Introduction and overview of the focus of the position paper
Inflammation is a central component of innate (non-specific) immunity. In generic terms, inflammation is a local response to cellular injury that is marked by increased blood flow, capillary dilatation, leucocyte infiltration, and the localised production of a host of chemical mediators, which serves to initiate the elimination of toxic agents and the repair of damaged tissue( Reference Calder, Ahluwalia and Albers 1 ). It is now clear that the termination (alternatively known as resolution) of inflammation is an active process involving cytokines and other anti-inflammatory mediators, particularly lipids, rather than simply being the switching off of pro-inflammatory pathways( Reference Ortega-Gomez, Perretti and Soehnlein 2 , Reference Serhan, Chiang and Dyke 3 ).
Inflammation acts as both a ‘friend and foe’: it is an essential component of immunosurveillance and host defence, yet a chronic low-grade inflammatory state is a pathological feature of a wide range of chronic conditions, such as the metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM) and CVD( Reference Hotamisligil 4 , Reference Libby 5 ). Although the association between inflammation and chronic conditions is widely recognised, the issue of causality and the degree to which inflammation contributes and serves as a risk factor for the development of disease remain unresolved. As will be discussed, part of this uncertainty is due to a general lack of sensitive and specific biomarkers of low-grade chronic inflammation that can be used in human trials( Reference Calder, Ahluwalia and Albers 1 ).
The present article results from an International Life Sciences Institute (ILSI) Europe Workshop held in September 2013 in Granada, Spain entitled ‘Low-grade inflammation a high grade challenge: biomarkers and modulation by dietary strategies’, and aims to serve as an update to existing reviews in the area of inflammation and health and its assessment and modulation( Reference Calder, Ahluwalia and Albers 1 , Reference Calder, Ahluwalia and Brouns 6 , Reference Calder, Albers and Antoine 7 ). In particular, the present article will focus on the latest research findings in the areas of inflammation and cardiometabolic, cognitive and gut health, and how early-life nutrition and the macronutrient and plant bioactive composition of the adult diet influence inflammatory processes. It will discuss existing and emerging methods used to quantify inflammatory status in humans. Importantly, the article will identify knowledge gaps and methodological limitations that need to be addressed.
Exploring the role of inflammation in health and chronic diseases
Low-grade inflammation in cardiometabolic disease
The role of inflammation in the early-stage pathophysiology of atherothrombotic events has been recognised for over 20 years. Leucocyte recruitment into the sub-endothelial compartment of damaged arteries initiates a cascade of events mediated by leucocyte-derived inflammatory mediators. In particular, chemokines and cytokines propagate atherosclerosis via (1) increased chemokine production and expression of endothelial adhesion molecules, stimulating further leucocyte recruitment, (2) promoting lipid-laden foam-cell formation, (3) initiating smooth muscle cell proliferation, and (4) inducing plaque instability and eventual rupture( Reference Hallenbeck, Hansson and Becker 8 , Reference Hansson 9 ). The ensuing thrombosis is also in large part dependent on the inflammatory status of the ruptured plaque.
In addition to a direct role on events within the arterial wall, inflammation is an important determinant of the multi-organ cardiometabolic dysfunction, and the increased risk of T2DM, NAFLD and CVD associated with obesity( Reference Harford, Reynolds and McGillicuddy 10 ). Adipose tissue hypertrophy is associated with immune cell infiltration, in particular that of macrophages and T cells, and a local pro-inflammatory milieu wherein key cytokines including TNF-α, IL-6 and IL-1β impede the insulin signalling cascade to induce insulin resistance( Reference Lumeng, DelProposto and Westcott 11 , Reference Weisberg, McCann and Desai 12 ). This ultimately leads to a dysregulation of glucose and lipid metabolism in adipose tissue, skeletal muscle and liver. However, up to 30 % of obese individuals are considered metabolically healthy (MHO)( Reference Wildman, Muntner and Reynolds 13 ), and there is evidence to suggest that a lack of the typical elevation in the inflammatory profile associated with obesity may underlie this ‘protected’ MHO phenotype. For example, in morbidly obese individuals, Barbarroja and co-workers observed mean homeostatic model assessment for insulin resistance (HOMA-IR) scores (insulin sensitivity index) of 3·31 and 11·48 in subjects with MHO (BMI 55 kg/m2) or who were metabolically unhealthy obese (BMI 56 kg/m2), respectively, which was associated with a 2- to 4-fold greater adipose expression of inflammatory cytokines (TNF-α, IL-1β and IL-6) between the two obese groups( Reference Barbarroja, Lopez-Pedrera and Mayas 14 ).
Inflammation plays a direct role in the progression of NAFLD, the most common liver disorder in Western countries. NAFLD comprises a spectrum of conditions ranging from benign steatosis to non-alcoholic steatohepatitis characterised by hepatocyte injury (hepatocyte ballooning and Mallory bodies) and necroinflammation, and potentially to progressive fibrosis that can lead to cirrhosis( Reference Fujii and Kawada 15 , Reference Smith and Adams 16 ). The pathological progression of NAFLD is considered to have a two-hit basis (Fig. 1). The first hit, hepatocyte accumulation of fat, is thought to arise due to an increased delivery of fatty acids to the hepatocyte, an increase in hepatocyte fatty acid and TAG synthesis, and decreased fatty acid oxidation. The resultant excess of fat may result in lipotoxicity and a pro-inflammatory and pro-oxidative state (the second hit), which ultimately induces cellular senescence, which, if unchecked, leads to fibrosis and cirrhosis. Hepatic inflammation is mediated via the activation of local macrophages called Kupffer cells. Currently, no medication or surgical procedure has been approved for treating NAFLD or non-alcoholic steatohepatitis with confidence. Considering the overall lack of success in curbing global trends in the prevalence of excess body weight, inflammatory processes are emerging as a strong therapeutic target to reduce the risk of T2DM, CVD and NAFLD in obese individuals.

Fig. 1 Two-hit model of non-alcoholic fatty liver disease. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Gut–systemic inflammatory associations
With recent significant advances in the ability to characterise the gut microbiota in increasing detail, comes the recognition of the importance of the microbiota not only in gastrointestinal health, but also in systemic metabolism and cardiometabolic health, with the immune system and inflammatory processes central to gut–systemic tissue ‘cross-talk’. The human intestine contains 1 × 1013 to 1 × 1014 bacterial cells, which outnumber human cells by a factor of 10 to 1 and contain approximately 150 times as many genes as the human genome( Reference Gill, Pop and Deboy 17 ). Increasing evidence indicates that the microbiota is significantly altered through the ageing process( Reference Power, O'Toole and Stanton 18 , Reference Toole and Claesson 19 ) and in obesity( Reference Power, O'Toole and Stanton 18 ), with a deleterious decline in microbiota ‘richness’ and gene expression diversity evident in both situations( Reference Power, O'Toole and Stanton 18 ).
Gastrointestinal tract–microbiota interactions influence host health, and in particular immune function, by promoting the development and maintenance of the mucosal immune system, protecting against pathogen invasion and maintaining gastrointestinal tract barrier integrity( Reference Kurashima, Goto and Kiyono 20 ). Gut permeability to bacterial lipopolysaccharides (LPS), a potent inflammatory stimulant, appears to be an important trigger for low-grade systemic inflammation. LPS are found on the outer membrane of Gram-negative bacteria such as Proteobacteria (e.g. Escherichia coli), and serve as an endotoxin. In the elderly, a higher count of LPS-producing bacteria in the colon, along with a lower abundance of bifidobacteria( Reference Cani and Delzenne 21 , Reference Collado, Isolauri and Laitinen 22 ), a combination which is thought to promote increased gut permeability( Reference Cani and Delzenne 21 ), is likely to lead to higher plasma levels of LPS (termed metabolic endotoxaemia). Through the interaction with Toll-like receptor 4 on mononuclear cells, microbiota-derived LPS may be an important trigger in the development of inflammation and metabolic diseases( Reference Miller, Ernst and Bader 23 ). In a recent dietary intervention study in male C57Bl/6 mice, the alteration in microbiota profiles as a result of a high-fat diet was strongly associated with gut permeability, endotoxaemia and adipose tissue inflammation( Reference Serino, Luche and Gres 24 ).
In addition to its role in low-grade inflammatory cardiometabolic conditions, emerging evidence is suggesting that the gut microbiota can influence the risk of high-grade autoimmune inflammatory conditions such as type 1 diabetes mellitus, coeliac disease, inflammatory bowel disease and rheumatoid arthritis( Reference Belkaid and Hand 25 – Reference Dunne, Triplett and Gevers 27 ), the incidence of which has risen dramatically since the 1940s. These conditions are now thought to affect 5–10 % of those in Western societies( Reference Miller, Pollard and Parks 28 ). Certain members of the gut microbiota have been shown to induce mimics of human antigens and trigger the production of autoantibodies responsible for aberrant immune responses to normal human proteins and hormones including leptin, peptide YY and ghrelin( Reference Fetissov, Sinno and Coeffier 29 ). It is not unreasonable to speculate that the adverse impact of the energy-dense, nutrient-poor Western-style diet on human gut microbiota and immune system, which have both been finely tuned and honed by high-fibre, high-polyphenol traditional diets over the millennia, may therefore be an important contributor to the environmental stimuli that trigger and progress autoimmune conditions( Reference Tuohy, Fava and Viola 30 ). A possible starting point when discussing the underlying mechanisms by which diets rich in whole plant foods or fermentable fibres can have an impact on immune function and tolerance may be the recent demonstration that butyrate, an important fermentation end product produced by the gut microbiota from fibre, controls human dendritic cell maturation, a key process in immune homeostasis, since dendritic cells are considered as ‘gate keepers’ of the immune system( Reference Liu, Li and Min 31 , Reference Millard, Mertes and Ittelet 32 ). In addition, butyrate induces murine peripheral regulatory T-cell generation( Reference Arpaia, Campbell and Fan 33 ), acetate affects neutrophil chemotaxis and oxidative burst, butyrate inhibits adipocyte–macrophage inflammatory interactions( Reference Ohira, Fujioka and Katagiri 34 ), and propionate reduces the inflammatory output of adipose tissue( Reference Al-Lahham, Roelofsen and Priebe 35 ). Probiotic, fibre or polyphenol up-regulation of microbial activities that control both the quantity and profile of bile acids returning to the liver via the enterohepatic circulation with their subsequent regulation of farnesoid X receptor and TGR5 is also emerging as an important pathway linking the gut microbiota with extra-intestinal physiological/immune function( Reference Arpaia, Campbell and Fan 33 , Reference Boesjes and Brufau 36 , Reference Shen, Gaskins and McIntosh 37 ).
Low-grade systemic inflammation and neuroinflammation
Communication between the systemic immune system and the central nervous system (CNS) is a critical but often overlooked component of the inflammatory response to tissue injury, disease or infection. Activation of highly conserved neuronal and hormonal communication pathways in mammals drives diverse CNS-regulated components of the inflammatory response, including fever, neurogenic inflammation, descending anti-inflammatory mechanisms and a coordinated set of metabolic and behavioural changes, including fatigue, anhedonia, depression and mild cognitive impairment. These behavioural changes are collectively referred to as ‘sickness behaviour’( Reference Dantzer and Kelley 38 – Reference Hart 40 ). Experimental studies have provided evidence that activation of microglia, the macrophages of the CNS, as well as the cerebral vasculature, plays a key role in the development of these behavioural changes, by inducing pro-inflammatory mediators, such as IL-1β, TNF-α and PGE2 in the CNS( Reference Dantzer and Kelley 38 , Reference Teeling, Felton and Deacon 41 , Reference Teeling, Cunningham and Newman 42 ).
Much of what we know is derived from studies using mimetics of bacterial and viral infection. Depending on the stimulus used, these mimetics induce a transient response in otherwise healthy subjects; for example, administration of LPS results in enhanced production of IL-6 (approximately 80-fold) and IL-1β (approximately 4-fold), peaking at 3 h after a challenge and returning to baseline at 24 h( Reference Millard, Mertes and Ittelet 32 ). CNS responses to (patho)physiological stimuli, such as genuine infections or low-grade inflammation as a result of the MetS, are less well described.
Development of sickness behaviour in response to an infection is part of the normal response to fighting infection, and can occur during low-grade sub-pyrogenic inflammation( Reference Teeling, Felton and Deacon 41 ); however, these adaptive responses are not always harmless. Microglia have a very low turnover, and it has been suggested that these long-lived cells have an innate memory, resulting in a prolonged and heightened response under neuroinflammatory conditions( Reference Krstic, Madhusudan and Doehner 43 ). A normal part of the homeostatic signalling from the periphery to the brain, therefore, has the potential to have a profound impact on brain disease initiation or progression( Reference Perry, Cunningham and Holmes 44 , Reference Perry and Teeling 45 ). In a recent prospective clinical study, Alzheimer's disease patients were followed for 6 months and assessed for the presence of circulating cytokines, episodes of microbial infection and cognitive decline. Patients with both high levels of TNF-α (>4·2 pg/ml) at baseline and microbial infection during the assessment period showed a 4-fold greater cognitive decline, relative to patients with low levels of TNF-α ( < 2·4 ng/ml) at baseline and no infections( Reference Hennessy, Barrett and Ross 46 ). Raised serum levels of TNF-α and IL-6, but not CRP, are also associated with increased frequency of other common neuropsychiatric symptoms observed in Alzheimer's disease patients, including apathy, anxiety, depression and agitation( Reference Holmes, Cunningham and Zotova 47 ).
Recently, the effects of LPS and a real infection (Salmonella typhimurium) on cerebral endothelial and microglial activation were compared. While LPS administration resulted in a robust but transient neuroinflammatory response, a genuine infection induced a prolonged pro-inflammatory cytokine response in the CNS, leading to microglial priming( Reference Puntener, Booth and Perry 48 ).
A detailed consideration of the impact and mechanistic basis for the association between neuroinflammation and neuronal and overall CNS function, cognition and the risk of age-related cognitive decline and dementia is outside the scope of the present review, and has been the topic of many recent expert review articles( Reference Thiel, Cechetto and Heiss 49 – Reference Heneka, Golenbock and Latz 54 ).
Collectively, these data highlight inflammatory pathways as important targets for strategies promoting healthy brain ageing and reducing the risk of age-related cognitive decline.
Dietary modulation of low-grade inflammation
There is a substantial amount of evidence to suggest that many foods, nutrients and non-nutrient food components modulate inflammation both acutely and chronically( Reference Calder, Ahluwalia and Albers 1 , Reference Calder, Ahluwalia and Brouns 6 ). However, dietary studies have been typically limited to measuring a small number of blood markers of inflammation, often in the fasting state, and these may not necessarily reflect inflammation in tissue compartments or what happens in response to inflammatory challenges. This presents a significant limitation to our understanding of diet/nutrient–inflammation interactions. Previous ILSI Europe activities have dealt extensively with the food/nutrition–inflammation interaction( Reference Calder, Ahluwalia and Brouns 6 , Reference Calder, Albers and Antoine 7 ), and it is beyond the scope of the present review to provide a systematic or extensive coverage of this area. Instead, some specific examples will be discussed.
Dietary fats and inflammation
Dietary fatty acids may affect inflammatory processes through effects on body weight and adipose tissue mass and via an impact on membrane and lipid raft composition and function. Within the cell, membrane-derived fatty acids and their derivatives can influence inflammation by serving as modulators of NF-κB and PPAR-α/γ transcription factor pathways( Reference Calder 55 ), and as precursors for a host of eicosanoid and docosanoid oxidation products produced via the action of epoxygenases, lipoxygenases and cyclo-oxygenases( Reference Wall, Ross and Fitzgerald 56 ). Also, recent advances in the field have uncovered NLRP3 (NACHT, LRR and PYD domains-containing protein 3) inflammasome activation and IL-1β signalling as a key sensor of SFA-mediated metabolic stress in obesity and T2DM( Reference Vandanmagsar, Youm and Ravussin 57 ) and EPA- and DHA-derived resolvins and protectins that actively ameliorate a pro-inflammatory state( Reference Norling and Serhan 58 ). Obesity significantly reduced DHA-derived 17-hydroxydocosahexaenoic acid, a resolvin D1 precursor, and protectin D1 in adipose tissue, which may in turn have pro-inflammatory consequences( Reference Neuhofer, Zeyda and Mascher 59 ). Also, dietary EPA/DHA supplementation within an obesogenic dietary challenge restored endogenous adipose resolvin and protectin biosynthesis, concomitant with attenuated adipose inflammation and insulin resistance( Reference Neuhofer, Zeyda and Mascher 59 ). An elegant human study showed that a relatively high dose of LC n-3 PUFA augmented anti-inflammatory eicosanoid secretion and attenuated inflammatory gene expression in the subcutaneous adipose tissue of severely obese non-diabetic patients( Reference Itariu, Zeyda and Hochbrugger 60 ). Thus, there is much recent information on novel mechanisms of action by which dietary fatty acids of different classes influence inflammatory processes, some acting in pro-inflammatory and others in anti-inflammatory or inflammation-resolving ways.
There is some evidence, albeit not always consistent, for pro-inflammatory effects of dietary SFA( Reference Calder, Ahluwalia and Albers 1 ). Much of this evidence comes from either in vitro or cross-sectional studies, and there are limited randomised controlled trial (RCT) examining changes in SFA intake and inflammation in humans. The LIPGENE RCT investigated the effects of substituting dietary SFA with MUFA or as part of a low-fat diet, with or without LC n-3 PUFA supplementation, in subjects with the MetS( Reference Shaw, Tierney and McCarthy 61 ). While a low-fat n-3 PUFA-enriched diet significantly reduced the risk of the MetS( Reference Paniagua, Perez-Martinez and Gjelstad 62 ), modifying dietary fat had no significant effect on key biomarkers of cardiometabolic risk including insulin sensitivity and the plasma inflammatory markers assessed( Reference Tierney, McMonagle and Shaw 63 ). However, there was clear modulation of NF-κB-mediated inflammation and oxidative stress in the postprandial state according to lipid composition( Reference Cruz-Teno, Perez-Martinez and Delgado-Lista 64 , Reference Pena-Orihuela, Camargo and Rangel-Zuniga 65 ). This lack of impact of LC n-3 PUFA on the fasting plasma inflammasome in humans( Reference Robinson and Mazurak 66 ) is in line with previous human studies( Reference Tierney, McMonagle and Shaw 63 , Reference Vessby, Uusitupa and Hermansen 67 ), but contradicts the effects observed in a wide variety of cell and animal models. However, as will be discussed in the section ‘Translating research into public health benefit and novel products’, it is difficult to know whether the output from these RCT truly demonstrates a lack of efficacy or reflects insufficient dose and/or duration or poor selection of fasting plasma biomarkers of inflammation, which are insensitive to physiologically meaningful changes occurring in key metabolic tissues such as the liver and adipose tissue.
As with other common phenotypes, there is evidence emerging that the associations between dietary fat composition and inflammation are influenced by common gene variants( Reference Madden, Williams and Calder 68 ). In the LIPGENE study, SNP in the genes encoding the anti-inflammatory peptide adiponectin (ADIPOQ) and its receptor (ADIPOR1) have been shown to interact with SFA to modulate the effect of dietary fat modification on insulin resistance( Reference Ferguson, Phillips and Tierney 69 ), and using a case–control approach, it was observed that a common SNP of the C3 gene was related to the risk of the MetS, but more importantly, the impact of this was greatly accentuated by high plasma levels of SFA( Reference Phillips, Goumidi and Bertrais 70 ). Also, the combination of polymorphisms in genes encoding IL-6, lymphotoxin α (LTA) and TNF-α had an additive effect, which interacted with plasma fatty acid status to modulate the risk of the MetS( Reference Phillips, Goumidi and Bertrais 71 ). Grimble et al. ( Reference Grimble, Howell and O'Reilly 72 ) demonstrated that the ability of LC n-3 PUFA to decrease TNF-α production is influenced by inherent TNF-α production and by polymorphisms in the TNF-α and LTA genes.
Inflammation in the postprandial state is likely to contribute to the pathological impact of exaggerated postprandial lipaemia( Reference Jackson, Poppitt and Minihane 73 ). Although there has been some investigation of the impact of meal fatty acid composition on non-fasting inflammatory biomarkers, the data thus far remain inconsistent( Reference Jackson, Poppitt and Minihane 73 ). It has been reported that in overweight men, plasma IL-6, TNF-α and soluble vascular adhesion molecule-1 concentrations decreased after an n-6 PUFA-rich meal, while markers were increased after a SFA-rich meal( Reference Masson and Mensink 74 ). In contrast, Manning et al. ( Reference Manning, Sutherland and McGrath 75 ) showed that high-fat meals increased IL-6, independent of the type of fatty acid, and had no impact on IL-8 and TNF-α concentrations.
Dietary carbohydrates and inflammation
Besides postprandial lipaemia, postprandial glucose is an independent predictor of diabetes and CVD, an effect which may be mediated through oxidative stress and inflammation( Reference Blaak, Antoine and Benton 76 ). Importantly, there appears to be no glycaemic threshold for reduction of either microvascular or macrovascular complications. The progressive relationship between plasma glucose and the risk of CVD extends well below the diabetic threshold( 77 , 78 ).
Acute glucose variations from peaks to nadirs include postprandial glucose excursions that can be described by two components. The first component, which is the duration of the postprandial glucose increment, is a major contributor to chronic sustained hyperglycaemia, while the second component, which is the magnitude of the postprandial rise, is more often a reflection of glucose variability. It is difficult to discriminate between the contributions of these two components of dysglycaemia. It seems that both contribute to the two main mechanisms that lead to diabetic and cardiovascular complications, namely excessive protein glycation and activation of oxidative stress and inflammation.
Although mechanistic evidence indicates a positive correlation between the glycaemic index and load of the diet and low-grade inflammation, intervention studies, to date, do not convincingly support this. Hu et al. ( Reference Hu, Block and Norkus 79 ) observed a stepwise relationship between dietary glycaemic index and oxidative stress markers in healthy adults. Furthermore, high-glycaemic index carbohydrates increase NF-κB activation and NF-κB binding in mononuclear cells of young, lean healthy subjects( Reference Dickinson, Hancock and Petocz 80 ). Diets low in glycaemic load and high in whole grains may have a protective effect against systemic inflammation in diabetic patients, as reviewed elsewhere( Reference Qi and Hu 81 ). Consistent with this, epidemiological studies have shown an inverse relationship between dietary fibre and CRP levels. Both the DASH diet (naturally high in fibre, i.e. 30 g fibre/d) and a fibre-supplemented usual diet (30 g psyllium fibre/d) decreased CRP concentrations in lean normotensive subjects( Reference King, Egan and Woolson 82 ). In contrast, a high-carbohydrate, low-fat diet with a relatively high dietary fibre and complex carbohydrate content, within the context of a lifestyle intervention programme, has been shown to reduce diabetes incidence in the long term by 50 %( Reference den Boer, Herraets and Stegen 83 ). The prominent role of the type of carbohydrate has also been illustrated in studies showing that dietary carbohydrate modification, i.e. an oat/wheat/potato diet, up-regulated sixty-two genes related to stress, cytokine–chemokine-mediated immunity and IL pathways compared with a rye–pasta diet( Reference Kallio, Kolehmainen and Laaksonen 84 ). These differences in the inflammatory response have been ascribed to differences in the early insulin response and the resultant late hypoglycaemia in the oat/wheat/potato group.
Taken together, studies have suggested that healthy eating patterns characterised by reduced postprandial glycaemia and lipaemia are associated with reduced concentrations of markers of low-grade inflammation.
Plant bioactive compounds and inflammation
Recent prospective cohort data suggest that improved cognitive function and a reduced risk of age-related neurodegenerative diseases, associated with increased fruit and vegetable intake( Reference Sofi, Abbate and Gensini 85 – Reference Barberger-Gateau, Raffaitin and Letenneur 87 ), may be in large part attributable to intake of specific flavonoids( Reference Barberger-Gateau, Raffaitin and Letenneur 87 ), and may involve an effect on inflammatory processes (Table 1). In particular, increased consumption of total flavonoids was positively associated with episodic memory in middle-aged adults( Reference Kesse-Guyot, Fezeu and Andreeva 88 ) and with a reduced rate of cognitive decline in adults aged 70 years and over( Reference Bakker, van Erk and Pellis 89 ). The anthocyanin group of flavonoids, with certain soft fruits providing the most significant dietary source, has emerged as being particularly potent. In the Nurses' Health Cohort, greater intakes of blueberries and strawberries were associated with slower rates of cognitive decline, with a high intake of soft fruits estimated to delay cognitive ageing by up to 2·5 years( Reference Devore, Kang and Breteler 90 ). Furthermore, a large cross-sectional study has also indicated that total flavonoid intake is inversely correlated with serum CRP concentrations( Reference Chun, Chung and Claycombe 91 ). In support of this association, a number of dietary intervention studies have provided evidence that dietary flavonoids are capable of modulating inflammatory cytokines (e.g. TNF-α) and CRP production( Reference Chun, Chung and Claycombe 91 – Reference Zern, Wood and Greene 94 ). However, there are relatively few human RCT investigating the anti-inflammatory and cognitive effects of flavonoids (Table 1).
Table 1 Dietary flavonoids and inflammation: evidence from epidemiological and intervention studies
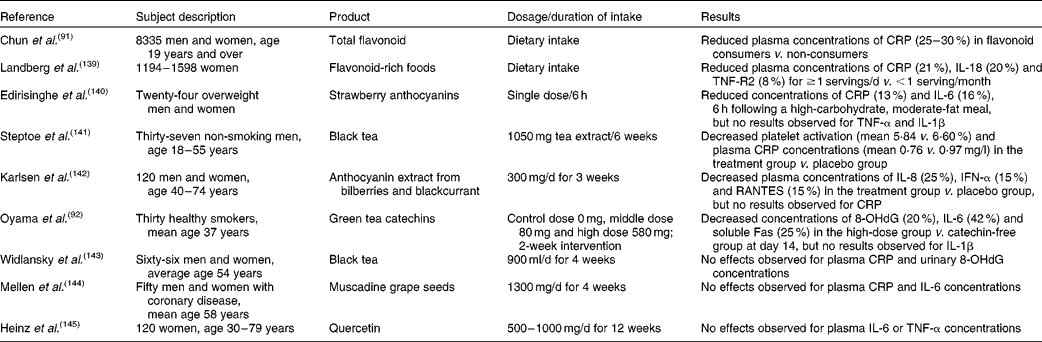
CRP, C-reactive protein; TNF-R2, TNF receptor 2; IFN-α, interferon-α; RANTES, regulated on activation, normal T-cell expressed and secreted; 8-OHdG, 8-hydroxydeoxyguanosine.
Although the effects of flavonoids were originally ascribed to an antioxidant action, it is now clear that levels achieved in biological tissues may not be sufficient to act in this way. Evidence indicates that flavonoids are capable of acting in a number of other ways that may result in their targeting of inflammation, including (1) the modulation of intracellular signalling cascades that control neuronal survival, death and differentiation; (2) an impact on gene expression and (3) interacting with the mitochondria( Reference Mandel, Amit and Kalfon 95 – Reference Vauzour, Vafeiadou and Rice-Evans 98 ). In particular, emerging evidence suggests that dietary flavonoids may exert neuroprotective effects by suppressing the activation of microglia, which mediate inflammatory processes in the CNS (see the earlier section). Although rather complex, the main anti-inflammatory properties of flavonoids include (1) an inhibitory role in the release of cytokines, such as IL-1β and TNF-α, from activated microglia; (2) an inhibitory action against inducible NO synthase induction and subsequent NO production in response to glial activation; (3) an ability to inhibit the activation of NADPH oxidase and subsequent generation of reactive oxygen species in activated glia; and (4) a capacity to down-regulate the activity of pro-inflammatory transcription factors, such as NF-κB, through their influences on a number of glial and neuronal signalling pathways( Reference González-Gallego, García-Mediavilla and Sánchez-Campos 99 , Reference Spencer, Vafeiadou and Williams 100 ). However, almost all mechanistic studies have been carried out in vitro at rather supraphysiological concentrations, with limited research on animal models and scarce data from human RCT.
Early-life nutrition and inflammation
During development, the human embryo and fetus undergo an enormously complex series of changes in both cell type and cell number. Each of these changes takes place in a strictly choreographed series, and disruption of the process can lead to dramatic and long-lasting consequences. There are many recent summaries of the processes involved( Reference British Nutrition Foundation 101 ). In humans, this was most clearly demonstrated in the Second World War, when Dutch women were placed under famine conditions following a railway workers' strike (the Dutch Hunger Winter)( Reference Franzek, Sprangers and Janssens 102 , Reference Heijmans, Tobi and Stein 103 ). Studies on the offspring of women who were pregnant at this time have shown clearly that women who were pregnant in the first trimester gave birth to babies who would go on to develop a much wider spectrum of health problems than babies born to women who were in the second or third trimester, though these offspring still would continue to show health problems( Reference Roseboom, van der Meulen and Ravelli 104 ).
Factors other than undernutrition can also have both short- and long-term consequences. Of particular relevance, obese women give birth to babies with a higher risk of both small for gestational age and large for gestational age, of complications at birth and of developing the MetS( Reference Cottrell and Ozanne 105 , Reference McMillen, Rattanatray and Duffield 106 ). All these cannot be explained by postnatal events, and are at least partly explained by the phenomenon known as ‘fetal programming’ or ‘developmental programming’( Reference Frias and Grove 107 , Reference Hales, Barker and Clark 108 ). This hypothesis states that nutrition-related exposures in utero ‘programme’ the baby to expect a postnatal nutritional environment, and if a different one is experienced, then there is a risk of the development of metabolic complications. There have been refinements to the basic hypothesis and to our understanding of the mechanisms involved( Reference Hales and Barker 109 – Reference Gluckman, Cutfield and Hofman 112 ); however, the fundamental observations remain unchanged and unchallenged. How these associations are mediated is not yet clearly demonstrated, but several hypotheses are being tested. There is substantial support for nutrition altering the epigenetic profile of the offspring, including hypermethylation of cytokine receptors. Evidence indicates that low Fe status at birth, which is associated with impaired lung function in children, can result in reduced nephron number and decreased levels of cell-cycle enzymes( Reference Swali, McMullen and Hayes 113 ), suggesting that nutritional deficiency during a critical phase of development can inhibit organ growth. This fits with data showing that thymus growth is reduced, and that this leads to changes in the cytokine profile.
Maternal obesity also has dramatic effects on pregnancy outcome. Again, there are many detailed reviews dealing with this topic( Reference Frias and Grove 107 ). The mechanisms seem to involve inflammatory responses, and increased cytokine levels have been reported in the placenta and cord blood of babies born to obese mothers. Whether, in humans, the placenta alone is responsible is not clear, and it is quite likely that adipose tissue itself, which becomes infiltrated with macrophages, will produce increased amounts of pro-inflammatory cytokines( Reference Lazar 114 ). The situation becomes more complex in obesity, because in addition to the cytokines, or possibly because of the cytokines, inflammation results in changes in Fe metabolism( Reference McClung and Karl 115 ), and there is abundant evidence to show that decreased Fe status during pregnancy has adverse effects on the offspring( Reference Gambling, Danzeisen and Gair 116 – Reference Siddappa, Georgieff and Wewerka 119 ). Obesity results in increased hepcidin production( Reference Bekri, Gual and Anty 120 , Reference Zafon, Lecube and Simo 121 ). Hepcidin is a negative regulator of Fe absorption( Reference Laftah, Ramesh and Simpson 122 ), and lower Fe status in the mother before birth is associated with an increased risk of wheezing in the children (W Bright, G Devereux, HJ McArdle, unpublished results). Thus, decreased Fe status may be an additional risk factor in obese mothers.
Translating research into public health benefit and novel products
Biomarkers of inflammation in human nutrition studies
As explained previously, inflammation is a normal process, and there are a large number of cells and mediators involved; measurement of these is often used as a ‘biomarker’ of inflammation, i.e. an indicator that inflammation is occurring. These cells and mediators are largely involved in, or are produced as a result of, the inflammatory process, irrespective of the trigger or its location in the body, and are common to all inflammatory situations( Reference Calder, Ahluwalia and Albers 1 ). To monitor inflammation in a meaningful way, the markers used must be valid: they must reflect the inflammatory process under study and must be predictive of future health status. The range of potential biomarkers of inflammation was considered by an Expert Group of ILSI Europe, with the aim of identifying robust and predictive markers, or patterns or clusters of markers, which can be used to assess inflammation in human nutrition studies in the general population; markers indicative of a specific inflammatory pathology (e.g. rheumatoid arthritis) and/or in less accessible tissue sites (e.g. in lung lavage fluid or in intestinal biopsy material) were not considered to be relevant to more healthy populations( Reference Calder, Albers and Antoine 7 ). Currently, there is no consensus as to which markers best represent low-grade inflammation( Reference Calder, Ahluwalia and Brouns 6 ), or differentiate between acute and chronic inflammation or between the various phases of inflammatory responses( Reference Calder, Albers and Antoine 7 ). Therefore, a range of blood cellular markers (e.g. total leucocytes, granulocytes and activated monocytes) and soluble mediators (cytokines and chemokines (TNF, IL-1, IL-6, IL-8, CC chemokine ligand 2 (CCL2), CCL3, CCL5), adhesion molecules (vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E-selectin), adipokines (adiponectin) and acute-phase proteins (CRP, serum amyloid A, fibrinogen)) are frequently measured. Some of these are associated with future risk of CVD and with cardiometabolic health( Reference Calder, Ahluwalia and Albers 1 , Reference Calder, Ahluwalia and Brouns 6 , Reference Calder, Albers and Antoine 7 ). However, there are several key issues concerning the use of these markers as determinants of low-grade inflammation. First, they are non-specific acute-phase response and pro-inflammatory response markers, and, by themselves, do not represent metabolic low-grade inflammation. Second, even in healthy individuals, there is wide variation in the measurements made. This is because there are a number of modifying factors that affect the concentration of an inflammatory marker at a given time. These modifying factors include age, diet, body fatness, physical fitness and genetics, among others( Reference Calder, Ahluwalia and Albers 1 ).
One can question whether static measurements of single or complex biomarkers are truly informative about health status, reasoning from the concept that health is defined by the ability to adequately adapt to everyday challenges( Reference Huber, Knottnerus and Green 123 ). Measuring the concentration of inflammatory markers in the bloodstream under basal conditions is probably less informative and relatively insensitive compared with measurements of the concentration change in response to a challenge. A number of inflammatory challenges have been described. These include an oral glucose load( Reference Dickinson, Hancock and Petocz 80 ), an oral fat load( Reference Nappo, Esposito and Cioffi 124 , Reference Esposito, Nappo and Giugliano 125 ), acute exercise, administration of bacterial LPS( Reference Michaeli, Berger and Revelly 126 ), exposure to UV irradiation( Reference Rhodes, Darby and Massey 127 , Reference Pilkington, Massey and Bennett 128 ) and vaccination. Although each of these challenges has been used in nutritional studies, many are poorly standardised, limiting the comparisons that can be made. Most often, the markers measured in response to challenges are those mentioned earlier in the context of static basal measurements. Currently, a number of large European consortia, i.e. PhenFlex (http://www.nugo.org/everyone/42 701/7/0/30), NutriTech (http://www.nugo.org/nutritech) and BioClaims (http://bioclaims.uib.es), are developing and validating the metabolic challenge test concept for application in the assessment of health status, including the study of inflammatory process markers( Reference Wopereis, Wolvers and van Erk 129 ).
The past decade has seen huge growth in innovation in ‘omics’ technologies that provide enormous opportunities for high-throughput biological sample characterisation, with patterns and clusters of markers (signatures or fingerprints) emerging as robust biomarkers of inflammation( Reference Bakker, van Erk and Pellis 89 , Reference Rudkowska, Paradis and Thifault 130 ). The enormous challenge in this era of big data is making biological sense of different levels of data, including the transcriptome, proteome, metabolome and clinical chemistry data. Novel data analysis methodologies, such as machine learning, offer large potential for identifying relevant data for specific biological outcomes based on complex multidimensional datasets( Reference Swan, Mobasheri and Allaway 131 ). In addition, bioinformatic tools have been developed to interpret these complex data in the context of existing biological knowledge in the literature and databases, also termed network biology( Reference Kelder, Conklin and Evelo 132 , Reference Barabasi and Oltvai 133 ). These technologies will be instrumental to the discovery of relevant biomarker signatures that reflect ‘low-grade inflammation’ based on inflammatory response networks connected to organ-specific metabolic derailment.
With the coming of age of the ‘omics’ technologies and bioinformatic tools, a large increase in the number, specificity and sensitivity of candidate biomarkers of inflammation can be expected in the next decade( Reference van Gool, Henry and Sprengers 134 ). A screening of the ‘Thomson Reuters IntegritySM Biomarker Database’ reveals that as of May 2014, 945 candidate biomarkers of inflammation have been described, of which only seventeen, including CRP, TNF-α, serotransferrin and erythrocyte sedimentation rate, have been developed into biomarker assays approved and recommended by regulatory bodies for use in clinical studies. This represents the classical biomarker gap: many candidate biomarkers are identified based on preclinical and clinical studies; however, due to relatively limited efforts in validation and assay development, these are subsequently not further developed( Reference Kumar, van Gool, Horvatovich and Bischoff 135 ). To accelerate biomarker development, a paradigm shift in this area is needed; instead of single companies developing a single biomarker assay, pre-competitive collaborations between different industrial, academic, and research and technology organisations have the advantage of a more efficient development process time- and cost-wise, by combining a wide diversity of expertise, in the development of a harmonised, standardised and accepted assay. In these consortia, ideally, companies from nutrition, pharma and diagnostics join forces in a pre-competitive way.
A major concerted effort should comprise (1) the discovery of context-based biomarker signatures for the assessment of the status of low-grade inflammation, (2) the development of challenge tests that determine the inflammatory response functionality in the context of metabolic stress-induced low-grade inflammation, and (3) the development of the identified biomarkers towards application in a clinically accepted assay, with normative data.
Low-grade inflammation and health claims
The European Food Safety Authority (EFSA) guidance document on scientific requirements for health claims related to gut and immune function( 136 ) specifically states that chronic inflammation is associated with the development of a number of diseases, and that ‘altering levels of markers of inflammation might indicate a beneficial effect in the context of “a reduction of disease risk claim”, if it can be demonstrated that altering the levels of inflammatory markers is accompanied by a reduced incidence of a disease for a specific dietary intervention’. No additional specificity is added for chronic low-grade inflammation. At present, the European Union health claim register (http://ec.europa.eu/nuhclaims) does not contain any authorised or non-authorised health claims that specifically address the health benefit area of suppression or control of low-grade inflammation.
To build strong health claims on nutrition for improving inflammation control in the future, one of the key focus areas should be the need for clinically relevant prognostic marker(s) or marker signatures that reflect the inflammatory state in a context-specific manner, which have been well validated and for which a robust standardised assay is available. The lack of health claims is probably attributable to the fact that, although numerous biologically plausible mechanisms have been established to explain inflammation–disease associations, no single biomarker or cluster of biomarkers of inflammation has yet been robustly demonstrated to be sufficiently predictive of future disease. Based on the EFSA guidance on this topic( 136 ) and the classification of candidate biomarkers as described by the expert group of ILSI Europe( Reference Albers, Bourdet-Sicard and Braun 137 ), the suggested strategy for building a EFSA health claim dossier (Fig. 2) comprises (1) a definition of the composition of the product; (2) a well-founded selection of the target population; (3) the selection of a clinically relevant composite biomarker panel representing inflammation as well as the selected health benefit (or disease risk) endpoints; and (4) a number of sufficiently powered and well-controlled human studies assessing the effect of the test material (nutrient, food, product) on the relevant biomarkers in the relevant target population.

Fig. 2 Schematic of topics to be addressed when building a dossier for a European Food Safety Authority (EFSA) health claim on control of chronic low-grade inflammation. The blue boxes indicate the main topics to be addressed; the white boxes state the actual content topics. Building a strong EFSA health claim dossier requires (1) a definition of the composition of the nutritional component including manufacturing procedures in scope and out of scope for the claim, (2) a clear definition of the target population, being the general population or a specific subpopulations at risk, including the defining parameters, (3) a definition of biomarkers measured to assess the health effects of the nutritional component, including a description of the proof of clinical relevance, or the clinical validity of the combination of inflammation biomarkers and related clinically relevant biomarkers for health benefit endpoints associated with the health claim, and (4) a full description of clinical study design for all studies included in the dossier, including statistical power analysis and safety evaluation. The red arrow indicates the primary hurdle for functional health claims in the area of chronic low-grade inflammation, which is the lack of (combinations of) inflammation biomarkers with established and therefore accepted clinical relevance. This is primarily the consequence of inflammatory responses being non-specific normal physiological responses to tissue damage, and discrimination between normal and abnormal levels or combinations has not been well established in relation to chronic low-grade inflammation. The description of the classification of clinical relevance of biomarkers (categories A–D) was adapted from Albers et al. ( Reference Albers, Bourdet-Sicard and Braun 137 ). RCT, randomised controlled trial. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Summary and suggestions for the way forward
Inflammation is a normal component of host defence; however, elevated unresolved chronic inflammation is a core perturbation in a range of chronic diseases and is an important determinant of the pathological impact of excess adiposity. Cell, animal and human epidemiological studies have identified a number of potential diet derived anti- and pro-inflammatory components, some of which have been discussed here; this topic has been dealt with more extensively elsewhere( Reference Calder, Ahluwalia and Albers 1 , Reference Calder, Ahluwalia and Brouns 6 , Reference Calder, Albers and Antoine 7 ). Available human RCT evidence is more limited and sometimes conflicting or inconsistent, in part attributable to under-powered studies where inflammation was not specified as a primary study outcome. Furthermore, research tends to take a reductionist approach and examine the impact of individual dietary components in isolation, despite the identification of numerous potential diet-derived anti-inflammatory and inflammation-resolving bioactive compounds, with likely additive or synergistic effects. There is a need to take a more holistic approach and consider the impact of combinations of components of foods and dietary patterns, with a likely greater overall benefit than each single component might have on its own. Moreover, although it is evident that the inflammatory response is highly variable, a full understanding of the source of heterogeneity is distinctly lacking. More extensive profiling of participants in human studies and consideration of potential key variables such as age, sex, genotype and lifestyle factors in statistical models is needed in order to help understand the aetiology of the variation in both inflammation itself and in its response to dietary change. This approach will also allow for the identification of population subgroups that may particularly benefit from interventions that target inflammation.
Establishing and quantifying reliable, precise diet–inflammation–health associations is reliant on the availability of approved, standardised biomarkers with normative data for use in human observation studies and RCT. Biomarker research is a highly active area with significant advances to be expected in the coming years( Reference de Vries, Antoine and Burzykowski 138 ). Rather than rely on a limited number of generic markers common to both acute and low-grade chronic inflammation, future inflammation ‘testing’ is likely to involve quantifying clusters or signatures of markers with some tissue specificity. Such biomarkers should generally be measured in the challenged state( Reference Calder, Ahluwalia and Albers 1 ), with the choice of the physiological stressor dependent on the tissue, and research question of interest. The biomarkers assessed are likely to include those already typically measured (cytokines, chemokines, soluble adhesion molecules, etc.), but are also likely to include tissue-specific markers and fingerprints based on gene expression profiles (e.g. in blood mononuclear cells), cell or plasma proteomics, and microRNA.
The research focus on the establishment of a robust diet–inflammation–health association is justifiable, considering the substantial role of low-grade inflammation in the pathology of numerous chronic diseases, thereby making it a key future preventative and therapeutic target.
Acknowledgements
The present review results from a workshop organised by the European Branch of ILSI Europe. This publication was coordinated by Dr Peter Putz, Scientific Project Manager at ILSI Europe. The workshop was funded by the ILSI Europe Obesity and Diabetes Task Force, the ILSI Europe Metabolic Imprinting Task Force, ILSI Brazil, ILSI North America and ILSI Southeast Asia Region. Industry members of the task forces are listed on the ILSI Europe website at http://www.ilsi.eu. For further information about ILSI Europe, please email [email protected] or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies.
The authors thank the members of the Organising Committee: Professor Jean-Louis Bresson and Professor Ascension Marcos for their invaluable contribution to this work through their enthusiastic and generous participation. The authors are also grateful to Ms Belinda Antonio, Ms Toula Aslanidis, Ms Ruth Marquet, Mr Pierre Mouelhi and Mr Alex Rankin for their administrative support. The authors thank Dr Lorraine Gambling, Dr Helen Hayes and Ms Val Stevens for technical assistance.
The authors' contributions are as follows: A. M. M. and P. C. C. had responsibility for producing the final version of the manuscript. All authors contributed to the discussion and had input into the writing of the manuscript.
L. S. is an employee of Newtricious and S. V. is an employee of Mondelēz International. A. B. was an employee of ILSI Europe. The remaining authors have no conflicts of interest.






