Zinc is important for the function of numerous biological processes including enzymatic reactions, regulation of gene expressions (e.g. by the zinc finger motif of DNA-binding proteins) and maintenance of membrane structure and function(Reference Walsh, Sandstead and Prasad1–Reference Prasad3). Zinc-containing proteins play a crucial role in growth and reproduction and in maintaining the integrity of the immune system and the DNA(Reference Walsh, Sandstead and Prasad1–Reference Prasad3). Zinc deficiency is frequently observed among the elderly and has been associated with infections, cancer, atherosclerosis and other age-related degenerative diseases(Reference Walsh, Sandstead and Prasad1–Reference Zhou, Park and Liu7). Experimental data suggest that oxidative stress and immune dysfunction may at least in part mediate the link between zinc deficiency and these diseases(Reference Walsh, Sandstead and Prasad1–Reference Beattie and Kwun5). Only few prospective studies about serum zinc concentrations and mortality have been performed(Reference Kok, Van Duijn and Hofman8–Reference Soinio, Marniemi and Laakso13). Results from these studies are partially inconsistent, but largely support the hypothesis that zinc deficiency indicates an increased risk of fatal cardiovascular and/or non-cardiovascular events. Recent evidence that zinc supplementation may reduce mortality have further strengthened the need for large prospective studies designed to clarify the prognostic value of zinc status for the maintenance of human health(Reference Clemons, Kurinij and Sperduto14). The purpose of the present work was to examine whether low serum zinc concentrations are associated with total, cardiovascular and non-cardiovascular mortality. To the best of our knowledge, this is the first study to address this research question in a cohort at intermediate to high risk for future cardiovascular events, namely in patients referred to coronary angiography.
Methods
Study population
The LURIC study was designed to evaluate the environmental and genetic risk factors for atherosclerosis and related metabolic diseases(Reference Winkelmann, März and Boehm15). Baseline examinations were performed from July 1997 to January 2000 and included 3316 Caucasians who were referred to coronary angiography at the Heart Centre of the Ludwigshafen General Hospital in Southwest Germany. Inclusion criteria were clinical stability with the exception of acute coronary syndromes, the availability of a coronary angiogram and German ancestry (in order to limit genetic heterogeneity). All study participants were born in Germany to parents of German ancestry and were living in the Rhine Valley area(Reference Winkelmann, März and Boehm15). Patients were excluded from the present study if there was any acute illness other than acute coronary syndromes, any predominant non-cardiac disease or a history of malignancy within the past 5 years. The present study was approved by the Institutional Review board at the ‘Ärztekammer Rheinland–Pfalz’ (Mainz, Germany) and all the study participants gave their written informed consent.
Coronary artery disease was diagnosed in accordance with the classification of the American Heart Association if the maximal luminal narrowing in at least one of the fifteen coronary segments showed a stenosis of 20 % or more(Reference Austen, Edwards and Frye16). Diabetes mellitus was diagnosed by a fasting glucose level >1·25 g/l or with a 2 h value greater than 2 g/l in an oral glucose tolerance test (American Diabetic Association criteria) and in patients with a documented history of diabetes or drug and/or insulin therapy for diabetes mellitus. Hypertension was diagnosed if the systolic and/or diastolic blood pressure exceeded 140 and/or 90 mmHg or if the patient was on antihypertensive medication. Glomerular filtration rate was calculated according to the abbreviated Modification of Diet in Renal Disease study equation(Reference Levey, Coresh and Balk17).
Laboratory analyses
A detailed description of the baseline examination including laboratory methods has been published previously(Reference Winkelmann, März and Boehm15). Blood collection was performed after a 10–h overnight fast in the early morning before coronary angiography. After a 40 min pause during which the blood was allowed to clot at room temperature, it was centrifuged at 4°C with 3800 rpm for 15 min. Routine clinical parameters were measured immediately and other samples were distributed in 1 ml storage tubes that were filled with argon gas before closing them. Afterwards, the tubes were frozen in liquid nitrogen and stored at − 80°C for further laboratory analyses. Extreme care was taken to avoid any contamination of the blood samples and we used zinc-free materials for handling of the samples. Serum zinc concentrations were determined as part of the baseline measurements on a weekly basis by a highly sensitive colorimetric method (Wako Chemicals GmbH, Neuss, Germany) with a CV for within-day and day-to-day reproducibility of 4·2–5·2 % and 2·3–7·8 %, respectively(Reference Winkelmann, März and Boehm15, Reference Makino, Saito and Horiguchi18). Serum zinc levels determined by this colorimetric method are closely correlated with the values obtained by atomic absorption spectrophotometry: correlation coefficient r 0·98, n 58(Reference Makino, Saito and Horiguchi18) and r 0·996, n 105(Reference Johnsen and Eliasson19). The values obtained by the colorimetric method were on average 6(Reference Makino, Saito and Horiguchi18) and 2·4 %(Reference Johnsen and Eliasson19) lower than the results of the atomic absorption spectrophotometry. N-terminal pro-B-type natriuretic peptide, an established and clinically used parameter to assess cardiovascular risk and myocardial dysfunction(Reference März, Tiran and Seelhorst20), was measured by electrochemiluminescence on Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). High-sensitive C-reactive protein was also measured by immunonephelometry (N High Sensitive C-reactive protein, Dade Behring, Marburg, Germany). Homocysteine and glutathione were determined with HPLC-based methods (Waters Millennium chromatography, Chromsystems Instruments & Chemicals GmbH, Martinsried, Germany)(Reference Winkelmann, März and Boehm15). Retinol and α-tocopherol were measured with the HPLC method of Aebischer et al. (Reference Aebischer, Schierle and Schüep21).
Follow-up
Information about vital status was obtained from local person registries. We used medical records of local hospitals, death certificates and autopsy data to classify the causes of death into cardiovascular and non-cardiovascular. Classification of the causes of death was independently done by two experienced physicians who were blinded to any data of the study subjects except of those that were necessary for the coding of the causes of death. In the event of a disagreement regarding a specific case, it was discussed, and the final classification was done by one of the principal investigators of LURIC (W. M.).
Statistical analysis
Considering that there is no general consensus about the cut-off values for zinc deficiency, we established quartiles of serum zinc concentrations according to the values of the whole study cohort(Reference Walsh, Sandstead and Prasad1, Reference Yokoi, Egger and Ramanujam22). Continuous variables were tested for normal distribution by descriptive statistics including the Kolmogorov–Smirnov test. Variables following a non-normal distribution were logarithmically transformed before being used in parametric procedures. Comparisons between groups were performed by ANOVA (with P for trend) for continuous data and by χ2 test (with P for linear-by-linear test) for categorical data. Simple and partial correlation analyses between serum zinc concentrations and other parameters were performed to calculate Pearson's correlation coefficients. Kaplan–Meier curves followed by a log-rank test were used to graph and examine differences in survival across quartiles of serum zinc concentrations. Cox proportional hazard ratios (HR) with 95 % CI for mortality were calculated for each of the serum zinc quartiles and for study subjects with serum zinc concentrations below 700 μg/l using the fourth quartile as the reference(Reference Yokoi, Egger and Ramanujam22). Additionally, we also included zinc quartiles as a continuous variable in these analyses. We adjusted our Cox proportional hazard models for possible confounding variables, and the results of the final step using the backward stepwise LR selection method are shown. A P < 0·05 was considered statistically significant. The SPSS 15.0 statistical package (SPSS Inc., Chicago, IL, USA) was used.
Results
All continuous variables except LDL- cholesterol, total cholesterol, systolic and diastolic blood pressure were non-normally distributed and were thus logarithmically transformed before use in parametric procedures. Zinc values were available in all 3316 subjects and baseline characteristics stratified by serum zinc quartiles are depicted in Table 1. Beer and wine consumption evaluated by a questionnaire at baseline (four categories: never, sometimes, regular and often) were not significantly associated with serum zinc quartiles as calculated with χ2 tests (P = 0·217 for beer and P = 0·298 for wine consumption). Only 2·4 % of the present study subjects took vitamin supplements and their serum zinc concentrations were not significantly different when compared with the other study subjects (850 (720–940) v. 860 (770–960) μg/l; P = 0·300), so that we included the vitamin supplement users in all statistical analyses.
Table 1 Baseline characteristics according to quartiles of serum zinc concentrations*

BP, blood pressure; CAD, coronary artery disease; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; GFR, glomerular filtration rate; ACE, angiotensin-converting enzyme.
* Continuous variables are presented as medians with interquartile range (IQR) or as means and standard deviations, and categorical data are presented as percentages.
† ANOVA with P for trend was used for continuous variables, and χ2 test with P for linear-by-linear test for categorical variables.
Eighteen patients were lost during the follow-up period and were therefore excluded from the Cox proportional hazard analyses. In the remaining study cohort of 3298 patients, 769 (23 % of the study population) had died, after a median time of follow-up of 7·75 years. In twenty-four of the deceased patients, we could not obtain sufficient information to classify their causes of death and they were thus included in the analyses for total mortality, but excluded from the analyses for cardiovascular and non-cardiovascular mortality. From the 3274 study participants who were followed up for differentiated mortality analyses, 484 (15 %) died due to cardiovascular causes and 261 (8 %) due to non-cardiovascular causes. Kaplan–Meier curves followed by a log-rank test show that the risk for total, cardiovascular and non-cardiovascular mortality significantly increased across serum zinc quartiles (P < 0·001 for all; see Fig. 1). HR for total, cardiovascular and non-cardiovascular mortality according to the quartiles of serum zinc concentrations are shown in Table 2. Unadjusted HR for total mortality for the first when compared with the fourth zinc quartile, and the per quartile decrease were 2·36 (95 % CI 1·91, 2·92; P < 0·001) and 1·35 (95 % CI 1·26, 1·44; P < 0·001), respectively. Accordingly, unadjusted HR were 2·12 (95 % CI 1·63, 2·77; P < 0·001) and 1·30 (95 % CI 1·19, 1·41; P < 0·001) for cardiovascular mortality and 3·09 (95 % CI 2·11, 4·52; P < 0·001) and 1·49 (95 % CI 1·33, 1·68; P < 0·001) for non-cardiovascular mortality. HR for total and cardiovascular mortality remained highly significant even after adjustment for several possible confounders. In the analyses of cardiovascular mortality, significance was lost when we compared the first with the fourth zinc quartile in the fully adjusted model (HR: 1·24 (95 % CI 0·92, 1·66); P = 0·162), but remained significant for decreasing zinc quartiles as a continuous variable (HR: 1·10 (95 % CI 1·01, 1·21; P = 0·038).
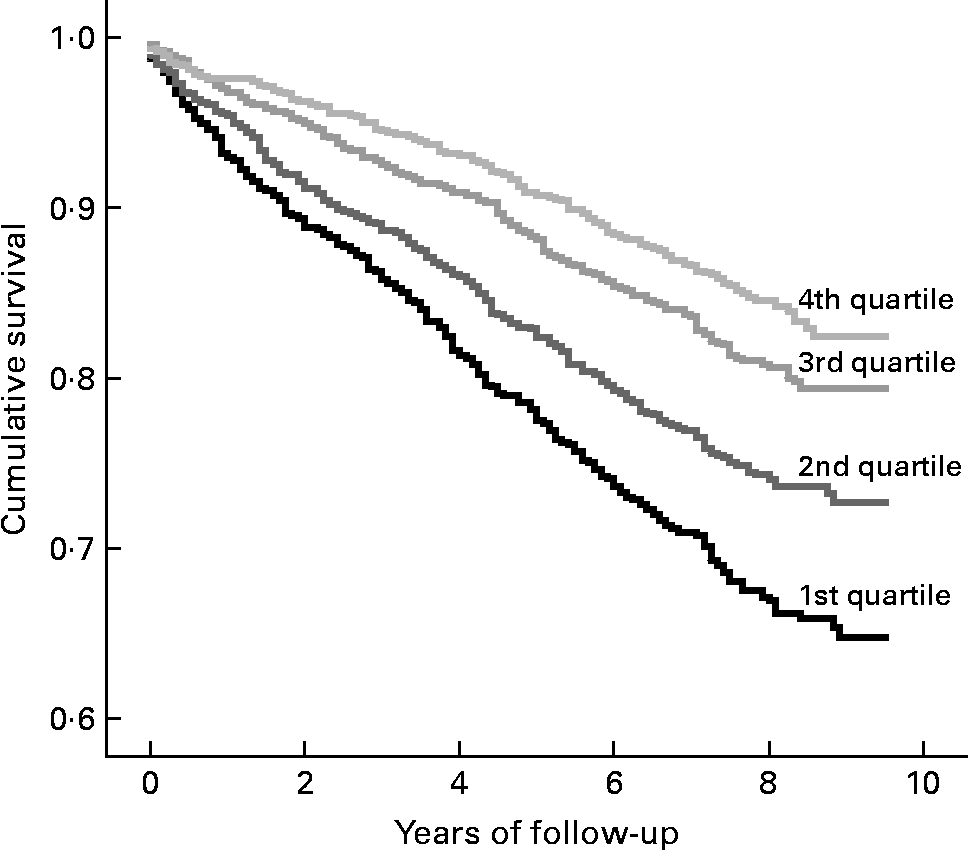
Fig. 1 Kaplan–Meier curve according to serum zinc quartiles for total mortality.
Table 2 Hazard ratios (HR) with 95 % CI for total, cardiovascular and non-cardiovascular mortality according to quartiles of serum zinc concentrations

* Model 1: unadjusted.
† Model 2: adjusted for age.
‡ Model 3: adjusted for age, sex, BMI, HbA1c, systemic hypertension, active smokers (yes/no), HDL- and LDL- cholesterol, TAG, glomerular filtration rate, C-reactive protein, N-terminal pro-B-type natriuretic peptide, copper, albumin, Hb, homocysteine, angiotensin-converting enzyme inhibitors and diuretics (yes/no).
Unadjusted and fully adjusted (according to model 3 in Table 2) HR of individuals with serum zinc concentrations below 700 μg/l (n 379) compared with those in the fourth zinc quartile were 3·12 95 % CI 2·46, 3·95; P < 0·001) and 2·03 (95 % CI 1·54, 2·68, P < 0·001) for total mortality, 2·85 (95 % CI 2·12, 3·84, P < 0·001) and 1·50 (95 % CI 1·04, 2·17, P = 0·030) for cardiovascular mortality and 3·97 (95 % CI 2·60, 6·05; P < 0·001) and 3·22 (95 % CI 1·97, 5·27, P < 0·001) for non-cardiovascular mortality.
In simple and partial correlation analyses with adjustment for age, sex and glomerular filtration rate, serum zinc concentrations were significantly correlated with antioxidants and were inversely correlated with inflammatory parameters (Table 3). Furthermore, we observed a significant negative correlation between age and serum zinc concentrations (unadjusted Pearson's correlation coefficient: − 0·189, P < 0·001).
Table 3 Simple and partial correlation analyses of serum zinc concentrations
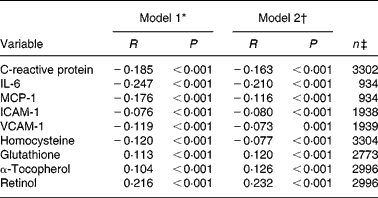
MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cellular adhesion molecule-1.
* Model 1: unadjusted.
† Model 2: adjusted for age, sex and glomerular filtration rate.
‡ Probands with available values for correlation analyses.
Discussion
In this large, well-defined cohort of patients referred to coronary angiography, zinc deficiency at baseline was associated with total, cardiovascular and non-cardiovascular mortality. After careful adjustments for several cardiovascular risk factors and possible confounders related to zinc metabolism, low serum zinc concentrations remained an independent and highly significant predictor for total and non-cardiovascular mortality. The association between zinc deficiency and cardiovascular mortality was attenuated to a marginal level of significance after these adjustments. We further implicated that serum zinc concentrations are negatively correlated with age and inflammatory parameters and positively with antioxidants.
Results from previous studies of the association between serum zinc and mortality in adults are inconclusive(Reference Kok, Van Duijn and Hofman8–Reference Soinio, Marniemi and Laakso13). In a prospective study in patients with type 2 diabetes and in a nested case–control study within a population-based study in men, low serum zinc concentrations were significantly associated with increased cardiovascular mortality, even after controlling for relevant established risk factors(Reference Reunanen, Knekt and Marniemi9, Reference Soinio, Marniemi and Laakso13). These findings are further supported by a study in postmenopausal women who showed an inverse association between dietary zinc intake and cardiovascular mortality(Reference Lee, Folsom and Jacobs23). Other authors of population-based prospective studies reported only about a non-significant trend for a protective effect of high serum zinc levels on total(Reference Leone, Courbon and Ducimetiere12), cardiovascular(Reference Kok, Van Duijn and Hofman8, Reference Leone, Courbon and Ducimetiere12) and cancer mortality(Reference Kok, Van Duijn and Hofman8, Reference Wu, Sempos and Freudenheim11, Reference Leone, Courbon and Ducimetiere12). However, one prospective study among 344 community-living elderly found neither a significant association nor a noteworthy trend between zinc and mortality, but the results of the present study may be limited by the relatively low number of study participants(Reference Marniemi, Järvisalo and Toikka10). The present results extend the present knowledge about the predictive value of serum zinc concentrations because we were the first to address this issue in patients referred to coronary angiography and we were able to adjust our analyses for a variety of possible confounders including, for instance, the use of diuretics and angiotensin-converting enzyme -inhibitors, HbA1c, albumin, Hb, glomerular filtration rate and C-reactive protein, which could not all be considered in previous studies. Interestingly, we noticed that controlling for age reduced HR for the association between zinc and mortality far more than all other covariates taken together (Table 2). This can mainly be attributed to the negative correlation between age and zinc concentrations and supports previous reports that the elderly are at increased risk to develop zinc deficiency(Reference Sandstead, Henriksen and Greger24). Therefore, food choices become very important in the elderly to ensure an adequate intake and bioavailability of zinc(Reference Sandstead, Henriksen and Greger24). Several factors may contribute to the reduced zinc concentrations in the ageing population, involving low dietary intake, malabsorption or comorbidities in particular inflammatory and metabolic diseases(Reference Walsh, Sandstead and Prasad1–Reference Beattie and Kwun5, Reference Sandstead, Henriksen and Greger24). We therefore carefully adjusted our mortality analyses for these possible confounders, in order to reduce the probability that the association between zinc and mortality is only observed due to medical conditions with a secondary decline of serum zinc concentrations, a process that might partially occur due to a redistribution of plasma zinc to other tissues (e.g. in inflammatory diseases)(Reference King25). However, low serum zinc remained a significant predictor of mortality even after these adjustments, suggesting that zinc deficiency may directly contribute to a reduced life expectancy.
The mechanisms underlying the association between zinc and mortality might involve oxidative stress, immune dysfunction and inflammatory processes, which have all been associated with zinc deficiency and may contribute to age-related degenerative diseases such as cancer, infections and atherosclerosis(Reference Walsh, Sandstead and Prasad1–Reference Beattie and Kwun5, Reference Bogden, Oleske and Munves26). Regarding this, it has recently been shown that zinc supplementation in elderly individuals decreased inflammatory cytokines and markers of oxidative stress, and was associated with a reduced incidence of infections(Reference Prasad, Beck and Bao27). The present results are in line with these data by showing a negative correlation between zinc and inflammatory cytokines and a positive correlation of zinc and antioxidants (Table 3).
The association between zinc and CVD appears to be less clear in the present study with a marginal significance for cardiovascular mortality and only a non-significant trend for a higher prevalence of coronary artery disease in patients with low serum zinc concentrations (Table 1). In this context, it has been shown that zinc may prevent early lesion formation in atherosclerosis, in particular by antioxidant effects on endothelial cells(Reference Beattie and Kwun5, Reference Minqin, Watt and Huat28, Reference Hennig, Meerarani and Toborek29). Inconsistent results on the association between zinc and atherosclerosis in advanced stages of CVD might therefore be explained by the fact that zinc may be more important in early phases of atherogenesis(Reference Alissa, Bahjri and Ahmed30–Reference Singh, Niaz and Rastogi33). Towards this, it is important to note that zinc supplementation decreased the development of atherosclerosis in rabbits(Reference Ren, Rajendran and Ning34, Reference Jenner, Ren and Rajendran35).
We believe that our data are in favour of approaches to avoid and treat zinc deficiency in old individuals as a promising target for disease prevention(Reference Maret and Sandstead36). Reviewing the current literature, Maret & Sandstead(Reference Maret and Sandstead36) have concluded that indications for zinc supplementation may override concerns about side effects, in particular, since adverse health effects of zinc intake are mainly attributed to the induction of a secondary copper deficiency that can be prevented by a proportional intake of zinc and copper. Results from the Age-Related Eye Disease study further underline the possible public health benefit of zinc supplementation in the elderly, because in that study multivariate-adjusted total mortality was significantly reduced in patients randomly assigned to receive zinc when compared with patients receiving placebo(Reference Clemons, Kurinij and Sperduto14). Large zinc supplementation trials in the ageing population, as previously performed to reduce infectious diseases in young children of developing countries, which are at high risk for zinc deficiency, are still needed and warranted to establish the public health benefit of the treatment and/or prevention of zinc deficiency in adults(Reference Tielsch, Kathry and Stoltzfus37–Reference Krebs and Hambidge39). Notably, a recent meta-analysis of zinc supplementation trials in children aged over 12 months showed a significant 18 % reduction in total mortality (relative risk 0·82; 95 % CI 0·70, 0·96)(Reference Tielsch, Kathry and Stoltzfus37). Importantly, zinc should be given with other limiting micronutrients to ensure the most efficacious response to zinc supplementation(Reference Sandstead40, Reference Solomons, Ruz and Gibson41).
The present results are limited because serum zinc concentrations may not be the best parameter for whole-body homoeostasis of this mainly intracellularly located trace element, and it may therefore be hypothesised that indices of zinc status reflecting both intracellular zinc levels and zinc function are more likely to mirror the association between zinc deficiency and adverse health outcomes (Reference King25, Reference Thompson42–Reference Feillet-Coudrey, Meunier and Rambeau44). However, moderate long-term zinc supplementation significantly increased and experimental zinc deficiency decreased serum zinc concentrations, although it should be noted that this was observed only in accelerated stages and not as an initial response to low dietary intake of zinc(Reference King25, Reference Thompson42–Reference Feillet-Coudrey, Meunier and Rambeau44). Apart from this, it was shown that plasma zinc is a valid indicator of whole-body zinc status at least in healthy men(Reference Lowe, Woodhouse and Sutherland45), but according to an extensive review of epidemiological zinc data, serum zinc concentrations can be regarded as a valid indicator of zinc status and its response to dietary intervention on a population level rather than on an individual level(Reference Hess, Peerson and King46). Furthermore, it is important for the interpretation of zinc status to consider that serum zinc concentrations are maintained within a fairly narrow range by homoeostatic mechanisms and are only significantly altered under extreme dietary conditions(Reference Hess, Peerson and King46). However, due to changing hormonal activities related to the time of the day, nutrition (zinc decreases after meals), exercise or stress, individual serum zinc concentrations fluctuate by as much as 20 % during a day and it has been shown that ageing, low albumin levels and inflammatory processes are associated with reduced serum zinc concentrations(Reference Hess, Peerson and King46). Thus, we aimed to reduce a possible bias of the present results by these potential influence factors on serum zinc concentrations by performing a standardised fasting blood sampling in the morning and by multivariate adjustments of our analyses, but we cannot exclude residual confounding. Concerning the zinc measurement at our laboratory, we have to acknowledge that we did not perform an accurate long-term storage of the quality control data of our zinc determinations (analysed from 1997 to 2000), and we could therefore not present these data, which can be regarded as a further limitation of the present study results. Another limitation is that we did not pursue a precise classification of non-cardiovascular deaths and that we did not perform a detailed nutritional assessment in the LURIC study. Zinc deficiency remained an independent predictor of mortality after adjustments for some parameters of malnutrition including albumin, BMI and Hb, but we cannot completely rule out that low zinc levels are only indicators of a general malnutrition or specific nutritional inadequacies other than zinc deficiency which contribute to the increased mortality(Reference Alberda, Graf and McCargar47). This is, however, very unlikely because of the high prevalence of zinc deficiency compared with other micronutrient deficiencies, except of iron.
In summary, the present results show that low serum zinc concentrations predict mortality in patients scheduled for coronary angiography and thus support considerations for supplementation of zinc plus other micronutrients in ageing individuals with a deficiency for this essential trace element.
Acknowledgements
The LURIC study was funded by grants from the Deutsche Forschungsgemeinschaft (GRK 1041, SFB 518 and Exzellenzzentrum Stoffwechselforschung Baden-Würtemberg). The authors thank the LURIC study team either temporarily or permanently involved in patient recruitment and sample and data handling and the laboratory staff at the Ludwigshafen General Hospital and the Universities of Freiburg, Ulm and Graz, and the German registration offices and local public health departments for their assistance. None of the authors had any personal or financial conflict of interest. B. M. W. R., B. O. B. and W. M. were responsible for the conception and design of the LURIC study. All the authors were responsible for collecting and/or organising the data. S. P. was responsible for statistical analyses and drafting of the manuscript. S. P., H. D., B. M. W. R., B. O. B. and W. M. were responsible for interpreting the data. All authors were responsible for revising the manuscript.






