Vitamin C plays a role in numerous biological reactions, many of which are only known in little detail. Over the years, it has been suggested that vitamin C be used as a remedy against many diseases as different as common colds and cancers. Even today, there is considerable controversy about the exact role of the vitamin in human health and no agreement has been reached on the amount needed to be consumed for optimum wellbeing. Thus, as little as 10 mg/d will largely prevent development of the most well-known clinical and ultimately mortal manifestation of severe vitamin C deficiency: scurvy(Reference Weber, Bendich and Schalch1). Nevertheless, the RDA for vitamin C was recently increased from 60 mg/d to 75 mg/d for women and 90 mg/d for men in the US, primarily based on biochemical evidence(2). Others have argued that the optimum plasma concentration is about the level of saturation (70 μmol/l), which would require a daily intake of about 200 mg(2–Reference Lykkesfeldt, Loft and Nielsen4), and still hypotheses on new specific roles of vitamin C in health and disease are being put forward(Reference Frikke-Schmidt and Lykkesfeldt5–Reference Tveden-Nyborg, Johansen and Raida7).
The potential benefit of vitamin C supplementation has been fueled in part by a considerable body of epidemiological literature suggesting a positive association between vitamin C status and health. Thus, several large cohort studies have shown an inverse relationship between plasma vitamin C status and risk of CVD and/or all-cause mortality(Reference Eichholzer, Stahelin and Gey8–Reference Singh, Ghosh and Niaz15). In contrast, large randomised controlled trials using antioxidant supplements have been less promising. None of the major clinical studies using mortality or morbidity as endpoints has found significant positive effects of supplementation with vitamin C(Reference Blot, Li and Taylor16–Reference Hercberg, Galan and Preziosi19). However, the vast majority of these trials have examined the effect a multi-component supplement and consequently not the effect of vitamin C itself.
The results from clinical trials in the last decades have shifted public opinion and that of health authorities towards antioxidants, including vitamin C, being generally unimportant. This development is likely to obscure a public health risk from deficiency, as several large cross-sectional population studies have shown that a considerable proportion (up to 50 %) of subpopulations of the Western world can have hypovitaminosis C, defined as a plasma concentration less than 23 μmol/l(Reference Jacob20, Reference Smith and Hodges21). While the clinical significance of this condition remains to be clarified – beyond the increased risk of developing scurvy – it is obvious that large subpopulations, for example, smokers, do not achieve the RDA of vitamin C(Reference Lykkesfeldt, Halliwell and Poulsen22).
It has been shown that those individuals most likely to benefit from supplements also are those least likely to get them(Reference Kirk, Cade and Barrett23–Reference Sinha, Frey and Kammerer25). So far, this discouraging finding is unfortunately also valid for most of the randomised controlled trials using vitamin C in their intervention. One frequently overlooked problem is that vitamin C uptake is highly dose dependent(Reference Levine, Conry-Cantilena and Wang3, Reference Lykkesfeldt, Christen and Wallock26). Thus, subjects already saturated with vitamin C through their daily diet will efficiently excrete any surplus and are therefore highly unlikely to benefit from further vitamin C supplementation. This and several other issues should be taken into account when designing and drawing conclusions from randomised controlled trials with the purpose of studying the effects of vitamin C. In view of the pharmacology and kinetics of vitamin C, the present review examines the current knowledge of the effect of vitamin C supplementation, evaluates the lessons to be learned from the many trials that have been conducted, and provides guidelines for future randomised trials.
Clinical significance and prevalence of vitamin C deficiency in observational studies
The definition of optimal vitamin C status remains a matter of controversy. However, current opinions appear to agree on a dose that gives saturated uptake, i.e. a dietary intake resulting in a plasma concentration of approximately 70 μmol/l(Reference Levine, Conry-Cantilena and Wang3, Reference Carr and Frei27–Reference Levine, Padayatty, Katz, Asard, May and Smirnoff30). Defining vitamin C deficiency is also complex since considerable individual variation apparently exists in the relationship between the plasma concentration of vitamin C and the development of scurvy, the classic hallmark of severe vitamin C deficiency(Reference Newton, Schorah and Habibzadeh31, Reference Schorah, Newill and Scott32). Moreover, the clinical significance of vitamin C deficiency – beyond that of scurvy – has not been clearly defined. Guidelines developed by the National Survey of Canada suggested categories of severe vitamin C deficiency (serum level < 11 μmol/l) and marginal vitamin C deficiency (serum levels between 11 and 23 μmol/l) and have largely been adopted(Reference Smith and Hodges21). Since these categories were put forward in 1987, the RDA for vitamin C has been increased in an attempt to reflect the now-believed optimal vitamin C level in plasma of 70 μmol/l. Therefore a new category (for serum levels between 23 and, for example, 50 μmol/l) is needed, and we suggest it to be termed suboptimal vitamin C status.
Severe vitamin C deficiency
Scurvy typically constitutes the ultimate clinical manifestation of prolonged and severe vitamin C deficiency. In non-smokers, scurvy is prevented by a daily intake of as little as 10 mg of vitamin C(Reference Weber, Bendich and Schalch1). Clinical symptoms include follicular hyperkeratosis, petechiae, ecchymoses, coiled hairs, inflamed and bleeding gums, perifollicular haemorrhages, joint effusions, arthralgia and impaired wound healing(Reference Chazan and Mistilis33). Other early symptoms include dyspnoea, weakness, fatigue and depression. Cases of scurvy are usually limited to the group of individuals with plasma concentrations lower than 11 μmol/l, i.e. those diagnosed with severe vitamin C deficiency. However, far from all individuals with plasma levels < 11 μmol/l develop clinical scurvy(Reference Newton, Schorah and Habibzadeh31, Reference Schorah, Newill and Scott32). Thus, other factors seem to be of importance and the relationship between plasma vitamin C status and scurvy is not entirely clear, when the diet is not totally depleted from the vitamin. However, older reports indicate that total deficiency over a prolonged time invariably leads to scurvy(2).
While the basic symptoms and cure of the disease have been known for centuries(Reference Lind34), a significant part of the population in developed countries continues to suffer from severe vitamin C deficiency and thus have increased risk of experiencing scurvy-like symptoms (Table 1). But the clinical significance of severe vitamin C deficiency may extend beyond that of scurvy. In clinical studies in which subjects were made vitamin C deficient, common complaints such as gingival inflammation, fatigue and depression were among the most sensitive markers of deficiency(Reference Levine, Conry-Cantilena and Wang3, Reference Leggott, Robertson and Rothman35). In a prospective population study, Nyyssönen et al. found a higher risk of myocardial infarction (relative risk 3·5) among men with severe vitamin C deficiency, constituting about 6 % of their Finnish cohort (1605 subjects)(Reference Nyyssönen, Parviainen and Salonen12). Moreover, Langlois et al. recently showed that 14 % of patients with peripheral arterial disease suffered from severe vitamin C deficiency compared with none of the healthy controls and suggested a relationship between vitamin C status and severity of atherosclerosis(Reference Langlois, Duprez and Delanghe36). In a study with advanced cancer patients, 30 % had severe vitamin C deficiency and these patients had shorter survival(Reference Mayland, Bennett and Allan37).
Table 1 Prevalence of vitamin C deficiency in larger cross sectional population studies
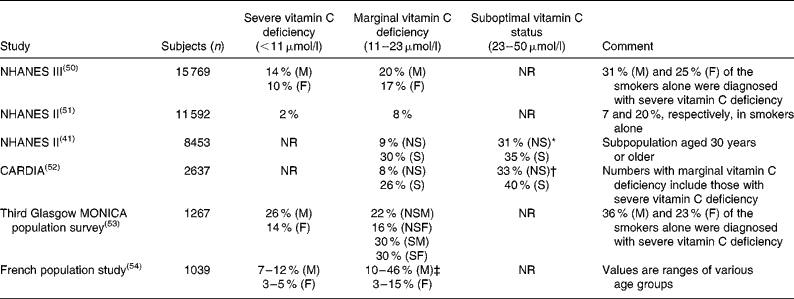
NHANES III, Third National Health and Nutrition Examination Survey; M, males; F, females; NR, not reported; NHANES II, Second National Health and Nutrition Examination Survey; NS, non-smokers; S, smokers; CARDIA, Coronary Artery Risk Development in Young Adults Study; MONICA, Monitoring of Trends and Determinants in Cardiovascular Disease; NSM, non-smoking males; NSF, non-smoking females; SM, smoking males; SF, smoking females.
* Range used: 23 to 55 μmol/l.
† Range used: 23 to 45 μmol/l.
‡ Range used: 11 to 19 μmol/l.
Marginal vitamin C deficiency
As defined above, a plasma concentration between 11 and 23 μmol/l is termed marginal vitamin C deficiency. Hypovitaminosis C has been characterised as having a plasma concentration of vitamin C < 23 μmol/l(Reference Schectman38), i.e. encompassing both severe and marginal vitamin C deficiency. As with severe vitamin C deficiency, smokers also have a markedly increased risk of marginal vitamin C deficiency (Table 1).
The clinical significance of marginal vitamin C deficiency – as different from severe vitamin C deficiency – has not been thoroughly investigated. In most studies, upper and lower tertiles, quartiles or quintiles are compared, making it difficult to compare groups between studies. Consequently, the category of marginal vitamin C deficiency can rarely be singled out from vitamin C deficiency or hypovitaminosis C. With respect to scurvy, clinical cases among individuals with marginal vitamin C deficiency are rare, but do occur(Reference Hodges, Hood and Canham39, Reference Reuler, Broudy and Cooney40). Probably more important though, considerable epidemiological evidence suggests that there may be other clinical consequences of marginal vitamin C deficiency. Thus, in a recent re-examination of the Second National Health and Nutrition Examination Survey (NHANES II) data combined with a follow up on vital status 12–16 years later, Loria et al. found that men in the lowest ( < 28·4 μmol/l) compared with the highest (>73·8 μmol/l) serum ascorbate quartile had a 57 % higher risk of death from any cause and a 62 % higher risk of dying from cancer(Reference Loria, Klag and Caulfield11). A similar conclusion was reached by Simon et al. who also found that severe or marginal vitamin C deficiency was significantly associated with all-cause mortality while being weakly associated with death from CVD(Reference Simon, Hudes and Tice41). In a 20-year follow-up study in Britain (730 subjects), a significantly higher risk of mortality from stroke was observed in elderly men and women with severe and marginal vitamin C deficiency separately compared with those with plasma concentrations of vitamin C>28 μmol/l(Reference Gale, Martyn and Winter9). The authors concluded that vitamin C status was as strong a predictor of death from stroke as diastolic blood pressure(Reference Gale, Martyn and Winter9). An inverse correlation between vitamin C status and stroke was also reported from a study (2121 subjects) in a rural Japanese population aged 40 years or older(Reference Yokoyama, Date and Kokubo42). In the 12-year follow up on the Basel Prospective Study, a significantly increased risk of IHD and stroke was found in individuals with plasma ascorbate < 22·7 μmol/l, corresponding to severe or marginal vitamin C deficiency(Reference Gey, Stahelin and Puska43–Reference Gey, Stahelin and Eichholzer45).
Suboptimal vitamin C status
Based on the increased RDA for vitamin C as well as the indication that a plasma concentration of vitamin C of about 70 μmol/l is currently considered optimal for health, we suggest a new category of suboptimal vitamin C status for those individuals with plasma concentrations between 23 and 50 μmol/l. An obvious rationale for this additional category could be that if 70 μmol/l is optimal, for example, 35 μmol/l is probably not, and therefore investigations into the clinical significance of a suboptimal vitamin C status are warranted. Moreover, a proper control group should be selected from individuals with optimal vitamin C status, i.e. excluding those with suboptimal status. However, limited data are available and need to be extracted from studies discriminating between the concentrations of suboptimal and optimal vitamin C status (Table 1).
As discussed above, several large prospective studies have shown an inverse relationship between plasma vitamin C status and risk of CVD and/or all-cause mortality(Reference Eichholzer, Stahelin and Gey8–Reference Singh, Ghosh and Niaz15). However, no studies have investigated the specific clinical significance of suboptimal vitamin C status as compared with optimal. Thus, it remains to be established if the biochemical evidence pointing towards an optimal plasma level of about 70 μmol/l can be backed up in larger epidemiological studies or ultimately in clinical trials. Clearly, the effects of suboptimal compared with optimal vitamin C status are likely to be at most moderate and presumably relevant only in the long term if at all. Thus, it is debatable if studies aimed at clarifying such a limited risk are feasible from a cost perspective. On the other hand, the problems potentially associated with suboptimal vitamin C status affect a large percentage of the population and can be readily and inexpensively cured(Reference Blot, Li and Taylor22).
Randomised controlled trials with vitamin C
Randomised clinical trials have evolved and been refined for testing drug effects. Their strength lies in eliminating or reducing bias by randomisation, blinding and control. In the case of drugs, this design is superior and regarded as the ‘gold standard’. The design is particular strong for testing the effects of a chemical that is normally not present in the organism, has a relatively short pharmacokinetic and pharmacodynamic half-life, and is used in a relatively short dose regimen. In the case of intervention with dietary components in prevention trials, a number of problems arise that are not prominent in drug testing.
In the present context, we will particularly discuss the testing of proper biological hypotheses in relevant cohorts.
In the 1980s and 1990s, the epidemiological evidence pointed towards the importance of antioxidant intake (vitamin C, vitamin E and β-carotene) in the prevention of, for example, cancer and arteriosclerosis. This led to the initiation of a large number of clinical intervention studies. The first large study published was the Linxian study that showed an inspiring preventive effect(Reference Blot, Li and Taylor16). The subsequent studies were all negative. At that time, the prevailing hypothesis was that dietary components were beneficial, without side effects, and the larger dose the better. Implicitly, it was also believed that cancer and arteriosclerosis were the result of ‘high-level deficiency’ of these substances. As a consequence, trials were mainly designed for the broad and little-selected population and doses were very high. Today, basic knowledge of the biological effects of the antioxidants has increased, and more importantly, their functions are no longer considered to be generally antioxidative, but rather as specific cofactors in biological reactions or direct signalling, signalling modifying, or gene-expression modifying compounds(Reference Azzi46).
Epidemiological evidence is sometimes at great variance with the evidence from randomised controlled trials, particularly if control is not extensive(Reference Poulsen, Andersen and Keiding47). It should be acknowledged that in the epidemiological studies on the relationship between vitamin C concentrations and diseases, there is no evidence that the relationship is due to vitamin C itself. Thus, it is possible that vitamin C concentrations in plasma are a proxy or surrogate for vegetable and/or fruit intake and it may be some other substance in these foods that provides the health benefit. It might even be that the individuals with a high vegetable and fruit intake have no or a reduced intake of other foods with deleterious health effects, in which case vitamin C is a marker of an absence of a negative factor.
Neither epidemiological studies nor randomised clinical intervention trials can test mechanisms, but randomised controlled intervention trials can confirm if the effect is due to a single substance.
Current knowledge based on randomised controlled trials and recommendations for future studies
A large number of randomised clinical studies on antioxidants are now available. They have recently been reviewed and tabulated for effect by Bjelakovic et al. (Reference Bjelakovic, Nikolova and Gluud48). That review, however, was done with the purpose of estimating risk of mortality for any antioxidant treatment, alone or in combination. The authors categorised the studies as high or low risk of bias. Thus, trials with adequate generation of the allocation sequence, adequate allocation concealment, adequate blinding and adequate follow-up were considered low-bias risk trials (high methodological quality), while trials with one or more unclear or inadequate quality components were classified as high-bias risk trials (low methodological quality)(Reference Kjaergard, Villumsen and Gluud49).
We searched the literature by using identical criteria to those above(Reference Bjelakovic, Nikolova and Gluud48) and reviewed the combined number of papers using vitamin C as an intervention (Table 2). We then added a new set of criteria specifically addressing vitamin C (Table 3). Thus, based on the well-established dose dependency of vitamin C pharmacokinetics, we believe that it is imperative that enrolled subjects have hypovitaminosis C at study entry and that this condition is used as an entry-level inclusion criterion in order to ensure a possibility of effect. To verify the vitamin C status at entry and during the study, plasma concentration needs to be measured before and during the study by a validated method. As discussed above, vitamin C needs to be used as a single supplement to be able to determine the effect of this supplement specifically. Major confounders are, for example, dietary vitamin C and smoking status, and these factors need to taken into account in the study design. A valid hypothesis or molecular mechanism should be proposed involving vitamin C and a mechanism-related hard clinical endpoint used as the primary outcome. Finally, inclusion and exclusion criteria should be reported.
Table 2 Randomised, controlled trials with vitamin C
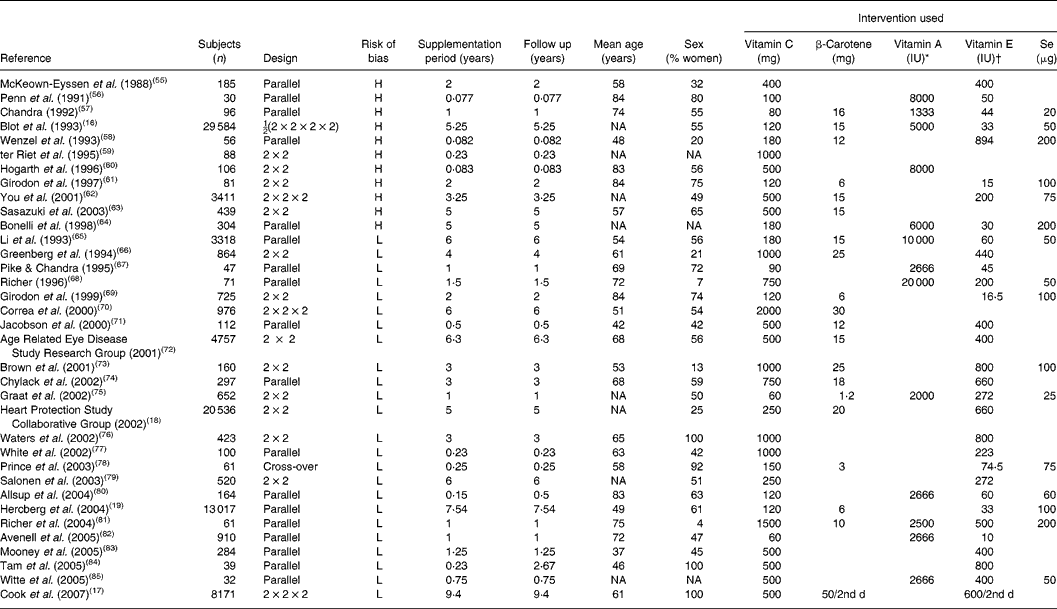
H, high-bias design; NA, not available; L, low-bias design.
* 1 IU vitamin A = 0·3 μg.
† 1 IU vitamin E = 0·667 mg.
Table 3 Compliance of randomised, controlled trials with the present set of guidelines*
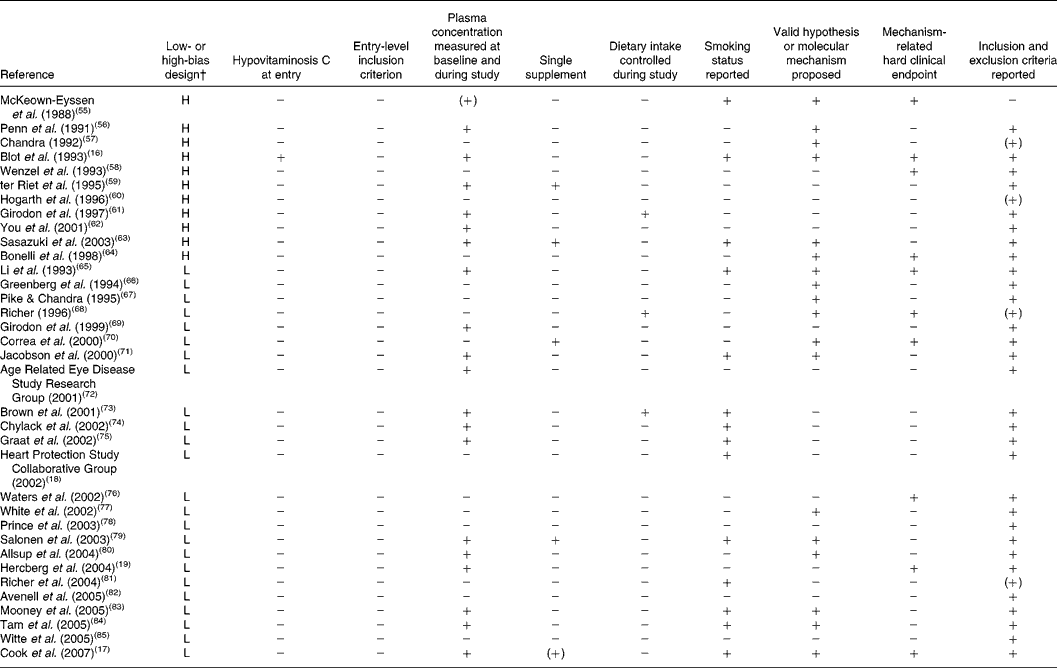
L, low-bias design; H, high-bias design.
* + indicates full compliance, (+) indicates partial compliance and − indicates that the criterion was not met by the study.
† As defined by Bjelakovic et al. (Reference Bjelakovic, Nikolova and Gluud48).
Reviewing the extracted literature, it is striking that no study has used vitamin C deficiency as an inclusion criterion. In contrast, reviewing those studies that have included a baseline determination of plasma vitamin C, only one of thirty-five studies (3 (95 % CI 0, 5) %) rendered it probable in a small sample that the subjects were in fact insufficient in vitamin C at the study start. Moreover, only five studies out of thirty-five studies (14 (95 % CI 2, 11) %) were available with data on vitamin C as a single substance.
This means that information from clinical trials with defined and verified vitamin C deficiency from a practical point of view is not available. In contrast, large and long-duration trials with β-carotene are available and show that ‘hypervitaminosis’ of β-carotene carries a risk for adverse effects on mortality (Alpha-Tocopherol, Beta-Carotene cancer prevention study, etc). It must therefore be concluded that at present we do not have the necessary scientific evidence to judge the effect on health – be that beneficial or deleterious – from vitamin C supplementation as a single substance. Dose–concentration relationships are largely available from pharmacokinetic evaluations, but no dose–response relationships for pharmacodynamic evaluation are available. For most of the available studies, the population status at entry with regard to vitamin C is unclear and may have been severely or marginally deficient, suboptimal or optimal. For the evaluation of the possible effect of vitamin C supplementation on human health, these studies are therefore largely irrelevant.
We had hoped that it would be possible to perform a meta-analysis of high-quality trials with vitamin C as a single substance based on the criteria suggested in Table 3, but have found that at this point this is not possible because such trials have not been performed.
In conclusion, we find that from a public health point of view, there is a dire need to examine the effect of vitamin C as a single supplement in populations which have been carefully defined with inclusion criteria of different levels of vitamin C status and with variable demand for vitamin C, for example, smokers v. non-smokers. The outcome markers should be defined and achieved targets, including plasma vitamin C concentration and relevant clinical endpoints.
Acknowledgements
The manuscript was written by J. L. and the draft was discussed and revised by J. L. and H. E. P.
J. L. has no conflicts of interest. H. E. P. is a consultant for the Danish company Ferrosan A/S that produces supplements.





