Low serum enterolactone concentration is an independent risk factor of acute coronary events and death for CHD and CVD in men( Reference Vanharanta, Voutilainen and Lakka 1 , Reference Vanharanta, Voutilainen and Rissanen 2 ). The inhibition of lipid peroxidation( Reference Kitts, Yuan and Wijewickreme 3 , Reference Vanharanta, Voutilainen and Nurmi 4 ), up-regulation of hepatic LDL receptor activity( Reference Owen, Roach and Abbey 5 ), inhibition of lipoprotein uptake by macrophages( Reference Owen and Abbey 6 ) and oestrogen-like effect( Reference Penttinen, Jaehrling and Damdimopoulos 7 ) are the proposed mechanisms behind the protective effect of enterolactone. Enterolactone is produced by intestinal metabolism of food lignans such as matairesinol, secoisolariciresinol, pinoresinol and lariciresinol( Reference Borriello, Setchell and Axelson 8 – Reference Peterson, Dwyer and Adlercreutz 10 ), non-energetic, phenolic plant compounds abundantly found in whole-grain cereals, vegetables and some fruits and berries( Reference Peterson, Dwyer and Adlercreutz 10 , Reference Adlercreutz 11 ). Enterolactone is the main circulating product of intestinal lignan metabolism( Reference Peterson, Dwyer and Adlercreutz 10 ) and has been proposed to be more potent in terms of biological properties than the parent compounds( Reference Clavel, Doré and Blaut 12 ). Although it is possible that some lignan-metabolising enzymes are present in the intestinal brush border( Reference Rowland, Faughnan and Hoey 13 , Reference Clavel, Henderson and Engst 14 ), intestinal microbiota, especially those in the colon, are mainly responsible for the metabolism of lignans and are major determinants of serum enterolactone concentration( Reference Clavel, Doré and Blaut 12 , Reference Kilkkinen, Pietinen and Klaukka 15 ). Enterolactone formation requires a network of sequential reactions involving several species, most of which are common members of dominant bacterial groups in the human intestine( Reference Clavel, Doré and Blaut 12 ). For enterolactone formation to take place, there need to be sufficient amounts of both lignan and bacteria capable of forming enterolactone.
The serum concentration of high-sensitivity C-reactive protein (CRP), a predictor of cardiovascular events among healthy individuals( Reference Ridker 16 ), has been reported in healthy participants to be inversely correlated with the total faecal microbial counts( Reference Tiihonen, Ouwehand and Rautonen 17 ) and bacterial populations with high DNA guanine+cytosine contents( Reference Brignardello, Morales and Diaz 18 ). Furthermore, reductions in CRP concentration have been associated with increases in the bacterial counts of lactobacilli and bifidobacteria, but not with those in the counts of any other bacteria, in the faeces of healthy humans( Reference Tzounis, Rodriguez-Mateos and Vulevic 19 ). Intestinal microbes can induce a cascade of immunological events either through pathogen-associated molecular patterns on their surface recognised by Toll-like receptors in the gut epithelial cells and antigen-presenting cells( Reference Tlaskalová-Hogenová, Stepánková and Hudcovic 20 ) or through products of their metabolism, such as SCFA( Reference Sanderson 21 , Reference Seifert and Watzl 22 ).
In the present study, we compared faecal microbiota between men with the lowest serum enterolactone concentration and those with the highest concentration at recruitment and carried out a randomised, double-blind intervention study with a cross-over design to investigate whether a synbiotic mixture, consisting of a specific probiotic combination (two Lactobacillus strains, one Bifidobacterium strain and one Propionibacterium strain) and prebiotic galacto-oligosaccharides (GOS), could elevate serum enterolactone concentration in men who have a low serum enterolactone concentration. The effect of the synbiotic mixture on serum sensitive C-reactive protein (sCRP) concentration was also investigated. The consumption of lignan-rich foods was monitored during the intervention periods using a FFQ. This particular synbiotic combination was chosen on the basis of our previous open-label study with sequential 2-week interventions( Reference Kekkonen, Holma and Hatakka 23 ), which suggested that in a subgroup of men with a serum enterolactone concentration < 20 nmol/l at run-in, the mean serum enterolactone concentration was doubled at the end of the intervention periods with this combination. In men with a serum enterolactone concentration ≥ 20 nmol/l at run-in, no change in the mean serum enterolactone concentration was observed. Therefore, we hypothesised that this synbiotic combination would increase serum enterolactone concentration in men with a value < 20 nmol/l when given for a longer period of time. To our knowledge, there are no other studies on the effects of probiotics, prebiotics or synbiotics on serum enterolactone concentration.
Materials and methods
Participants
Men, aged 20–65 years, were recruited from the personnel of four industrial companies in Southern Finland by an advertisement. The serum enterolactone concentration of eighty-four volunteers was determined at recruitment. In part I of the study, ‘Cross-sectional comparison of faecal microbiota in groups with low and high serum enterolactone concentrations’, ten men with the lowest serum enterolactone concentration and ten men with the highest concentration were selected (Table 1). In part II, ‘Cross-over intervention study’, those who had a serum enterolactone concentration < 20 nmol/l, in total fifty-six of the eighty-four volunteers, were selected. Among them, four withdrew from the study before it begun, resulting in fifty-two participants in part II (Table 1). Before recruitment into the study, the participants of both parts were interviewed for illness, medication, diet, smoking and alcohol use. Exclusion criterion was antibiotic treatment for 2 months before the interventions. All the participants consumed an omnivorous diet, except for one, who consumed a lacto-vegetarian diet. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa. Written informed consent was obtained from all the subjects.
Table 1 Characteristics of the study participants at recruitment in part I, ‘Cross-sectional comparison of faecal microbiota in groups with high and low enterolactone concentrations’ and in part II, ‘Cross-over intervention study’ (Mean values and ranges)
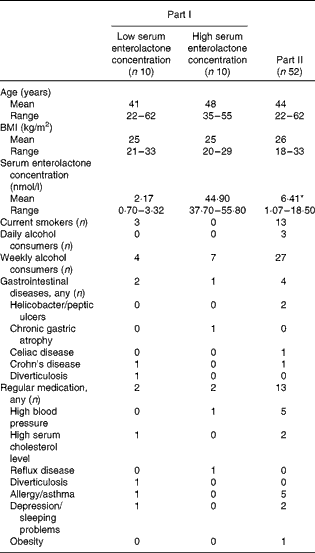
* Geometric mean.
Interventions
The intervention study was a randomised, double-blind, placebo-controlled, two-period cross-over trial with 6-week treatment periods and a 4-week washout period. Group A consumed one bacterial capsule together with 0·1 litres of GOS juice (a three-fruit juice (grape, orange and passion fruit), GOS 32 g/l) daily during the first treatment period, followed by consumption of placebo capsules and a placebo juice (fruit juice without GOS) during the second treatment period, and group B received the treatments in the reverse order. The participants were randomised into group A or group B according to a computer-generated random permuted-block method and a block size of four subjects. The participants were instructed to take the capsules and the juice once daily with the first meal of the day.
Bacterial capsules contained a probiotic mixture of two Lactobacillus rhamnosus strains, GG (ATCC 53 103, 5·9 × 1010 colony-forming units (CFU)/l) and LC705 (DSM7061, 1·3 × 1011 CFU/l), one Bifidobacterium strain, Bifidobacterium breve Bb99 (DSM 13 692, 5·7 × 109 CFU/l), and one Propionibacterium strain, Propionibacterium freudenreichii ssp. shermanii JS (DSM7067, 1 × 1011 CFU/l). In addition to the bacteria, the capsules contained microcrystalline cellulose and gelatine as a filler and were lactose free. The placebo capsules were of identical composition but without the bacteria.
Before the start of the intervention study, the participants were instructed not to consume products containing any lactic acid bacteria considered probiotic, including certain type of cheeses containing the investigated probiotics L. rhamnosus LC705 and P. freudenreichii ssp. shermanii JS (Emmental and Polar), seeds and nuts during the whole study period starting 4 weeks before the start of the study. The participants were instructed not to change their ordinary diet and habits in any other way.
Questionnaires
At recruitment, a FFQ was used to gain information about the participants' dietary habits during the previous 2 months. There were six consumption frequency options: not once; one to three times a month; one to two times a week; three to five times a week; every day or almost every day; several times a day. The participants were asked to fill in the portion size in numbers. The questionnaire included questions on a total of fifty-six foods and food groups. In part II, more detailed FFQ were administered after both treatment periods in order to monitor the consumption of lignan-rich foods, such as cereals, fruits, berries, vegetables, nuts and seeds, and of products containing probiotic bacteria during the study. The questions concerned foods consumed during the previous 4 weeks, and there were three consumption frequency options: X times a day/week/month. The participants were asked to fill in the frequency in numbers in only one frequency option. They were asked to fill in the portion size in numbers. The questionnaire included a total of eighty questions. In part II, the participants were asked about the use of antibiotics after each treatment period. After the second treatment period, the participants filled in a questionnaire about their gastrointestinal symptoms (stomach ache, abdominal distension, flatulence, heartburn, loose stools and hard stools: 0 = no symptoms; 1 = a few; 2 = moderately; 3 = a lot) during that period and also in their everyday life.
Blood and faecal samples
Blood samples for enterolactone analyses were collected at recruitment and four times during the experiment in part II, at the beginning and end of both the treatment periods. Serum was separated from the blood samples not more than 2 h after sampling. The samples were centrifuged and stored at − 20°C until analysis. Enterolactone analyses were carried out using a time-resolved fluoroimmunoassay( Reference Adlercreutz, Wang and Lapcik 24 , Reference Stumpf, Uehara and Nurmi 25 ). Serum sCRP concentration was determined in part II at the beginning and end of the first treatment period using a highly sensitive immunoturbidimetric assay (Tina-quant CRP high-sensitive assay reagent, Roche Hitachi 912 analyser; Roche Diagnostics GmbH).
Faecal samples for the microbiological analysis were collected at recruitment to study the differences in faecal microbiota in part I between men with the lowest serum enterolactone concentration (n 10) and those with the highest concentration (n 10). Men were instructed to freeze the faecal samples immediately and to keep them frozen until taken in a cooler with ice packs to the study centre, where they were immediately stored at − 70°C until analysis.
Fluorescence in situ hybridisation analysis of faecal samples was carried out as described previously( Reference Myllyluoma, Ahlroos and Veijola 26 ). Bacterial cells were counted visually with an epifluorescence microscope, examining a minimum of fifteen fields in each sample. DAPI (4′,6-diamidino-2-phenylindole) dye was used for counting total bacteria. Oligonucleotide probes used were Cy3-labelled Bfra602 (Bacteroides fragilis group), Bdis656 (Bacteroides distasonis), Erec482 (Eubacterium rectale–Clostridium coccoides group), Bif164 (bifidobacteria), Chis150 (Clostridium histolyticum) and Lab158 (lactobacilli and enterococci).
For bacterial counts analysed by cultivation, faecal samples were homogenised in 1:10 with the Wilkins–Chalgren broth (Oxoid Limited) in an anaerobic chamber. Subsequently, tenfold serial dilutions were plated on appropriate agars. Total anaerobes were cultivated anaerobically on Brain–Heart Infusion Agar (Oxoid) at 37°C for 5 d and total aerobes on the same agar aerobically for 3 d. Total lactobacilli were cultivated on De Man Rogosa Sharpe (MRS) agar for 3 d, bifidobacteria on Raffinose agar for 2 d and L. rhamnosus GG as well as Lactobacillus 705 on MRS Agar supplemented with vancomycin for 3 d, all anaerobically at 37°C, as described previously( Reference Kekkonen, Holma and Hatakka 23 ). B. fragilis was cultivated anaerobically on Bacteroides Bile Esculin Agar at 37°C for 2–4 d. Sulphite-reducing clostridia were cultivated anaerobically on Ferrous Sulphite Agar at 37°C for 1–2 d.
Statistical analysis
Part I. ‘Cross-sectional comparison of faecal microbiota in groups with low and high serum enterolactone concentrations’
Faecal bacterial plate and fluorescence in situ hybridisation counts were the primary variables. They were logarithmically (log10) transformed and are expressed as log10 CFU/g. The Mann–Whitney U test was used as a primary analysis to compare the groups of men with low and high serum enterolactone concentrations. ANCOVA was used as a secondary analysis in order to control the possible effect of the intake of lignan-rich foods. Based on the consumption of twenty lignan-rich foods given by FFQ, two summary statistics were derived. First, the total number of lignan-rich foods consumed was calculated. In addition, the total consumption was roughly estimated (consumption frequency × portion size). These two variables were included as covariates in separate ANCOVA models, in which the bacterial counts as log10 CFU/g were compared between groups with a high serum enterolactone concentration and those with a low concentration.
Part II. ‘Cross-over intervention study’
All data were analysed based on the intention-to-treat population, which included all fifty-two randomised men who were enrolled in the study and had at least one follow-up measurement of serum enterolactone or sCRP concentration. In this population, three men were on an antibiotic course during the second treatment period and discontinued the study. Their serum enterolactone and sCRP values at the end of the second treatment period were set as missing values. Serum enterolactone and sCRP concentrations were the primary variables. As the distributions were skewed to the right, they were logarithmically (loge) transformed before analysis. Serum enterolactone concentration was measured at the beginning and end of treatment periods 1 and 2. The repeated-measures ANOVA for cross-over designs was used to analyse the concentrations at the end of the treatment periods and to analyse the within-period changes. The results are given as ratios (synbiotic:placebo) and mean difference (synbiotic − placebo) with 95 % CI, respectively. The within-period changes (enterolactone concentration at the end of the treatment period minus that at the beginning of the treatment period) were calculated using untransformed values. The period and carry-over effects were non-significant for both analyses.
Serum sCRP concentration was measured at the end and beginning of treatment period 1. The synbiotic and placebo groups were compared at the end of the treatment period using ANCOVA in which the value at the beginning was included as a covariate. The result is given as a ratio (synbiotic:placebo) with 95 % CI. In addition, the absolute within-period changes were analysed using ANOVA.
The percentage of users of different lignan-rich foods and the number of weekly servings were compared between treatment periods 1 and 2 in order to assess the stability of diet during the study period. Changes in the percentage of users and the number of weekly servings were analysed using the McNemar test and Wilcoxon signed-rank test, respectively.
Gastrointestinal symptoms (scores 0–3) during everyday life and during treatment period 2 were assessed. Logistic regression analysis was used to compare the treatment groups with respect to any grade (1–3) of symptoms during treatment. The ordinary symptoms of any grade were included as a categorical covariate. In addition, score (0–21) of the sum of symptoms = stomach ache+abdominal distension+flatulence+heartburn+diarrhoea+loose stools+hard stools) was calculated. The treatment groups were compared using ANOVA and ANCOVA, in which the sum of symptom scores during everyday life was included as a continuous covariate.
All tests were carried out as two sided, and P values < 0·05 were considered statistically significant. Statistical analysis was carried out using IBM SPSS version 20.0 (SPSS, Inc.).
Results
Part I
Faecal bacterial counts in participants with low and high serum enterolactone concentrations
The mean (range) serum enterolactone concentrations of the ten lowest and ten highest concentrations at recruitment were 2·17 (0·70–3·32) and 44·90 (37·70–55·80) nmol/l, respectively (Table 1). Faecal bacterial counts of these two groups are given in Table 2. The participants with a low serum enterolactone concentration had significantly less total bacteria, both aerobic and anaerobic, in faeces than those with a high serum enterolactone concentration. The difference in medians 6·2 v. 7·7 in the counts of aerobic bacteria in the groups with low and high serum enterolactone concentrations indicated approximately thirtyfold difference in the medians of untransformed bacterial counts. Similarly, the difference in medians 8·4 v. 9·2 in the counts of anaerobic bacteria indicated more than sixfold difference in untransformed bacterial counts. The Lactobacillus–Enterococcus group was the only one that was found to have significantly different counts between the two participant groups, with approximately threefold larger medians of untransformed bacterial counts in those with a high serum enterolactone concentration when compared with those with a low concentration. In the low-enterolactone concentration group, one participant had Crohn's disease and one had diverticulosis, and in the high-enterolactone concentration group, one participant had chronic gastric atrophy (Table 1). Their exclusion from the analysis did not affect the results obtained, so the results are presented for all the participants. None of these twenty men reported constipation.
Table 2 Faecal bacterial plate and fluorescence in situ hybridisation (FISH) counts in groups with low and high serum enterolactone concentrations at recruitment in part I, ‘Cross-sectional comparison of faecal microbiota in groups with high and low enterolactone concentrations’ (Medians and interquartile ranges (IQR))

CFU, colony-forming units; Lactobacillus LC705, Lactobacillus rhamnosus LC705; Lactobacillus GG, Lactobacillus rhamnosus GG.
* Mann–Whitney U test.
Even when the results were adjusted in separate models for the total number of lignan-rich foods consumed and the total consumption of lignan-rich foods during the previous 2 months, the total bacterial counts and the anaerobic bacterial counts were significantly higher in the group with a high serum enterolactone concentration (P= 0·001 and 0·002 for total bacteria; P= 0·012 and 0·016 for anaerobic bacteria, respectively). The counts of the Lactobacillus–Enterococcus group were also significantly higher in the group with a high serum enterolactone concentration when the results were adjusted for the number of lignan-rich foods consumed (P= 0·029). However, when the consumption of lignan-rich foods was included as a covariate, the difference between these groups was non-significant (P= 0·124), although the adjusted means of 8·7 v. 8·3 in the log scale indicated a 2·5-fold difference in the original counts. The aerobic bacterial counts were only marginally significantly higher in the group with a high serum enterolactone concentration when the results were adjusted for the number of lignan-rich foods consumed and for the consumption of lignan-rich foods (P= 0·056 and 0·086). After adjustment for the number of lignan-rich foods consumed, the adjusted means 7·6 v. 6·5 in the log scale indicated more than tenfold difference in the original counts. Similarly, after adjustment for the total consumption of lignan-rich foods, the adjusted means 7·7 v. 6·5 indicated more than tenfold difference.
Part II
Compliance
During the second treatment period, three men were withdrawn from the study because of the use of antibiotics (two during placebo treatment and one during synbiotic treatment). Thus, forty-nine men completed the study. None of them used antibiotics during the study period. After treatment period 2, one of them failed to reply to the FFQ and gastrointestinal symptom questionnaire. Compliance with the interventions was good, with only five men being reported as acting contrary to the instructions given at some point during the study period. Mostly, these participants did not comply with the instructions for 1–2 d during the 6-week periods, but during the intervention periods, one participant did not consume the juice with GOS for ten consecutive days and one participant for three consecutive days. In addition, one participant did not consume the juice with GOS or the capsules for five consecutive days during the intervention periods. Instructions to avoid the consumption of certain products were more often disobeyed, but concerned mostly one to two incidents during the 6-week intervention periods. The consumption of products contrary to instructions was approximately the same during the two interventions. However, during placebo treatment, one participant consumed L. rhamnosus GG-containing sour milk every day and one participant Lactobacillus reuteri-containing yogurt once weekly. The exclusion of these two men from the analysis of main variables did not change the results, so the results are presented for all the participants. No other products containing probiotics were reported. During placebo treatment, one person reported to have lost 7 kg weight.
Dietary habits
There were no significant differences in the percentage of users of different lignan-rich foods between treatment periods 1 and 2 (Table 3). The users of lignan-rich foods or food groups remained the same in at least 70 % of the study population between treatment periods 1 and 2. No significant differences were detected in the weekly servings of different lignan-rich foods either, except for beer, the consumption of which was slightly higher during treatment period 1 than during treatment period 2 (median 0·75 v. 0·53 litres weekly, respectively, P= 0·004).
Table 3 Users and number of servings of different lignan-rich foods during treatment periods 1 and 2 according to a FFQ in part II, ‘Cross-over intervention study’ (Medians and interquartile ranges (IQR))
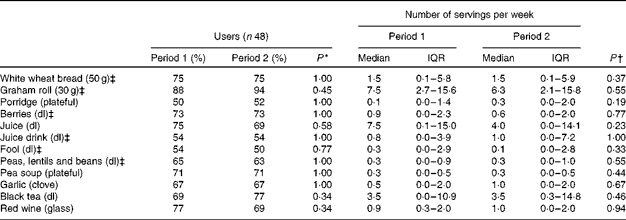
* McNemar's test.
† Wilcoxon signed-rank test.
‡ Combined variable: white wheat bread = white wheat bread or roll; graham roll = graham bread or roll, yeast bread or mixed-grain bread; juice drink = homemade or commercial juice drink; fool = berry soup or fool, or fruit fool.
Foods whose consumers were at least 90 % the same individuals when comparing the two treatment periods are not reported in Table 3. These were rye bread, fruits, vegetables, potatoes, onions and beer. Foods that were used by an insufficient number of users to conduct statistical tests of the number of weekly servings are not reported in Table 3 either. These were rye bran, any bran (rye, oat or wheat), muesli, breakfast cereals, berry porridge, berry quark, berry jam, dried fruits, pickles, nuts, almonds, seeds (linseeds, sesame seeds or sunflower seeds), germs, soya flour, green tea, black tea, whiskey and white wine. Their consumers were at least 70 % the same individuals when comparing the two treatment periods, and no significant differences were found.
Gastrointestinal symptoms
The occurrence of gastrointestinal symptoms (stomach ache, abdominal distension, flatulence, heartburn, loose stools and hard stools) during treatment period 2 (n 25 given synbiotics; n 23 given placebo) was not significantly different between the two treatment groups (data not shown). The differences between the treatment groups were not significant when the symptoms in their everyday life were or were not included as a covariate.
Serum enterolactone and sensitive C-reactive protein concentrations
Synbiotic treatment did not have a significant effect on serum enterolactone concentration (Table 4; Fig. 1). There was no significant difference in serum sCRP concentration between synbiotic and placebo treatment groups (Table 4).
Table 4 Serum enterolactone and sensitive C-reactive protein (sCRP) concentrations* in part II, ‘Cross-over intervention study’ (Geometric means for concentrations and means for changes and 95 % confidence intervals)
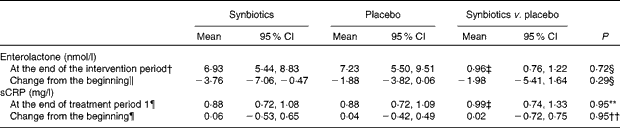
* Enterolactone and sCRP concentrations were logarithmically transformed before analysis.
† Only those whose enterolactone concentrations were analysed at the end of both the treatment periods were included (n 47).
‡ Values are ratios.
§ ANOVA for repeated measures.
∥ Only those whose enterolactone concentrations were analysed at the beginning and end of both the treatment periods were included (n 40).
¶ Only those whose sCRP concentrations were analysed both at the beginning and end of period 1 were included (synbiotics n 23; placebo n 22).
** ANCOVA, where the value at the beginning was included as a covariate. Results are given as adjusted geometric means and 95 % CI.
†† ANOVA.

Fig. 1 Serum enterolactone concentration in part II, ‘Cross-over intervention study’. Group A (○) received synbiotics during treatment period 1 and placebo during treatment period 2 (n 24) and group B (●) received placebo during treatment period 1 and synbiotics during treatment period 2 (n 25). The dotted line indicates the washout period. Values are geometric means, with 95 % CI represented by vertical bars.
Discussion
The aims of the present study were to compare faecal microbiota between men with a low serum enterolactone concentration and those with a high concentration and to investigate whether a specific synbiotic mixture affects serum enterolactone and sCRP concentrations in men who have a low serum enterolactone concentration. The total counts of bacteria and those belonging to the Lactobacillus–Enterococcus group in faeces were significantly lower in participants with a low serum enterolactone concentration than in those with a high concentration. However, the particular synbiotic mixture used in the present study had no significant effect on serum enterolactone and sCRP concentrations. The consumption of lignan-rich foods appeared to remain stable during the intervention periods.
To our knowledge, this is the first study to compare faecal microbiota between men with a high serum enterolactone concentration and those with a low concentration. The faecal counts of anaerobic and aerobic bacteria were approximately six and thirty times higher, respectively, in those with a high serum enterolactone concentration than in those with a low concentration. This is not surprising since enterolactone is produced by intestinal microbiota( Reference Borriello, Setchell and Axelson 8 – Reference Peterson, Dwyer and Adlercreutz 10 ) and oral antimicrobial use decreases serum enterolactone concentration according to an epidemiological study( Reference Kilkkinen, Pietinen and Klaukka 15 ). The Lactobacillus–Enterococcus group was the only one to exhibit a significant difference between the two participant groups in the present study. It was found approximately three times more in men with a high serum enterolactone concentration than in those with a low concentration. Enterococcus faecalis strain PDG-1, a strain isolated from human faeces, has been reported to be highly capable of transforming pinoresinol to lariciresinol, the first step in the synthesis of enterolactone from pinoresinol( Reference Xie, Akao and Hamasaki 27 ). Lactobacilli, on the other hand, have not been reported to be involved in enterolactone formation. However, human faecal lactobacilli, as well as enterococci, have β-glucosidase activity( Reference Mroczyńska and Libudzisz 28 ) and, therefore, have the potential to hydrolyse plant lignans that are glucosides( Reference Clavel, Henderson and Engst 14 , Reference Xie, Akao and Hamasaki 27 ). Since plating results did not show differences in the counts of lactobacilli, enterococci might be responsible for the difference in fluorescence in situ hybridisation counts of the Lactobacillus–Enterococcus group between men with a high serum enterolactone concentration and those with a low concentration.
It is possible that high counts of the Lactobacillus–Enterococcus group are not involved in enterolactone production, but merely reflect a characteristic that is also associated with a high serum enterolactone concentration such as a certain type of diet or bowel habits. Whole-grain wheat, for instance, when compared with wheat bran, has been reported to increase faecal counts of lactobacilli, as well as bifidobacteria( Reference Costabile, Klinder and Fava 29 ), and is also a dietary source of enterolactone precursors( Reference Adlercreutz 11 ). Constipation is associated with both increased serum enterolactone levels( Reference Kilkkinen, Stumpf and Pietinen 30 ) and alterations in the colonic microbiota( Reference Khalif, Quigley and Konovitch 31 ). None of the men in the present study reported constipation, ruling out the possibility of it being a major explanatory factor for the differences in faecal microbiota between the two groups.
The lack of an effect of the synbiotics on serum enterolactone concentration in the present intervention study is in agreement with a similar result obtained in an earlier synbiotic intervention study in postmenopausal women with or without a history of breast cancer( Reference Nettleton, Greany and Thomas 32 ). Our recent results obtained in an open-label study also showed that a synbiotic combination similar to the one used in the present study does not significantly affect serum enterolactone concentration during a short intervention period in men with a normal serum enterolactone concentration( Reference Kekkonen, Holma and Hatakka 23 ). In the previous study, the faecal counts of total lactic acid bacteria were not significantly increased during the consumption of this synbiotic mixture, although the counts of both the administered strains of lactobacilli were (as well as of total bifidobacteria and propionibacteria). It can, therefore, be speculated that the synbiotic mixture used in the present study did not increase the faecal lactobacilli counts in total, although modification at the strain level most probably occurred. Faecal bacteria were not assessed at the end of the intervention periods, which can be considered as a limitation of the present study. The administered probiotics, however, most probably survived through the gastrointestinal tract, since each of these probiotic bacteria has previously been detected in the faeces after administration of capsules similar to those used in the present study (without GOS) with metabolic effects in the colon( Reference Kajander, Krogius-kurikka and Rinttilä 33 ).
Previous studies have shown that most of the increase in serum enterolactone concentration occurs during the first 2–6 weeks of the intervention period with a lignan-rich diet, although it continues to increase until 12 weeks( Reference Stumpf, Pietinen and Puska 34 , Reference Jacobs, Pereira and Stumpf 35 ). This suggests that the intervention of 6 weeks was probably long enough to detect the effect of the synbiotic intervention.
The results of the present study that the synbiotic mixture used had no significant effect on serum sCRP concentration are similar to those of a recent intervention using another synbiotic combination (three Lactobacillus strains, one Bifidobacterium strain, Streptococcus thermophilus and fructo-oligosaccharides) in healthy adults( Reference Nova, Viadel and Wärnberg 36 ). A synbiotic mixture the same as that used in the present study has increased serum CRP concentration in allergy-prone infants( Reference Marschan, Kuitunen and Kukkonen 37 ), but a similar probiotic combination, with the only difference being in the Bifidobacterium strain used, without GOS did not affect serum CRP concentration in irritable bowel syndrome( Reference Kajander, Myllyluoma and Rajilić-Stojanović 38 ). Serum sCRP in adults without immunological disturbances is possibly resistant to modifications of intestinal microbiota by probiotics.
In conclusion, men with a low serum enterolactone concentration have less faecal bacteria, especially those belonging to the Lactobacillus–Enterococcus group, than those with a high concentration. However, the synbiotic mixture, composed of two strains of lactobacilli and one strain of bifidobacteria and propionibacteria together with GOS, did not significantly affect serum enterolactone concentration in men with a low serum enterolactone concentration. This suggests that these strains participate in enterolactone synthesis, but do not colonise in sufficient amounts to have an impact, do not provoke a major modification in the resident microbiota participating in enterolactone synthesis, or simply are not taking part in enterolactone formation.
Acknowledgements
The present study was supported by Valio Limited, Helsinki, Finland, and the Finnish Funding Agency of Technology and Innovation. The authors' contributions are as follows: R. H. and R. A. K. analysed the data and wrote the paper; R. H. had primary responsibility for the final content; R. K., K. H., H. A., R. A. K. and H. V. designed and conducted the research; T. P. conducted the statistical analysis; R. K. directed the research. All authors read and approved the final manuscript. R. K. is Professor of Medical Nutrition Physiology at the Institute of Biomedicine, University of Helsinki, and a former employee of Valio Research Centre, Helsinki, Finland. R. A. K. and K. H. are employees of Valio Research Centre. R. H. and T. P. received compensation from Valio Research Centre. H. V. and H. A. have no conflicts of interest.







