The observation that foods and food groups cluster within different cultures such as Mediterranean, Western and Asian cultures has led to the development of dietary pattern analysis, which affords a much better opportunity than that provided by a single-nutrient or -food approach to study the synergistic effects of diet. Pattern analyses are frequently being used now for studying the relationships between diet and risk factors or diseases( Reference Iqbal, Anand and Ounpuu 1 – Reference Williams, Prevost and Whichelow 3 ). Methods employed for patterning include the following: defining dietary patterns using current nutrition knowledge (dietary indices and dietary scores) and using multivariate techniques (principal component analysis (PCA), factor analysis and cluster analysis) and reduced rank regression( Reference Hoffmann, Schulze and Schieenkiewwitz 4 – Reference Schoenaker, Dobson and Soedamah-Muthu 7 ). The relationship between food synergy and chronic disease is a well-established concept, and the same argument could apply to the synergistic effects of other lifestyle factors( Reference Hu, Rimm and Stampfer 8 , Reference Garduno-Diaz and Khokhar 9 ).
Independent effects of risk factors on chronic disease outcomes have been studied in detail, with most studies looking at one or a few risk factors while controlling for the effects of other variables( Reference Iqbal, Anand and Ounpuu 1 , Reference He, Li and Lai 10 – Reference Bankoski, Harris and McClain 12 ). By studying the association between dietary patterns and type 2 diabetes risk in a Japanese population, Nanri et al. ( Reference Nanri, Mizoue and Yoshida 13 ) concluded that although a small protective effect of the prudent dietary pattern could not be excluded, dietary patterns were not appreciably associated with type 2 diabetes risk. However, in a large nationally representative Chinese study, associations between a healthy dietary pattern and parameters of active lifestyle were found to be beneficial for preventing the onset of the metabolic syndrome, while a ‘Western/new affluence’ dietary pattern characterised by higher consumption of beef/lamb, fruits, eggs, poultry and seafood was found to be significantly associated with the metabolic syndrome( Reference He, Li and Lai 10 ). A study among South Asian migrants in the UK did not find a significant association between vegetarianism and the metabolic syndrome( Reference Garduno-Diaz and Khokhar 9 ). Though some of these studies have also examined associations with physical activity, they have not included physical activity in the pattern analysis( Reference Iqbal, Anand and Ounpuu 1 , Reference Villegas, Yang and Gao 14 ).
Non-communicable diseases are multifactorial in nature, and where the risk factors are known, it is no longer useful to study their independent effects on disease outcomes. A shift in focus towards studying the total lifestyle, by including diet and physical activity/inactivity together in the pattern analysis, would lead to a greater understanding of the true multifactorial nature of risk and yield more holistic information. The co-linear associations often observed among different lifestyle risk factors can be used to generate patterns using mathematical techniques, which could explain the variance in diet and physical activity in a population. The only study in this direction has been that of clustering of energy-related behaviour in children through PCA( Reference Gubbels, Kremers and Stafleu 15 ). Our previous analysis of physical activity in urban women has shown that dysglycaemia is prevalent despite high levels of physical activity and low sedentary behaviour, necessitating further study of lifestyle in its entirety( Reference Waidyatilaka, Lanerolle and Wickremasinghe 16 ). PCA has traditionally been used for analysing variables obtained through frequency questionnaires, but conceptually quantification such as the use of food portion sizes and metabolic equivalents for physical activity would allow a better representation of actual real-life patterns.
South Asians appear to have the greatest risk of diabetes mellitus, and the disease is highly prevalent in both residents of Asian countries and migrant Asians( Reference Ramachandran, Ma and Snehalatha 17 ). This demands more information on the lifestyle patterns of Asians. Studying lifestyle patterns among dysglycaemic individuals within a community, especially in those who are unaware of their glycaemic status, would yield vital information regarding the clustering of risk factors associated with dysglycaemia. To our knowledge, there are no published data on pattern analysis incorporating both diet and physical activity among adults. The objective of the present study was to examine the anthropometric and biochemical markers of cardiometabolic risk in relation to lifestyle patterns identified using PCA.
Materials and methods
Study population
The study participants comprised urban women aged 30–45 years living in the Colombo Municipal Council area. Using cluster sampling, 2800 women were screened for dysglycaemia from all the fifty-five Grama Niladhari divisions (the smallest administrative unit) within the Colombo Municipal Council area in Sri Lanka. From each cluster, defined as the Grama Niladhari division, fifty-one women (aged 30–45 years) were randomly selected for screening. All the 272 dysglycaemic women detected through screening who were previously unaware of their glycaemic status and a further 345 normoglycaemic women randomly selected from the entire screened sample with representation from all Grama Niladhari areas were enrolled in a study to validate a questionnaire to detect dysglycaemia to be used by field health workers. Data from all these individuals were used in the present study to determine community lifestyle patterns assuming that the dysglycaemic individuals being unaware of their glycaemic status previously would not have altered their lifestyle patterns before data collection and were representative of the community at large. Women who were pregnant or breast-feeding, having an acute infection, on long-term steroids or reporting significant weight loss within the last 3 months were excluded from screening.
Methodology
Demographic information of the study population and family history was obtained using an interviewer-administered questionnaire. The International Physical Activity Questionnaire (IPAQ) validated for use in Sri Lanka was used to assess time (in min) spent on walking, moderate physical activity and vigorous physical activity on weekdays and weekends( Reference Craig, Marshall and Sjostrom 18 , Reference Arambepola 19 ). Weekly time spent on each activity was calculated by multiplying the frequency (number of days) and duration (min/d) of each activity on a typical day. Values of weekly time obtained for each category were multiplied by their estimated value in metabolic equivalent of tasks and summed up to obtain an overall estimate of physical activity in a week as per IPAQ guidelines( Reference Craig, Marshall and Sjostrom 18 ). All questions referred to the week immediately preceding the interview.
A comprehensive, pretested, content-validated, interviewer-administered FFQ was used to obtain quantitative information regarding the weekly frequency of consumption of common food items (n 96). The participants were asked to report the number of times a food item was consumed per day and the number of days it was consumed per week (one to seven). In addition, for each food item, the amount usually consumed was recorded using a food atlas for estimation of portion sizes. Each colour photograph in the food atlas contained a food item presented in three portion sizes. To record their individual responses, the participants were not only asked to identify the most commonly used portion size per food item from the given sizes, but also given the option to select virtual sizes among the given sizes.
Waist circumference (WC) was measured in the standing position at the end of normal expiration in the horizontal plane at the level of the narrowest point between the lower border of the last palpable rib and the iliac crest, to the nearest 0·1 cm, using a non-stretchable measuring tape (Seca 200). All measurements were taken in duplicate and the mean was calculated. Height was measured to the nearest 0·1 cm using a stadiometer (Seca 225, telescopic height measurement). Weight was measured to the nearest 0·1 kg using a calibrated electronic scale (Seca 813), and BMI was calculated as weight/height2. All measurements were taken by the same trained observer according to the International Society for Advancement of Kinanthropometry protocol( Reference Stewart, Marfell-Jones and Olds 20 ). Venous blood samples were collected under aseptic conditions after an overnight fast (10–12 h). Glycosylated Hb (HbA1c) was assayed by HPLC (NGSP certified and standardised to the Diabetes Control and Complications Trial assay) using a Bio-Rad D-10 analyser (Bio-Rad Laboratories Inc.) and fasting blood glucose by the glucose oxidase (GOD-PAP; glucose oxidase/phenol+aminophenazone) method on a Hitachi 911 analyser (Hitachi Instruments, Inc.) using reagents obtained from Roche Diagnostics. Serum total cholesterol, TAG and HDL-cholesterol (HDL) concentrations were measured using the cholesterol oxidase (CHOD-PAP; cholesterol oxidase/phenol+aminophenazone), GPO-PAP triglyceride and HDL plus 3rd generation assays of Roche Diagnostics on a Hitachi 911 analyser (Hitachi Instruments, Inc.) using reagents obtained from Roche Diagnostics. High-sensitivity C-reactive protein (hs-CRP) was assayed using an IMMULITE high-sensitivity CRP solid-phase enzyme-labelled chemiluminescent assay.
Multi-frequency bioelectrical impedance analysis was carried out using a SFB7 ImpediMed analyser (ImpediMed Limited) and the software version 5.2.4.0. Women were instructed to lie supine on a non-conductive surface. Their hands were kept in a prone and slightly abducted position away from the trunk. Their legs were abducted to a minimum of 20 cm between the two medial malleoli and thighs. Surface electrodes were attached to the hand and foot in accordance with the manufacturer's instructions and connected to the bioelectrical impedance analysis machine. The instrument was routinely checked on each day of data collection. Fat mass percentage (FM%) and fat-free mass percentage (FFM%) values were obtained directly from the bioelectrical impedance analysis instrument.
Statistical analysis
The Statistical Package for the Social Sciences for Windows (version 18.0; SPSS, Inc.) was used for data analysis. Food items listed in the FFQ were grouped into ten food groups (rice and rice flour-based products, wheat flour-based products and tubers, pulses, vegetables and dark green leafy vegetables, fruits, seafood, red meat and poultry, processed meat, snacks, and dairy products) according to their nutritional characteristics. Physical activity in metabolic equivalent of tasks-min was computed by adding walking, vigorous activity and moderate activity metabolic equivalent of tasks-min.
Lifestyle patterns were identified based on the frequency of consumption of each of the ten food groups and physical activity using exploratory factor analysis. The Bartlett test of sphericity and the Kaiser–Meyer–Olkin measure of sampling adequacy were used to assess data adequacy for factor analysis. The factor analysis model was appropriate as the Kaiser–Meyer–Olkin measure of sampling adequacy was >0·6 and the Bartlett test of sphericity showed a P value < 0·05( Reference Rodrigues, Pereira and Cunha 21 ). A correlation matrix was then constructed. PCA was used for the extraction of factors. The model was tested using an eigenvalue of 1·0. Food groups with factor loadings >0·30 and communality >0·3 were retained in the patterns identified.
Independent-samples t test was used to compare the means of factor scores. ANOVA was used to assess the association between independent variables (lifestyle patterns) and dependent variables (WC, FM%, FFM%, BMI, and HbA1c, fasting blood glucose, total cholesterol, TAG, HDL and hs-CRP concentrations).
Ethics
The study protocol was approved by the Ethics Review Committee of the Faculty of Medicine of the University of Colombo, Sri Lanka. Written informed consent was obtained from all participants before screening and recruitment. Women who were dysglycaemic were referred appropriately. All newly diagnosed dysglycaemic women were individually counselled and offered advice on diet and exercise.
Results
The general characteristics of the study participants according to their glycaemic status are given in Table 1. There were no significant differences in age or monthly family income between dysglycaemic and normoglycaemic women.
Table 1 General characteristics of the study population by glycaemic status (Mean values and standard deviations, n 617)
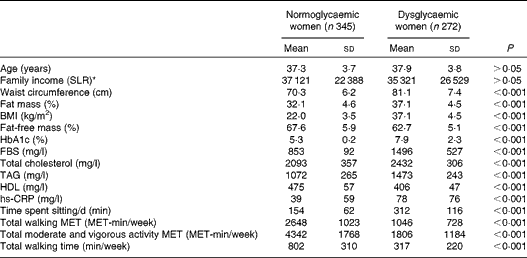
HbA1c, glycosylated Hb; FBS, fasting blood glucose; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalent of tasks.
* SLR = Sri Lankan rupee (1 US $ = 131 SLR).
Dietary pattern analysis
The Kaiser–Meyer–Olkin measure of sampling adequacy (0·679) and the Bartlett test of sphericity (P< 0·001) revealed that the data were adequate for factor analysis. A total of three patterns were identified, and factor loading matrices for the three patterns are given in Table 2. Pattern 1 explained 23·5 % of the model variance and was characterised by positive factor loadings for rice and rice flour-based products, pulses, seafood, fruits, vegetables and green leafy vegetables. Pattern 2 explained 14·0 % of the model variance and was characterised by positive factor loadings for wheat, wheat-based products and tubers, red meat and processed meat. Pattern 3 explained 10·4 % of the model variance and was characterised by positive factor loadings for snacks, dairy products and poultry and a negative factor loading for physical activity. Together, these three patterns explained 47·9 % of the model variance.
Table 2 Factor loadings and communalities estimated for the dietary patterns of urban Sri Lankan women aged 30–45 years (n 617)
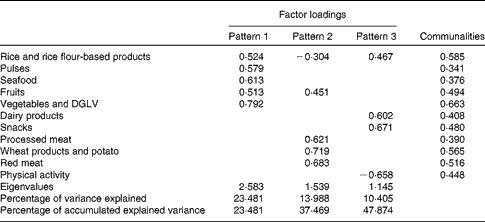
DGLV, dark green leafy vegetables.
The mean factor score for pattern 1 was not significantly different between normoglycaemic and dysglycaemic women. However, the mean factor scores for both pattern 2 (normoglycaemic women − 0·169; dysglycaemic women 0·214) and pattern 3 (normoglycaemic women − 0·481; dysglycaemic women 0·611) were significantly higher (P< 0·001) among dysglycaemic women (Table 3).
Table 3 Mean factor scores for normoglycaemic (n 345) and dysglycaemic (n 272) women
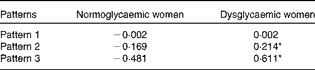
* Values were significantly different between normoglycaemic and dysglycaemic women (P< 0·001).
ANOVA adjusted for family history of diabetes mellitus, education, occupation and monthly family income revealed that for every one-unit increase in the factor score for pattern 2, there was a 1·4 cm increase in WC, a 0·6 % increase in FM%, a 0·6 kg/m2 increase in BMI and a 12 mg/l increase in hs-CRP concentrations (Table 4). For every one-unit increase in the factor score for pattern 2, HDL concentrations decreased by 6 mg/l and FFM% by 0·5 %. For every one-unit increase in the factor score for pattern 3, there was a 3·9 cm increase in WC, a 2·4 % increase in FM% and a 2·0 kg/m2 increase in BMI. For every one-unit increase in the factor score for pattern 3, there was a 0·8 % increase in HbA1c concentrations, a 193 mg/l increase in fasting blood glucose concentrations, a 71 mg/l increase in total cholesterol concentrations, a 96 mg/l increase in TAG concentrations and a 10 mg/l increase in hs-CRP concentrations; HDL concentrations decreased by 17 mg/l and FFM% decreased by 2·3 %. In pattern 1, a one-unit increase in the factor score resulted in a decrease in total cholesterol concentrations by 49 mg/l, TAG concentrations by 31 mg/l and hs-CRP concentrations by 4 mg/l; HDL concentrations increased by 6 mg/l.
Table 4 Effect of lifestyle patterns on cardiometabolic risk variables adjusted for family history of diabetes, education, occupation and monthly family income (Adjusted coefficients and 95% confidence intervals)
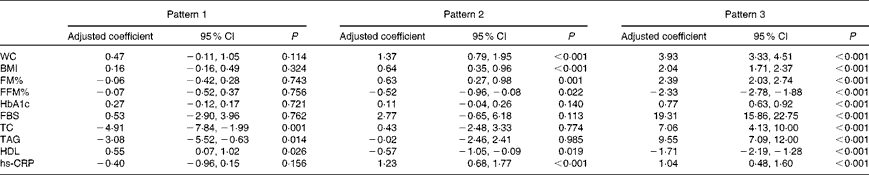
WC, waist circumference; FM%, fat mass percentage; FFM%, fat-free mass percentage; HbA1c, glycosylated Hb; FBS, fasting blood sugar; TC, total cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Discussion
Lifestyle patterning incorporating dietary and physical activity parameters has not been attempted previously in adults. We report the synergistic effects of physical activity/inactivity and dietary patterns and demonstrate the effects of three lifestyle patterns through their relative association with biochemical risk parameters and health outcomes in dysglycaemic and normoglycaemic urban Sri Lankan women. The patterns that we describe explain 48 % of lifestyle pattern variability, far exceeding that explained (only 17 to 35 %) by models developed previously( Reference Dugee, Khor and Lyn 5 , Reference Charreire, Kesse-Guyot and Bertrais 22 ).
Women who were predominantly physically inactive and consumed snacks and dairy products (pattern 3) had the greatest cardiometabolic risk. They had a higher likelihood of having unfavourable obesity indices (increased WC, FM% and BMI and decreased FFM%), glycaemic indices (increased HbA1c and fasting blood glucose concentrations) and lipid profile (increased total cholesterol/TAG concentrations and decreased HDL concentrations) and increased hs-CRP concentrations. Women with higher factor scores for pattern 2 (consuming more wheat, wheat-based products and tubers, red meat and processed meat) only had a higher likelihood of having unfavourable obesity (anthropometry) indices. Physical activity or inactivity did not contribute to pattern 2. However, in a previous analysis of physical activity in this population( Reference Waidyatilaka, Lanerolle and Wickremasinghe 16 ), the mean physical activity has been found to be higher than the recommended energy expenditure on physical activity for health benefits based on global guidelines across the population, which may possibly explain why physical activity did not emerge as a component of pattern 2 or 1.
Pattern 1 appeared to offer only limited protection. Women with higher factor scores for pattern 1 (rice and rice flour-based products, pulses, seafood, fruits, vegetables and green leafy vegetables) did not exhibit significant differences in obesity or glycaemic indices, although they had a more favourable lipid profile and a lower hs-CRP concentration. A possible explanation could be that in a person predominantly consuming food groups belonging to pattern 1, there would also be some contribution from patterns 2 and 3 and vice versa; therefore, the unfavourable response to wheat/potato/processed meat and snacks, sweetened dairy products and inactivity may override the benefits conferred by the food groups belonging to pattern 1.
None of the patterns including pattern 1 clustered with high physical activity. However, lack of activity was significant in pattern 3, indicating that physical inactivity clusters with unhealthy food habits and is associated with dysglycaemia. The advantage of patterning, over other methods of assessing lifestyle, is that it allows us to recognise that although pattern 3 (snacks, dairy products and low physical activity) and to a lesser extent pattern 2 (wheat, wheat-based products and tubers, red meat and processed meat) predispose an individual to risk, this may occur across a background of healthful patterns. Our findings indicate that the synergistic effects of diet and physical activity taken together may behave differently from what would be expected if only food groups are considered in isolation.
We included a disproportionate number of dysglycaemic women in this sample assuming that their lifestyle patterns would not have changed as they were unaware of their dysglycaemic status before the study and at the time of data collection. Hence, it can be assumed that their lifestyle patterns are a true reflection of community patterns. Including a disproportionate number of dysglycaemic women in the present study ensured that the risk factors that we were interested in were prevalent in sufficient numbers for a meaningful analysis. This can be considered a strength of the present study. The FFQ estimation though limited is a necessity for PCA. However, the use of a quantitative FFQ, accounting for foods that are consumed less frequently but in greater quantity and vice versa, is a further advantage in the present study. The limitations of the present study were its cross-sectional design and the inherent subjectivity that results from the empirical decisions made by the investigators in data-driven patterning methods such as PCA. These need to be borne in mind when interpreting the results of the present study.
The present study paves the way for future longitudinal analyses of lifestyle patterns such as those documented in the present study and their effects on disease outcomes. More recently, a few studies( Reference Gubbels, Kremers and Stafleu 15 , Reference Lioret, Touvier and Lafay 23 ) have identified energy balance-related behaviour patterns in children and included measures of diet together with activity and sedentary behaviour. Although comparisons with these studies are difficult due to differences in design, the underlying message is that the synergistic effects of diet and physical activity warrant further study into lifestyle patterns as indicated by our findings.
In conclusion, we successfully generated PCA models for lifestyle patterns and demonstrated the synergistic effects of physical activity/inactivity and diet by identifying clustering of factors that are associated with biochemical risk parameters. In South Asian women, intervention strategies that promote reduction of physical inactivity, sedentary behaviour and snacking would be a pragmatic option with potentially far-reaching implications. Our findings emphasise that studying lifestyle patterns as opposed to diet alone or physical activity alone affords a unique opportunity to greatly increase our understanding of high-risk behaviours within real-life complexities that exist in communities. It is apparent that in the Sri Lankan community reducing the identified risk behaviours would be more important for reducing risk than improving desired behaviours alone.
Acknowledgements
The present study was funded by the International Atomic Energy Agency (IAEA) – RC 15920. IAEA had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: I. W., P. L., A. d. S., S. A. and R. W. participated in the design of the study; I. W., P. L., A. d. S. and S. A. conducted the study, collected the data and wrote the manuscript; I. W., P. L., A. d. S., M. d. L.-D., S. A. and R. W. contributed to the analysis; I. W., P. L., A. d. S., S. A., M. d. L.-D., N. S. and R. W. reviewed and edited the manuscript.
None of the authors has any conflicts of interest to declare.






