cell membrane permeability(Reference Krogdahl, Bakke-McKellep and Baeverfjord48,Reference Bakke-McKellep, Penn and Salas108) . Though salmonids are carnivorous and eventually more prone to have intestinal problems when dealing with plant feedstuff-based diets(Reference Oliva-Teles, Enes, Peres and Davis109), enteritis was also reported in omnivorous fish, such as common carp(Reference Urán, Gonçalves and Taverne-Thiele110) or zebrafish(Reference Hedrera, Galdames and Jimenez-Reyes111). On the contrary, carnivorous species such as the European sea bass or gilthead seabream seem to be more tolerant of soyabean anti-nutritional factors(Reference Couto, Kortner and Penn112,Reference Couto, Kortner and Penn113) . Nevertheless, although no overt inflammation was noticed, when fed diets containing soyabean meal gilthead seabream and European sea bass showed some alterations in the distal intestine morphology(Reference Bonaldo, Roem and Fagioli114,Reference Guerreiro, Serra and Enes115) .
Instead, the accumulation of vacuoles in the intestine of fish fed lower or none DDGS diets seems to be related to lipid accumulation and/or inhibition of re-esterification of fatty acid, common in plant ingredients(Reference Caballero, Izquierdo and Kjørsvik116), it was noticed an excess of lipid drops and clear irregularity on the size of the vacuole, which can modify the absorptive capacity of the enterocyte in the brush border(Reference Escaffre, Kaushik and Mambrini117). Reduction in GC promoted by DDGS inclusion and soyabean meal replacement is also evidence of a reduction in intestine damage since GC are involved in the synthesis of mucus as lubricants against mucosa degradation(Reference Ofelio, Cohen and Adriaens118).
Adding to that, yeast cells have been shown to improve gut morphology of some fish species and this may also contribute to explain the improved gut histology of pacu fed the DDGS diets. For instance, in common carp, Omar et al.(Reference Omar, Merrifield and Kühlwein119) observed increased GC number in the posterior gut of fish fed increasing levels of yeast protein concentrate, and Huang et al.(Reference Huang, Ran and He120) observed increased microvillus length, which suggests higher nutrient absorption capability. In Nile tilapia, live yeast (but not inactivated yeast) also improved gut microvilli length and density(Reference Ran, Huang and Liu121). The beneficial effects of yeast on gut morphology may be obtained not only with live yeast cells but also with yeast polysaccharides, which may function as prebiotics(Reference Guerreiro, Serra and Pousão-Ferreira122). Further, the reduction of oxidative stress of pacu intestines fed DDGS diets can also contribute to explain the improvement of the intestinal histological morphology, as ROS imbalance can cause cell structures damages(Reference Cabiscol, Piulats and Echave123–Reference Salmon, Evert and Song125).
In summary, maize DDGS inclusion in diets for pacu juveniles reduced DM, energy and lipids digestibility, and the intestinal amylase, lipase trypsin and chymotrypsin activities. However, the lack of differences in protein digestibility and total proteases activity, together with the improvements of intestine morphology and oxidative status, may contribute to explain the improvements on feed conversion ratio and protein efficiency ratio reported by Oliveira et al.(Reference Oliveira, Segura and Oliveira51) in pacu fed the same diets. Thereafter, the negative effects induced by soyabean meal were decreased by dietary replacement with maize DDGS, which may have a prebiotic effect, contributing to the improvement of the intestine health. Further studies evaluating maize DDGS for fish should include analysis of microbiota, to clarify DDGS effects in nutrient metabolism and immunity.
Acknowledgements
This work was supported by the National Council for Technological and Scientific Development (CNPq) (grant number 130664/2014-6) and São Paulo Research Foundation (FAPESP) (grant number 2014/16685-5; 2015/21245-7).
K. R. B. O. conceived, design research, performed experiments, analysed data and drafted the manuscript; J. M. performed the experiment; A. F. D. and R. R. P. performed laboratory analysis; A. O. T. and H. P. interpreted results of experiments, edited and revised the manuscript and E. M. M. V. and A. O. T. approved the final version of the manuscript. E. M. M. V. supervised the project.
The authors declare that they have no conflicts of interest.