In eukaryotic cells, the p38 mitogen-activated protein kinase (MAPK) signalling pathway, which was originally shown to be activated by environmental stress and pro-inflammatory cytokines, is a component of several important signalling pathways that relay extracellular cues to transcription factors in the nucleus(Reference Herskowitz1–Reference Robinson and Cobb3). The MAPK family primarily consists of three subfamilies: p38 MAPKα; extracellular-regulated kinase (ERK); Jun NH2-terminal kinase (JNK). These pathways are known to have an important role in the development of skeletal muscle(Reference Lluis, Perdiguero and Nebreda4–Reference Bandyopadhyay, Yu and Ofrecio6).
Myogenesis is a multistep process in which myoblasts cease to divide, elongate and fuse into multinucleated myotubes. The cellular changes include elongation, polarisation, aggregation and fusion and ultimately give rise to functional muscle. Myogenesis involves two families of transcription factors. One family is the myogenic regulatory factors (MRF), which include myogenic differentiation (MyoD), myogenic factor 5, myogenic regulatory factor 4 (MRF4) and myogenin(Reference Braun, Buschhausen-Denker and Bober7–Reference Edmondson and Olson9). MRF are skeletal muscle-specific transcription factors. The other family is the myocyte enhancer factors (MEF) and includes MEF2A, B, C and D. The MRF and the MEF contribute to myogenesis by binding to regions within the promoters of muscle-specific genes(Reference Gossett, Kelvin and Sternberg8).
Reactive oxygen species (ROS) function as secondary messengers for the signal transduction induced by many cytokines and contribute to proliferation and differentiation(Reference Janower10, Reference Chiarugi, Pani and Giannoni11). It has been shown that both exogenous and endogenous ROS activate the MAPK pathway(Reference Gupta, Rosenberger and Bowden12, Reference Klotz, Pellieux and Briviba13). Several studies have shown that low ROS concentrations enhance the growth of many types of mammalian cells via MAPK activation, which is often associated with proliferation and differentiation(Reference Kim, Lee and Lee14, Reference Su, Mitra and Gregg15).
Since the identification of fucoidan, it has been broadly studied for its numerous biological properties(Reference Beress, Wassermann and Tahhan16–Reference Bochkov, Tkachuk and Philippova20). Fucoidan, a sulfated polysaccharide, is primarily extracted from brown seaweeds such as Fucus vesiculosus, Ecklonia kurome and Undaria pinnatifida. It consists of l-fucose, together with xylose, galactose and mannose(Reference Lee, Hayashi and Hashimoto21–Reference Nishino, Aizu and Nagumo23). Some of the effects of fucoidan on smooth muscle proliferation have been reported to involve the p38 MAPK pathway(Reference Religa, Kazi and Thyberg24), but the detailed biological effects of low-molecular-weight fucoidan (LMWF) on ROS production, the expression of MRF and p38 MAPK-mediated signalling during MyoD and ROS have not been thoroughly investigated. Fucoidan preparations have been proposed as an alternative to the anticoagulant heparin. As with heparin, it has been shown that fucoidan affect many biological activities, such as anticoagulation, inflammation, cell proliferation and adhesion(Reference Berteau and Mulloy25). However, we use heparin as a negative control in the present study.
In the present study, the potential effects of LMWF on muscle differentiation were examined in vitro by analysing the effects of LMWF on gene expression and protein activity during myoblast differentiation into myotubes.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, horse serum, PBS and trypsin-EDTA were acquired from Gibco (Gaithersburg, MD, USA). Penicillin–streptomycin was purchased from JBI (Daegu, Chungchengnamdo, South Korea). Fucoidan was provided by Heawon Company, Inc. (Seoul, South Korea) and has been shown to be non-toxic by toxicity tests(Reference Kim, Lee and Lee14, Reference Kim, Lee and Lee26). It was hydrolysed by the method of Nardella et al. (Reference Nardella, Chaubet and Boisson-Vidal27) to produce LMWF. Heparin was purchased from Sigma (St Louis, MO, USA). The RNAlater was purchased from Ambion (Austin, TX, USA). Total RNA extraction and purification were done using the QIAGEN RNeasy Mini Kit (Qiagen, Hilden, Germany). The First-Strand cDNA Synthesis Kit and Taq DNA polymerase were obtained from Invitrogen (Carlsbad, CA, USA) and Solgent (Daegeon, Chungchengnamdo, South Korea), respectively. Primers were supplied by Bioneer (Daejon, Chungchengnamdo, South Korea).
Cell culture
Myotubes were cultured from the murine skeletal muscle-derived C2C12 myoblast line from American Type Culture Collection (Rockville, MD, USA) as described previously(Reference Gredinger, Gerber and Tamir28). Briefly, C2C12 cells were cultured in DMEM supplemented with 10 % fetal bovine serum and 1 % penicillin and streptomycin at 37°C in the presence of 5 % CO2. MyoD was initiated by replacing the growth medium with differentiation medium (DMEM supplemented with 2 % horse serum). This medium was then replaced every day. Differentiation was allowed to continue for 72 h before the addition of LMWF. To investigate the effects of LMWF on MyoD, LMWF concentrations of 0, 1, 10 and 100 μg/ml were applied to the cells. The same concentrations of LMWF were added at 24 h intervals when the culture medium was replenished. Cells left untreated with LMWF were used as controls in these experiments.
Cell viability
Cell viability was determined using a sodium 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis (4-methoxy-6-nitro)benzene sulfonic acid hydrate (XTT) reduction assay according to the method described by Roehm et al. (Reference Roehm, Rodgers and Hatfield29). The tetrazolium salt XTT used in the colorimetric proliferation assay was purchased from Sigma (Taufkirchen, Germany). Briefly, exponentially growing cells were plated in ninety-six-well microplates at a density of 1 × 104 cells/well in the DMEM/fetal bovine serum medium and then incubated at 37°C under 5 % CO2 for 48 h before the treatment. Cells were then treated with various concentrations of LMWF after which the absorbance was measured at 570 nm using an ELISA reader (Bio-Tek Instruments, Winooski, VT, USA). All measurements were collected five times (n 5), and the effect of LMWF on cell viability was assessed by calculating the optical density (OD) values at 570 nm for the LMWF-treated samples as a percentage of control values.
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted from C2C12 cells using the Qiagen RNeasy Kit (Qiagen, Valencia, CA, USA). RNA samples with an OD260/OD280 ratio greater than 2·0 were used for semi-quantitative RT-PCR. Total RNA (1 μg) was used to produce cDNA in an RT-PCR system. The sequences of the oligonucleotide primers were as follows: GAPDH sense (5′-AACTTTGGCATTGTGGAAGG-3′) and antisense (5′-ACACATTGGGGGTAGGAACA-3′); p38 MAPKα sense (5′-CCTTGACCAAGAAGAAATG-3′) and antisense (5′-ACAGACGAACAGACAGACAC-3′); ERK sense (5′-GCTCACCCTTACCTGGAACA-3′) and antisense (5′-GGACCAGATCCAAAAGGACA-3′); JNK sense (5′-AATGGTTTGCCACAAAATCC-3′) and antisense (5′-GAGTCAGCTGGGAAAAGCAC-3′); myogenin sense (5′-CAGGAGGAGCGCGATCTCCGCTAC-3′) and antisense (5′-CAGAAGTGATGGCTTTGACACCA-3′); MRF4 sense (5′-ATGGACCTTTTTGAAACTGGCTCC-3′) and antisense (5′-CTGACCTGGGCAGTCGGGTGGCTG-3′); MnSOD sense (5′-GCACCACAGCAAGCACCAT-3′) and antisense (5′- TGTCCCCCACCATTGAACT-3′); glutathione reductase sense (5′-GGGCAAGGTGCTGCTCATTG-3′) and antisense (5′-AGAGCGGGTGAGCCTTCTCA-3′); glutathione peroxidase (GPx) sense (5′-CTCGGTTTCCCGTGCAATCAG-3′) and antisense (5′- GTGCAGCCAGTAATCACCAAG-3′); catalase sense (5′-TCTGCAGATACCTGTGAACTG-3′) and antisense (5′-TAGTCAGGGTGGACGTCAGTG-3′). The PCR products were run on 1·0 % agarose gels, stained with ethidium bromide and photographed. Expression levels were quantified by scanning the gels and using an analysis system (Vilber Lourmat, GI-070AP, Marne la Vallée, France).
Western blot analysis
Cells were collected in lysis buffer (50 mm-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 137 mm-NaCl, 1 mm-MgCl2, 1 mm-CaCl2, 10 mm-Na2P2O7, 10 mm-NaF, 2 mm-EDTA, 10 % glycerol, 1 % Igepal CA-630, 2 mm-vanadate, leupeptin (10 mg/ml), aprotinin (10 mg/ml) and 2 mm-phenylmethylsulfonyl fluoride, pH 7·4). After lysis, the lysates were clarified by centrifugation at 12 000 g for 20 min at 4°C; the protein concentration in the supernatants was determined using the Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were directly solubilised in Laemmli sample buffer. Equal amounts of protein were separated by SDS-PAGE and were transferred to Immobilon-P membranes. Membranes were blocked overnight at 4°C and incubated with the indicated antibody for 2 h at room temperature. Specifically bound primary antibodies were detected with a peroxidase-coupled secondary antibody and enhanced chemiluminescence (Amersham Biosciences, NJ, USA).
Statistical analysis
All measurements are expressed as means and standard deviations. The results were statistically analysed by a one-way ANOVA followed by the Duncan multiple comparison test. Statistical significance was accepted at a level of P < 0·05 (SAS Institute, Inc., Cary, NC, USA).
Results
Cell morphology and viability of C2C12 myotubes
To determine the effects of LMWF on morphology and MyoD, C2C12 myoblasts were treated for 72 h with increasing concentrations of LMWF (0, 1, 10 and 100 μg/ml) in the growth media. As shown in Fig. 1, increasing the concentration of LMWF led to increased disturbances in the morphology and MyoD of C2C12 cells. XTT was used to determine the cytotoxicity of LMWF in C2C12 myoblast cells. According to the test results, the different concentrations of LMWF had no significant effect on cell viability after 12, 24 and 48 h incubation periods (Fig. 2). The absorbance values obtained in basal (without LMWF) culture media were set at 100 %, and the mean cell viability values obtained after the LMWF treatment were 97·3, 93·8 and 93·7 % of the basal values at LMWF concentrations of 1, 10 and 100 μg/ml, respectively, after 48 h of incubation. Thus, we demonstrated that LMWF had no cytotoxic effect over the entire concentration range up to 100 μg/ml.

Fig. 1 Morphology of the differentiated C2C12 myotube cells. (A) Control cells treated with deionised/distilled water; (B) cells treated with 1 μg/ml low-molecular-weight fucoidan (LMWF); (C) cells treated with 10 μg LMWF/ml; (D) cells treated with 100 μg LMWF/ml.

Fig. 2 Effects of low-molecular-weight fucoidan (LMWF) on the cell viability of C2C12 myoblasts treated for 12, 24 and 48 h. Viability was determined using the sodium 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis (4-methoxy-6-nitro)benzene sulfonic acid hydrate assay. Values are means of five independent experiments, with standard deviations represented by vertical bars. –●–, 0 μg LMWF/ml; –○–, 1 μg LMWF/ml; –▾–, 10 μg LMWF/ml; –Δ–, 100 μg LMWF/ml.
Effects of low-molecular-weight fucoidan on the key genes involved in myogenic differentiation
We tested the effects of LMWF on the differentiation of C2C12 myoblasts. C2C12 cells were differentiated in 2 % horse serum medium containing DMEM with LMWF at concentrations of 0, 1, 10 and 100 μg/ml. The differentiation medium was replaced every 24 h, and cells were further incubated for 72 h. Expression of the myogenic markers myogenin and MRF4, which are crucial for muscle development, was then examined. RT-PCR analysis revealed that the mRNA expression of myogenin and MRF4 was induced during myogenesis when the C2C12 cells became fully differentiated; however, this induction was eliminated in the presence of LMWF. LMWF at concentrations of 1, 10 and 100 μg/ml decreased the expression of myogenin during the MyoD of C2C12 cells by approximately 5·18, 11·9 and 19·4 %, respectively, compared with the control cells. Additionally, the MRF4 mRNA levels were decreased by approximately 7·3, 38·7 and 56·4 %, respectively, compared with the control cells. Moreover, the reduced protein expression of myogenin, MEF2 and MyoD in C2C12 cells paralleled the reduced mRNA expression levels in the presence of LMWF during differentiation. Taken together, these results suggest that LMWF inhibited MyoD, as shown in Fig. 3.

Fig. 3 Low-molecular-weight fucoidan (LMWF) suppressed key markers in C2C12 cells undergoing myogenic differentiation (MyoD). (A) mRNA expression of myogenin (■) and muscle regulatory factor 4 (MRF4, ![]() ) in C2C12 cells. (B) Protein expression of myogenin, myocyte enhancer factor 2 (MEF 2) and MyoD in C2C12 cells. Cells were cultured for 72 h with LMWF at concentrations of 0, 1, 10 and 100 μg/ml. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different within each treatment group (P < 0·05). C, control; F, fucoidan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WB, Western blotting.
) in C2C12 cells. (B) Protein expression of myogenin, myocyte enhancer factor 2 (MEF 2) and MyoD in C2C12 cells. Cells were cultured for 72 h with LMWF at concentrations of 0, 1, 10 and 100 μg/ml. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different within each treatment group (P < 0·05). C, control; F, fucoidan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WB, Western blotting.
Effects of low-molecular-weight fucoidan on mitogen-activated protein kinase expression during myogenic differentiation
To evaluate the effect of LMWF on the regulation of the MAPK pathways, we used RT-PCR to examine whether LMWF could activate p38 MAPKα, ERK and JNK (Fig. 4(A)). Expression of the p38 MAPKα gene decreased upon treatment with LMWF in C2C12 cells undergoing MyoD. We then investigated whether LMWF has a role in the inactivation of molecules downstream of p38 MAPKα, such as ERK and JNK. LMWF reduced the expression of ERK and JNK during the MyoD of C2C12 cells. Treatment with 1, 10 and 100 μg LMWF/ml decreased ERK by approximately 7·0, 53·2 and 58·8 %, respectively, relative to the controls. Additionally, the JNK mRNA levels decreased by approximately 0·6, 23·75 and 36·4 %, respectively, compared with the controls. Furthermore, as shown in Fig. 4(B), an increase in LMWF concentration resulted in a further decrease in the phospho-ERK and phospho-JNK levels in C2C12 cells.

Fig. 4 Low-molecular-weight fucoidan (LMWF) suppressed the expression of mitogen-activated protein kinase (MAPK) expression in C2C12 cells undergoing myogenic differentiation. (A) LMWF inhibited the mRNA expression of p38 MAPKα (■), extracellular-regulated kinase (ERK, ![]() ) and Jun NH2-terminal kinase (JNK,
) and Jun NH2-terminal kinase (JNK, ![]() ) in C2C12 cells undergoing myogenic differentiation. (B) Phospho-ERK (p-ERK) and phospho-JNK (p-JNK) protein expression was inhibited in a dose-dependent manner by LMWF in C2C12 cells. Cells were cultured for 72 h with 0, 1, 10 and 100 μg LMWF/ml. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different within each treatment group (P < 0·05). C, control; F, fucoidan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WB, Western blotting.
) in C2C12 cells undergoing myogenic differentiation. (B) Phospho-ERK (p-ERK) and phospho-JNK (p-JNK) protein expression was inhibited in a dose-dependent manner by LMWF in C2C12 cells. Cells were cultured for 72 h with 0, 1, 10 and 100 μg LMWF/ml. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different within each treatment group (P < 0·05). C, control; F, fucoidan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WB, Western blotting.
Low-molecular-weight fucoidan represses key genes of myogenic differentiation better than heparin
Because heparin inhibits myoblast differentiation(Reference Fuentealba, Carey and Brandan30), we investigated the effect of both LMWF and heparin on myogenesis. Heparin alone was used as a positive/negative control. MRF4 and myogenin are both important MyoD markers that are crucial for muscle development, and both were examined. LMWF and heparin were given alone and in combination during differentiation for a total of 4 d. MRF4 and myogenin mRNA expression showed that the patterns were statistically significant. The expression of both genes was decreased in the 100 μg LMWF/ml treatment group compared with the untreated group. Decreased gene expression was also observed after the treatment with 10 μg heparin/ml (Fig. 5). The LMWF–heparin combination treatment group showed a decrease in the gene expression of MRF4 and myogenin.
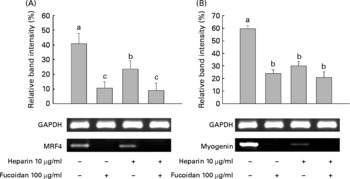
Fig. 5 Low-molecular-weight fucoidan (LMWF) and heparin suppressed the mRNA expression of (A) muscle regulatory factor 4 (MRF4) and (B) myogenin in C2C12 cells undergoing myogenic differentiation. LMWF and heparin were used in combination to treat C2C12 cells. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different within each treatment group (P < 0·05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Effects of low-molecular-weight fucoidan and heparin-mediated mitogen-activated protein kinase gene expression on myogenic differentiation
We then investigated whether treatment with LMWF and heparin leads to the inactivation of molecules downstream of p38 MAPKα, such as ERK and JNK. LMWF and heparin reduced the mRNA expression of ERK and JNK during myogenesis. During MyoD, C2C12 cells were treated with 100 μg LMWF/ml and/or 10 μg heparin/ml. Fig. 6 illustrates that the mRNA expression of p38 MAPKα, ERK and JNK was significantly decreased upon treatment with LMWF and heparin in combination. There was a strong repression of these genes compared with the control group. Interestingly, the ERK mRNA levels decreased more when treated with LMWF alone than in combination with heparin.
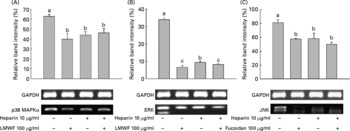
Fig. 6 Low-molecular-weight fucoidan (LMWF) and heparin suppressed the mRNA expression of (A) p38 mitogen-activated protein kinase α (p38 MAPKα), (B) extracellular-regulated kinase (ERK) and (C) Jun NH2-terminal kinase (JNK) in C2C12 cells undergoing myogenic differentiation. LMWF and heparin were used in combination to treat C2C12 cells. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different within each treatment group (P < 0·05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Response of antioxidant enzymes
After the cells were treated with LMWF and/or heparin for 4 d, the mRNA expression levels of MnSOD, catalase, glutathione peroxidase and glutathione reductase decreased significantly in the LMWF and/or heparin groups compared with the control group (Fig. 7(A) and (B)). These data showed that treatment with LMWF inhibited the expression of MnSOD, catalase and glutathione peroxidase better than treatment with heparin alone. The expression levels of these genes after the LMWF and combination treatments (LMWF and heparin) were repressed to levels significantly below those of the control group. Although a significant reduction in gene expression was also observed in the group treated with heparin alone, LMWF more strongly repressed antioxidant-related gene expression. Glutathione reductase expression was found to be significantly decreased in the LMWF, heparin and combination treatment groups.

Fig. 7 Semi-quantitative RT-PCR was performed on (A) the Mn superoxide dismutase (MnSOD), catalase, glutathione peroxidase (GPx) and glutathione reductase (GR) mRNA transcripts in (B) the control (■), low-molecular-weight fucoidan (LMWF, 100 μg/ml; ![]() ), heparin (10 μg/ml;
), heparin (10 μg/ml; ![]() ) and LMWF–heparin combination (
) and LMWF–heparin combination (![]() ) treatment groups. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05, Duncan's multiple test). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
) treatment groups. Values are means of three replicates, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05, Duncan's multiple test). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Fucoidan, a sulfated polysaccharide, is primarily extracted from brown seaweeds. Fucoidan has been broadly studied in recent years due to its numerous biological properties. Additionally, the biological activities of different fucoidan species have been reported to be closely related to both the sulfate content and the molecular weight(Reference Qiu, Amarasekara and Doctor31). Due to these characteristics of fucoidan, we hydrolysed it to produce LMWF, as described in the Materials and methods section, and examined the biological properties of LMWF.
We examined the effects of LMWF on the differentiation of myoblasts. Previously, we showed that LMWF is mainly composed of carbohydrates (58·2 %) and sulfate (35·5 %) with a small amount of protein (1·2 %)(Reference You, Yang and Lee32). These previous measurements are in agreement with other studies, which indicated that LMWF from other brown seaweeds has comparable amounts (42–66 %) of carbohydrates but smaller amounts of sulfate (11·5–30·0 %) and a protein content that varies from 0 to 12 %(Reference Bilan, Grachev and Shashkov33, Reference Ponce, Pujol and Damonte34).
The development of muscle in somites is a multistep process in which pluripotent mesodermal cells become committed to the myogenic lineage by receiving signals from neighbouring tissues. These signals initiate the expression of transcription factors from the MRF family, specifically MyoD and MRF4, in cells that differentiate into myoblasts. Subsequently, the activities of MyoD and MRF4 are induced, which leads to the withdrawal of myoblasts from the cell cycle. Additionally, MyoD initiates the expression of other transcription factors from the MEF2 family and myogenin. Together, myogenin and MEF2 family members cooperate in the activation of many structural muscle genes during differentiation. The present study showed that LMWF strongly inhibited MyoD by markedly decreasing the mRNA expression of MRF4 and myogenin, as well as MyoD, myogenin and MEF2 protein expression. Moreover, LMWF inhibited myotube formation.
Of the four isoforms of p38 (α, β, γ and δ), the phosphorylation and activity of the α and β isoforms are gradually induced during the differentiation of myoblasts(Reference Cuenda and Cohen35, Reference Wu, Woodring and Bhakta36). The p38 pathway is essential for both the activation of the MyoD-dependent promoter and the MyoD-mediated conversion of myoblasts to myogenic cells, which suggests the possibility that MyoD might also be a target of p38. Penn et al. (Reference Penn, Bergstrom and Dilworth37) demonstrated that p38 MAPKα expression regulated the formation of a MyoD–MEF2 complex at a set of late promoters. The ERK pathway has also been implicated in the positive control of myogenesis(Reference Gredinger, Gerber and Tamir28). However, another report has proposed a negative regulatory function(Reference Coolican, Samuel and Ewton38). The concomitant decline in ERK activity and the stimulation of p38 MAPKα during the early phase of terminal differentiation may facilitate a reduction in cyclin D1(Reference Lavoie, L'Allemain and Brunet39), thereby leading to the activation of MyoD(Reference Skapek, Rhee and Spicer40) and other MyoD markers. Consistent with these reports, the present data show that p38 MAPKα controls the MyoD of skeletal muscle cells, and the expression of muscle differentiation markers is down-regulated by inhibiting the p38 MAPKα pathway. Some researchers have suggested that JNK controls ERK-mediated myogenic activity(Reference Mauro, Ciccarelli and De Cesaris41), while another report has proposed that JNK is not essential for the inhibition of muscle differentiation(Reference Gallo, Serafini and Castellani42). The results of the present study show that LMWF suppresses the phosphorylation of JNK in C2C12 myoblasts. The p38 MAPKα pathways have been previously demonstrated to be oxidant-sensitive in vascular smooth muscle cells with preferential activation of p38 MAPKα(Reference Su, Mitra and Gregg15, Reference Ushio-Fukai, Alexander and Akers43). Indeed, the increase in ROS is partially responsible for muscle differentiation. Here, we showed that LMWF suppresses the mRNA and protein expression of p38 MAPK as well as ROS-related transcription factors in myogenesis. Although we have shown that ROS are related to the transcription level, further experiments are needed to define the relationship of MyoD and ROS. A previous report has shown that myostatin inhibits myoblast differentiation by down-regulating MyoD(Reference Langley, Thomas and Bishop44). This result indicated that LMWF might regulate MyoD through the p38 MAPKα pathway by regulating ROS activity.
Heparin exerts a pronounced inhibitory effect on muscle growth in vitro, as determined by total protein, and myosin accumulation(Reference Kardami, Spector and Strohman45). It has been shown that heparin not only prevented MAPK activation(Reference Daum, Hedin and Wang46) but also reduced ROS production(Reference Dzieciuchowicz, Checinski and Krauss47). In the present study, we have shown that heparin suppressed MyoD marker (Fig. 5). Inhibition of the MAPK pathway and ROS-related gene expression is also shown in Figs. 6 and 7. Interestingly, these results showed a more strong inhibition of MyoD through the repression of MAPK-related gene expression and ROS generation by LMWF.
In conclusion, the present study showed a part of the mechanism of how LMWF regulates the myogenesis of C2C12 cells. The data obtained in the present study suggest for the first time that LMWF suppressed MyoD through the p38 MAPK pathway and a ROS-related transcription factor better than heparin, suggesting that LMWF might have an interesting effect on muscle differentiation.
Acknowledgements
The present study was supported by both the project titled ‘Development of lipid lowering food and drug biomaterials with Korean seaweed’ funded by the Ministry of Land, Transport and Maritime Affairs, Korea and grant no. RTI 05-01-02 from the Regional Technology Innovation Program of the Ministry of Knowledge Economy. K.-J. K. contributed to the experimental design, experimental procedures, data interpretation and manuscript writing. O.-H. L. gave an important contribution to the experimental procedures. B.-Y. L. contributed to the experimental procedures, data analysis, data interpretation and manuscript writing. All authors contributed to the critical revision of the manuscript. The authors declare that there are no conflicts of interest.









