CVD is the leading cause of mortality worldwide(1). Hypertension is a major risk factor for future CVD, and the incidence of hypertension is expected to continue increasing(2). Already 20–30 % of North Americans have been diagnosed as hypertensive(2, Reference Appel, Brands and Daniels3) and as many or more can be classified as prehypertensive(Reference Appel, Brands and Daniels3).
The most diagnosed form of hypertension, accounting for ≥ 90 % of diagnoses, is essential hypertension(Reference Appel, Brands and Daniels3, Reference Guyton, Hall, Schmidt and Gruliow4). Essential hypertension, also known as primary hypertension, is high blood pressure (BP) with no known aetiology. The reduction of BP, especially through lifestyle and dietary changes, reduces the risk of CVD, stroke and kidney failure(Reference Appel, Brands and Daniels3). Essential hypertension is inherently difficult to treat using targeted therapies such as those offered by pharmaceutical intervention. Pharmaceuticals, such as angiotensin-converting enzyme inhibitors or β-blockers, which target specific signalling pathways, can alleviate BP with continued dosing, but cessation of treatment usually results in a return to dysregulated BP levels. Additionally, the effectiveness of drug intervention in those people with prehypertension – estimated at over 30 % of the population – is unclear(Reference Pickering5). In fact, the seventh report on hypertension by the Joint National Committee suggests that prehypertensive people manage their disease through lifestyle changes, namely diet and exercise, and not through pharmaceutical use(Reference Chobanian, Bakris and Black6). Furthermore, people on drugs are often able to reduce medication doses with improved dietary habits(Reference Appel, Brands and Daniels3).
Dietary Approaches to Stop Hypertension diet intake is one such approach for attenuating hypertension in the at-risk or affected population(Reference Appel, Brands and Daniels3, Reference Sacks, Svetkey and Vollmer7). Similar diets have been suggested by the American Heart Association and the Public Health Agency of Canada(2, Reference Appel, Brands and Daniels3). Focusing on vegetables, fruits, nuts and low-fat dairy products and avoiding foods high in Na, processed foods and red meat have been shown to be beneficial in patients with hypertension(Reference Hummel, Seymour and Brook8). Increasing vegetable intake is especially prudent, as an estimated 50 % of adults and 70 % of children in Canada do not consume the daily recommended amounts of fruits and vegetables(Reference Garriguet9). Additionally, legumes are underconsumed by individuals on a typical Western diet, with only about one in eight people consuming pulses on a daily basis(Reference Mudryj, Yu and Hartman10).
Pulse crops, among the first cultivated crops(Reference Zohary11, Reference Lev-Yadun, Gopher and Abbo12), are leguminous plants that are grown for reasons other than their oil(Reference Thompson, Thompson and Brick13, Reference Singh and Basu14). Pulses are protein-dense, high-fibre foods that contain variable levels of phytochemicals and antioxidants depending on the genus, species, and year and region of growth(Reference Patterson, Maskus and Dupasquier15–Reference Dueñas, Hernández and Estrella21). In in vitro experiments, extracts prepared from pulses have been shown to affect several of the signalling pathways associated with hypertension and vascular remodelling, including the renin–angiotensin–aldosterone system through angiotensin-converting enzyme inhibition(Reference Faris, Takruri and Issa16, Reference Roy, Boye and Simpson22–Reference Barbana and Boye24). The consumption of pulse crops has been shown to decrease the risk of heart attack, CHD and overall CVD in human subjects(Reference Bazzano, He and Ogden25, Reference Rimm, Ascherio and Giovannucci26). However, no studies have been carried out to determine whether a specific pulse variety is more effective in vivo.
The spontaneously hypertensive rat (SHR) is the most common animal model of human essential hypertension(Reference Lemmer, Mattes and Bohm27, Reference Pinto, Paul and Ganten28). Before the onset of hypertension, SHR experience marked arterial stiffness and arterial remodelling due to aberrant vascular smooth muscle cell growth and/or remodelling(Reference Marque, Kieffer and Atkinson29–Reference Gündüz, Baskurt and Meiselman31). Following atherosclerosis, arterial stiffness is the best predictor of CVD and mortality(Reference Zhou, Vendrov and Tchivilev32). Therefore, in the present study, we examined the effects of pulse-based diets on hypertension, arterial stiffness and vascular remodelling in SHR.
Materials and methods
Animals and diet
For the present study, 15-week-old male SHR and Wistar–Kyoto (WKY) rats were obtained from Charles River. The rats were housed individually under controlled laboratory conditions (12 h light–12 h dark cycle and 40–60 % humidity). The rats were acclimatised for a minimum of 12 d using an American Institute of Nutrition 93 growth diet(Reference Reeves, Nielsen and Fahey33) (henceforth: ‘control’) diet and then fed the experimental diets for 4 weeks. Previous studies have shown that 4-week intervention with diets and/or concentrated plant extracts is sufficient for attenuating BP in SHR(Reference Potenza, Marasciulo and Tarquinio34–Reference Shizuka, Kido and Nakazawa36).
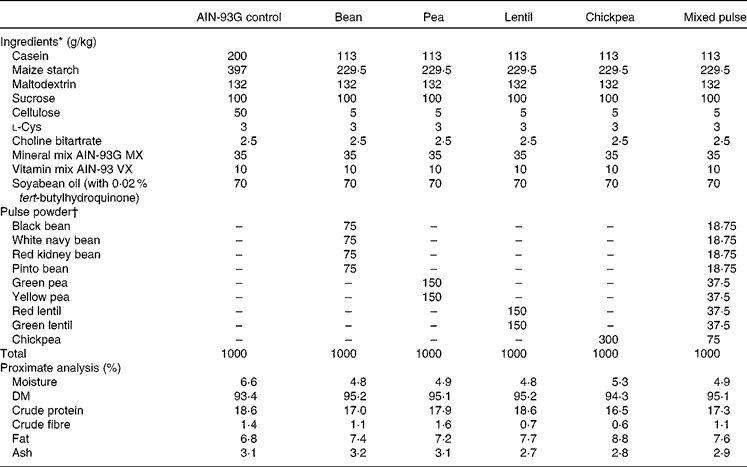
Pulses were soaked overnight (except lentils), cooked and then freeze-dried. Freeze-dried pulses were ground using a Retsch ZM 200 mill (Retsch) at 1200 rpm and passed through a 0·5 mm screen before incorporation into the diets. The cooked and freeze-dried pulse powder was added to the diets at 30 % w/w (Table 1). This level has been used previously in diets with no adverse effects on the growth of rats(Reference Thompson, Thompson and Brick13). The diets were formulated to be isonitrogenous, isofibrous and isoenergetic by appropriate adjustments of casein, maize starch and cellulose content. Samples of each diet were sent to Central Testing Labs Limited for proximate analysis (Table 1). SHR were assigned to one of the following six groups (n 8 per group): control (SHR-Ctrl); bean (SHR-B; white navy, black, pinto and red kidney); pea (SHR-P; green and yellow); lentil (SHR-L; red and green); chickpea (SHR-C); mixed pulse (SHR-M; combination of beans, peas, lentils and chickpeas). Normotensive WKY rats were fed the control diet (WKY). The protocol was approved by the University of Manitoba Protocol Management and Review Committee in accordance with proper animal care and experimentation as outlined by the Canadian Council on Animal Care.
Table 1 Diet formulations

AIN-93G, American Institute of Nutrition 93 growth diet.
* Ingredients ordered from Dyets, Inc.
† Pulse powders were prepared as described in the Materials and methods section.
Blood pressure measurements
BP measurements, using tail-cuff plethysmography, were taken at baseline and the week before the completion of the study. The rats were acclimatised to the BP apparatus the day before the measurements were taken. The rats were assessed six at a time with five acclimatisation cycles and ten measurement cycles using the CODA™ system (Kent Scientific).
Pulse wave velocity measurements
Pulse wave velocity (PWV) in the femoral artery was measured weekly using a 10 MHz electrocardiogram (ECG)-triggered Doppler probe (Indus Instruments). The rats were anaesthetised using isoflurane, and three measurements per rat were taken per session. Triplicate measurements had an average %CV < 7.
Fasting serum analysis
In week 4 of the study, the rats were subjected to a 12 h overnight fast. A saphenous blood sample was collected from the fasted rats, allowed to clot on ice for 1 h, and then spun for 15 min at 1000 g. Fasting serum was separated into aliquots and stored at − 80°C until analysed with a Cobas C111 autoanalyser (Roche Diagnostics GmbH). Serum was analysed for total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol, TAG, glucose, urea and creatinine levels.
Tissue weights
The rats were killed by sodium pentobarbital overdosing. The peri-renal, mesenteric and epididymal fat pads, along with the livers and hearts, were excised and weighed. The hearts were further dissected to isolate the left ventricle (LV), which was weighed separately. At the time of the completion of the study, a section of the descending aorta was placed into an optimal cutting temperature medium, then frozen in a dry-ice and ethanol bath, and stored at − 80°C.
Aortic histology
A cryotome was used to section the aorta into 5 μm sections, three per slide. The slides were kept at − 80°C until stained. The slides were fixed in paraformaldehyde for 8 min, washed with 1 × PBS (137 mm-NaCl, 3 mm-KCl, 10 mm-Na2HPO4 and 1·5 mm-KH2PO4) for 10 min and hydrated in double-distilled water for 15 min. Elastin Stain Kit (no. HT25A-1KT, lot no. 120M4344; Sigma Aldrich) was used according to the manufacturer's protocol to differentiate between elastin (black), muscle (yellow) and collagen (red). Differentiation in working ferric chloride was done for 3 min, followed by Van Geison's staining for 90 s to obtain the best contrast. The slides were mounted using VectaMount, and images were captured on an Evos® microscope (AMG) using 4 × and 20 × objectives. ImagePro Plus (Media Cybernetics) was used to measure the lumen circumference and outer-media circumference of the sections. Calculations to determine lumen diameter, media thickness, media:lumen ratio, media cross-sectional area and external diameter were carried out. On average, two images of non-consecutive aortic sections were analysed per rat.
Statistical analysis
Data were analysed using a repeated-measures ANOVA for BP and PWV and a one-way ANOVA for endpoint data using the Statistical Analysis Systems software package 9.2 (SAS). A post hoc Duncan's multiple range test was used to determine significance at the P< 0·05 level. When necessary, data were log-transformed to achieve normality and homogeneity. The data are reported as means with their standard errors.
Results
Body and tissue weights
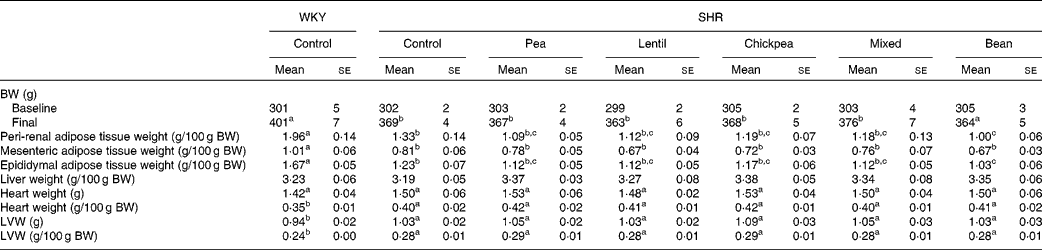
At baseline, there were no differences between any of the groups with respect to body weight (BW). At 4 weeks, the SHR groups weighed 8·2 % less than the WKY rat group; however, the pulse-based diets did not alter BW (Table 2). The WKY group had higher peri-renal, mesenteric and epididymal fat pad weights than all the SHR groups when normalised by BW. The SHR-B group had lower peri-renal and epididymal fat pad weights than the SHR-Ctrl group. The fat pad weights of all the other SHR groups were between those of the SHR-Ctrl and SHR-B groups and were not significantly different from each other. There were no differences between any of the groups with respect to liver weight.
Table 2 Body and tissue weights (Mean values with their standard errors)

WKY, Wistar–Kyoto; SHR, spontaneously hypertensive rats; BW, body weight; LVW, left-ventricle weight.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Blood pressure
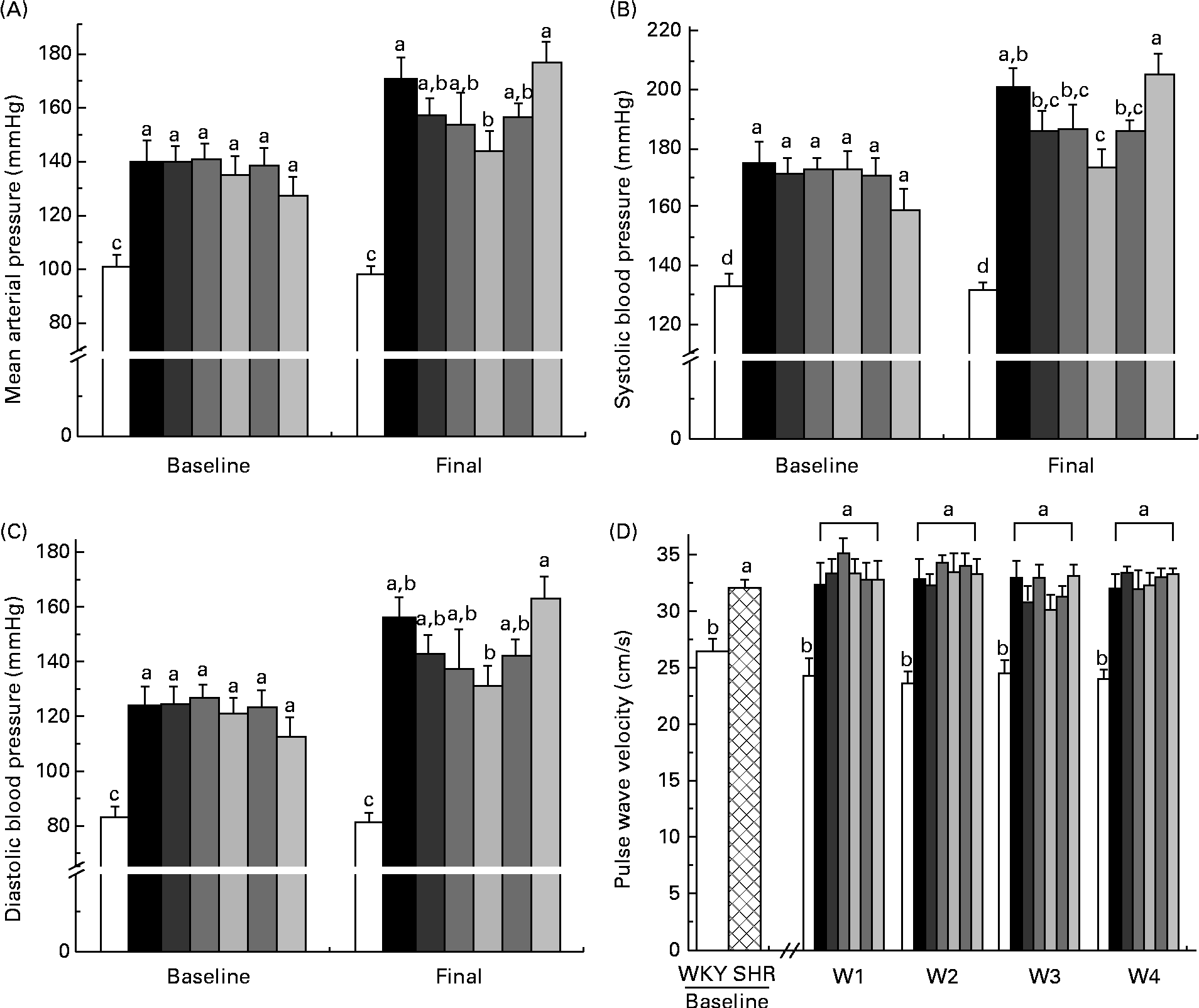
At baseline and week 4, the WKY group had significantly lower mean arterial pressure, systolic BP and diastolic BP than all the SHR groups (Fig. 1(A)–(C)). At week 4, the SHR-L group had lower mean arterial pressure than the SHR-Ctrl (144 (se 8) v. 171 (se 7) mmHg) and SHR-M (144 (se 8) v. 177 (se 8) mmHg) groups, with that of all the other SHR groups falling between the two extremes (Fig. 1(A)). The SHR-L group had lower systolic BP than the SHR-Ctrl group (174 (se 6) v. 201 (se 6) mmHg) (Fig. 1(B)) and lower diastolic BP than the SHR-M group (131 (se 8) v. 163 (se 8) mmHg) (Fig. 1(C)).

Fig. 1 (A) Mean arterial pressure, (B) systolic blood pressure and (C) diastolic blood pressure at baseline and week 4. (D) Pulse wave velocity at baseline and weeks 1–4. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). □, Wistar–Kyoto (WKY) rats; ![]() , SHR; ■, spontaneously hypertensive rat (SHR) controls;
, SHR; ■, spontaneously hypertensive rat (SHR) controls; ![]() , SHR-bean group;
, SHR-bean group; ![]() , SHR-pea group;
, SHR-pea group; ![]() , SHR-lentil group;
, SHR-lentil group; ![]() , SHR-chickpea group;
, SHR-chickpea group; ![]() , SHR-mixed group.
, SHR-mixed group.
Pulse wave velocity
At baseline, the SHR groups had a higher PWV than the WKY rat group, indicative of stiffer arteries. At weeks 1–4, the WKY group had a significantly lower PWV than all the SHR groups, with no significant changes due to diet being observed (Fig. 1(D)).
Cardiovascular remodelling
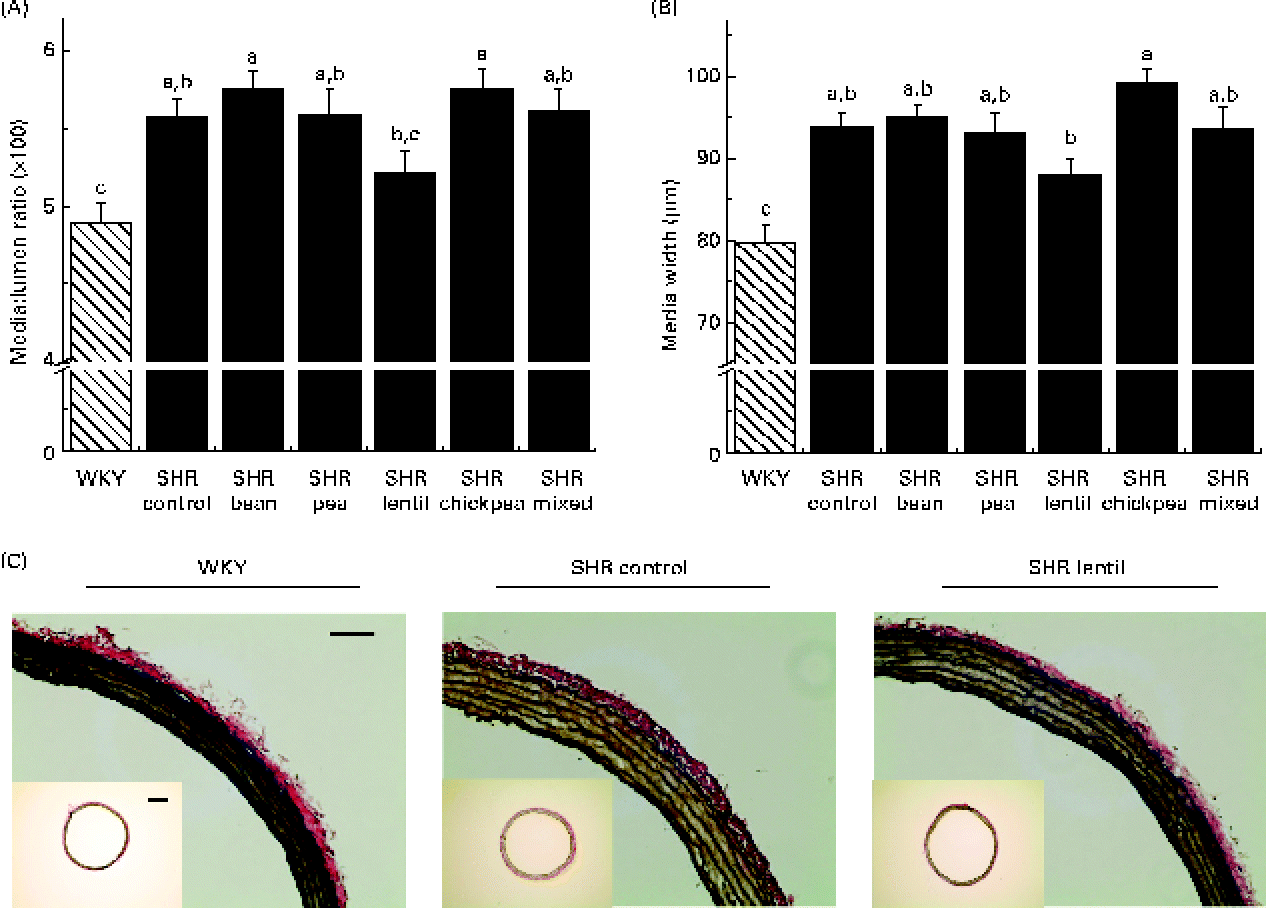
All the SHR groups had higher heart weight (6 %), heart weight:BW ratio (15 %), LV weight (10 %) and LV weight:BW ratio (15 %) than the WKY group (Table 2). No significant changes were observed for any of the dietary interventions. There were no differences between any of the groups with respect to lumen diameter or external diameter (data not shown). The WKY group had a smaller media width than all the SHR groups (Fig. 2(A)). The WKY group had a lower media:lumen ratio than all the SHR groups, except the SHR-L group. The SHR-L group had a significantly lower media:lumen ratio than the SHR-B and SHR-C groups (5·22 (se 0·14), 5·75 (se 0·11) and 5·75 (se 0·15), respectively), and the ratios of all the other SHR groups fell in between the values of these groups. The SHR-L group had the smallest media width among all the SHR groups, although it was not significantly different from that of the SHR-Ctrl group (Fig. 2(B)). In Fig. 2(C), representative aortic sections after staining are shown.

Fig. 2 (A) Media:lumen ratio and (B) media width of elastin-stained aortic sections. a,b,cMean values with unlike letters were significantly different (P< 0·05). (C) Sample aortic sections after elastin staining (bar = 0·1 mm). Inset: aortic sections used for vascular geometry calculations (bar = 0·05 mm). WKY, Wistar–Kyoto; SHR, spontaneously hypertensive rats. (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
Serum analysis
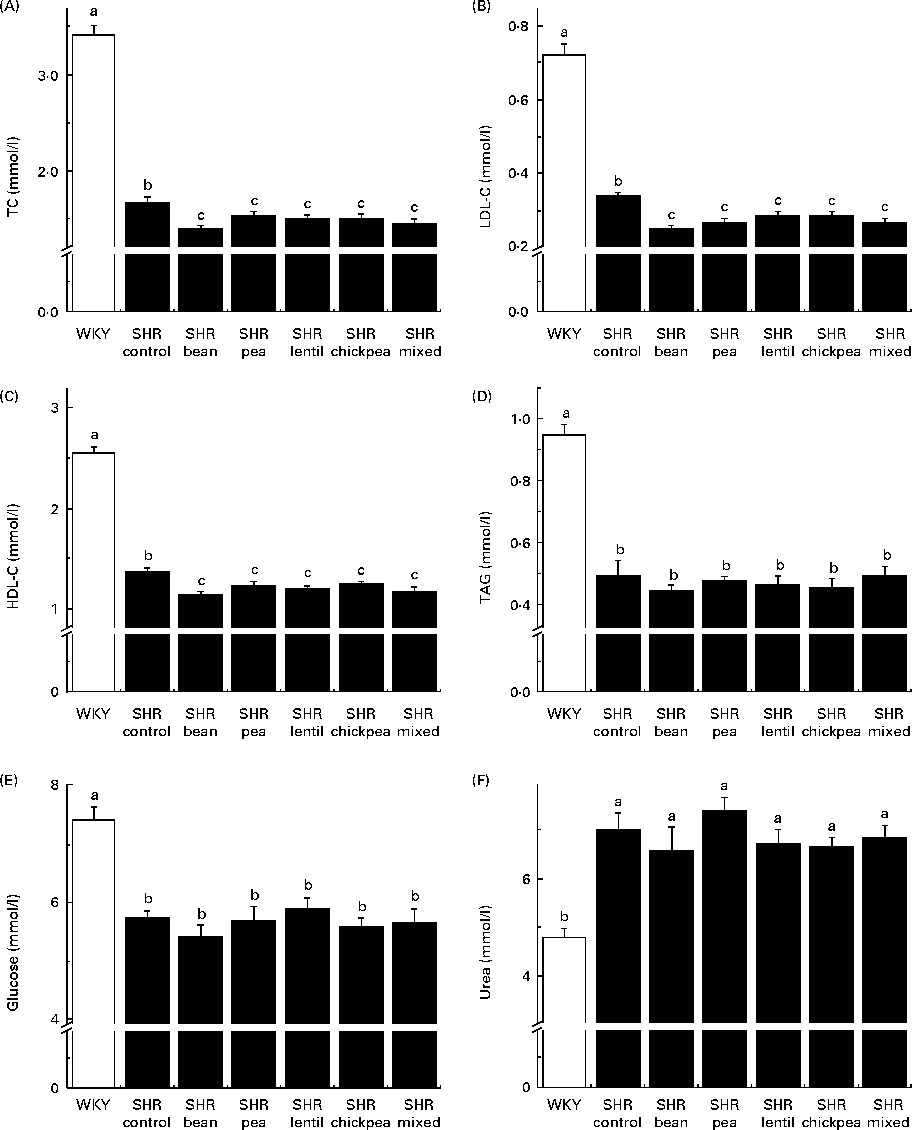
The WKY group had significantly higher fasting TC, LDL-C, HDL-cholesterol, TAG and glucose levels than all the SHR groups (Fig. 3(A)–(E)). The SHR-Ctrl group had significantly higher fasting TC, LDL-C and HDL-cholesterol levels than all the SHR groups on the pulse-containing diets (Fig. 3(A)–(C)). There were no differences among the SHR groups with respect to TAG or glucose levels. The WKY group had lower serum urea levels than all the SHR groups, with no dietary differences being observed (Fig. 3(F)). There were no significant differences between any of the groups with respect to serum creatinine levels (data not shown).

Fig. 3 Fasting serum (A) total cholesterol (TC), (B) LDL-cholesterol (LDL-C), (C) HDL-cholesterol (HDL-C), (D) TAG, (E) glucose and (F) urea levels. a,b,cMean values with unlike letters were significantly different (P< 0·05). WKY, Wistar–Kyoto; SHR, spontaneously hypertensive rats.
Discussion
Pulse crops and their phytochemical isolates have been widely studied in vitro for their effects on vascular remodelling and oxidative stress(Reference Amarowicz, Estrella and Hernández18, Reference Roy, Boye and Simpson22) and in vivo for their anti-nutritional components and effects on serum lipid profiles(Reference Faris, Takruri and Issa16, Reference Perez-Vizcaino, Bishop-Bailley and Lodi37). However, to the best of our knowledge, a study testing the anti-hypertrophic and BP-lowering effects in vivo or one determining which pulse variety has the greatest effect is yet to be carried out. We found that a mixture of red and green lentils had the greatest effect on BP and large-artery remodelling in SHR in the present study. The consumption of pulses at 30 % w/w in the diets had no adverse effects during the study period. The pulse-based diet-fed SHR gained weight at a similar rate to the SHR control group throughout the study period (data not shown), and there were no significant differences in BW at the completion of the study. Similar results have been reported previously for Sprague–Dawley rats fed diets containing 30 % w/w pulses(Reference Thompson, Thompson and Brick13). In the present study, the SHR consumed approximately 26 % of daily energy derived from pulses. With regard to human consumption, Mitchell et al. (Reference Mitchell, Lawrence and Hartman38) have reported that the highest quartile of pulse consumers in the USA ate 277 g of pulses per d and had a daily energy intake of 11 820 kJ; this represents approximately 16 % of daily energy derived from pulses based on 686 kJ/100 g of boiled chickpeas(39). A dose study is necessary to determine whether smaller quantities of lentils (e.g. 15 % w/w in a rodent diet) would also be effective for BP reduction and large-artery remodelling in SHR. Higher doses could be achieved in human diets by developing food products containing dehydrated pulse powders, as boiled and drained lentils contain approximately 70 % water.
The consumption of pulses has been shown to have a modest hypotensive effect in human subjects(Reference Hermsdorff, Zulet and Abete40–Reference Jenkins, Kendall and Augustin42). However, in the studies, the subjects were normotensive at baseline, and while consumption of pulses was indicated, the particular pulse variety was not important and it is, therefore, impossible to determine which pulse variety/varieties provided the benefits. In the present study, among all the pulse-based diets tested, the lentil-based diet was found to be able to attenuate the rise in BP experienced by the SHR model to the greatest extent. The lentil-based diet-fed rats experienced a 1 mmHg rise in systolic BP compared with the 26 mmHg rise experienced by the SHR control group. With respect to diastolic BP, the lentil-based diet-fed group exhibited an increase of 10 mmHg compared with that of 30 mmHg observed in the control group. This is a step towards supporting anti-hypertensive claims for lentils based on in vitro data. Pulses contain phenolic compounds exhibiting antioxidant effects when assayed in vitro (Reference Amarowicz, Estrella and Hernández18, Reference Stocklet, Chataigneau and Ndiaye43), but they may also act as selective inhibitors of angiotensin-converting enzyme, endothelin-1 and matrix metalloproteinases(Reference Stocklet, Chataigneau and Ndiaye43, Reference Halliwell44). These effects could explain the reduced BP and remodelling observed in the pulse-based diet-fed SHR, as angiotensin II activity and oxidative stress are among the major driving forces behind both these events(Reference Rajagopalan, Kurz and Münzel45, Reference Griffin, Brown and MacPherson46).
The fact that there was no change in arterial stiffness, as indicated by PWV measurements, is puzzling, given that PWV measurements have been shown to be closely associated with BP parameters (systolic BP, mean arterial pressure, diastolic BP and pulse pressure) in rats(Reference Cosson, Herisse and Laude47, Reference Fitch, Vergona and Sullivan48) and human subjects(Reference Gribbin, Steptoe and Sleight49, Reference Kim, Park and Park50). The study carried out by Cosson et al. (Reference Cosson, Herisse and Laude47) involved simultaneous, invasive measurements of PWV and BP in SHR and WKY rats and generated significant correlations between PWV and BW, heart rate and mean blood (arterial) pressure. Similar correlations have been shown in human subjects when BP and PWV parameters were obtained simultaneously(Reference Kim, Park and Park50). In the present study, BP and PWV measurements were not taken simultaneously; PWV was measured in anaesthetised rats and BP was measured through a non-invasive procedure in conscious rats. As a result, it was not possible to normalise PWV data against BP data. BP fluctuations during PWV measurements might explain the reason for not observing potential, small reductions in PWV in the present study, but this cannot be determined due to the non-simultaneous nature of the data collection process. It is also possible that there were no changes to detect, although lentils did attenuate aortic remodelling, which also contributes to PWV.
Rodent models are typically not as susceptible to atheromatous plaque development as are human arteries(Reference Zadelaar, Kleemann and Verschuren51), but rodent arteries still undergo remodelling in a manner similar to human arteries despite their morphological differences. The lentil-based diet was able to attenuate aortic remodelling to the greatest extent among all the pulse-based diets, leading to the smallest media:lumen ratio in all the SHR groups, which was not significantly different from that of the normotensive control group. The media:lumen ratio has been shown to increase as a result of hypertension, diabetes and arterial stiffness(Reference Rizzoni and Agabiti-Rosei52). Although obtaining these data requires an invasive approach, the media:lumen ratio is the best marker of vascular remodelling and has been proposed as a possible indicator of hypertension treatment effectiveness(Reference Rizzoni and Agabiti-Rosei52).
Despite the significant changes in arterial remodelling observed in the aorta on consumption of the lentil-based diet, there was no effect on heart or LV weights as a result of consuming any pulse-containing diet. LV remodelling is a common morphological response to chronic hypertension, with a more muscular wall required to pump blood into the higher-pressure environment in the arteries(Reference Gardin and Lauer53). The absence of a dietary response in this regard could be attributed to individual variability, time or duration of the intervention. It is possible that the 4-week duration was not long enough to observe changes in LV remodelling or that remodelling in the rats at this age was past the point of reversal by diet alone.
All the pulse-based diets were able to reduce serum TC and LDL-C levels in SHR compared with the control diet. Similar results with respect to cholesterol levels have been observed in human interventions, with an 8 % reduction in TC levels when 150 g of pulses were consumed per d(Reference Abeysekara, Chilibeck and Vatanparast54). This is probably due to the high fibre content of the pulses(Reference Abeysekara, Chilibeck and Vatanparast54). However, according to proximate analysis, the pea-based diet was the only diet containing more amounts of crude fibre than the control diet. The fibre content of the control diet is mainly attributable to cellulose, an insoluble fibre. The pulse-based diets had some amounts of cellulose, but also had a mixture of soluble and insoluble fibres derived from the pulses themselves. The addition of soluble fibre could be the reason for the decreased serum cholesterol levels observed in the pulse-based diet-fed rats, as soluble fibre has been reported to be associated with lower circulating cholesterol levels(Reference Abeysekara, Chilibeck and Vatanparast54). Pulse crops also contain anti-nutritional compounds – such as saponins – that have been reported to be associated with hypocholesterolaemia(Reference Singh and Basu14–Reference Faris, Takruri and Issa16, Reference Roy, Boye and Simpson22). This is especially important because serum LDL-C is a well-researched risk factor for atherogenesis and future CVD in humans and reducing circulating LDL-C level is considered to be an important clinical outcome for treatment(Reference Hansson55). Whether the observed changes were mediated through antioxidant(Reference Faris, Takruri and Issa16, Reference Amarowicz, Estrella and Hernández18, Reference Yao, Sun and Chang56), anti-remodelling(Reference Yao, Sun and Chang57) and/or angiotensin-converting enzyme inhibitory effects remains to be determined(Reference Boye, Roufik and Pesta23, Reference Barbana and Boye24). However, the fact that LDL-C levels were decreased by all the diets without a parallel improvement in vascular function clearly indicates that cholesterol is not the target through which lentils are able to positively affect blood vessel remodelling.
Conclusions
A diet rich in pulses should be part of a healthy, well-balanced lifestyle. All pulses were able to decrease the levels of circulating cholesterol, a known risk factor for CVD; however, only lentils were able to affect the pathways involved in vascular remodelling and hypertension. Since the mechanism by which these changes are mediated has yet to be determined, additional studies to determine whether lentils can affect resistance-artery remodelling, endothelial dysfunction and the mechanism of action for these changes are warranted.
Acknowledgements
The authors cordially thank Kate Molnar and Sheri Bage for assistance with PWV and BP data collection, Li Ren for running the Cobas instrument, and Leslie Rech for help with animal care.
The present study was supported by the Natural Sciences and Engineering Research Council of Canada Strategic Grant (P. Z., C. G. T.; STPGP 397306) and a Food Advancement Through Science and Training graduate scholarship (M. G. H.). The Natural Sciences and Engineering Research Council of Canada/Food Advancement Through Science and Training had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions were as follows: M. G. H. and C. G. T were responsible for animal care and tissue collection; M. G. H was responsible for sample collection, measurements and statistical analysis. All authors equally contributed to the study design and writing and editing of the manuscript.
The authors declare no conflicts of interest.