Undernutrition is a significant public health problem in early childhood, accounting for about one-fifth of global deaths and disability-adjusted life years in children younger than 5 years old(Reference Black, Allen and Bhutta1). It is currently estimated that 80 % of the world's undernourished children live in just twenty countries and almost half of these countries are in sub-Saharan Africa(Reference Bryce, Coitinho and Darnton-Hill2). Recent reports suggest that these countries also have the highest proportion of developmentally disadvantaged children worldwide but current estimates exclude those with sensory disabilities due to the dearth of relevant data from the developing world(Reference Grantham-McGregor, Cheung and Cueto3). Available evidence has undoubtedly shown that infants with congenital or early-onset sensorineural hearing loss (CESHL) in any population are at risk of irreversible deficits in speech, language, cognitive and psychosocial development with lifelong consequences(4, Reference Karchmer and Allen5). While the impact of undernutrition on cognitive and psychosocial development as well as educational attainment is well documented(Reference Ivanovic, Leiva and Perez6, Reference Pollitt, Golub and Gorman7), limited evidence still exists on the relationship between undernutrition and communication disorders in young infants, in sharp contrast with other sensory disabilities such as visual impairment which has been extensively linked with vitamin A deficiency(Reference Black, Allen and Bhutta1). Yet, such evidence is crucial for achieving optimal developmental outcomes in infants with CESHL, as effective intervention may be limited to the first year of life(Reference Kennedy, McCann and Campbell8, Reference Moeller9). For instance, in line with the resolution of the World Health Assembly urging for early hearing detection programmes for infants(10), the Joint Committee on Infant Hearing recommends that CESHL be detected by the age of 3 months to facilitate enrolment in appropriate family-oriented intervention ideally by the age of 6 months(4). Various reports have also shown that growth faltering or undernutrition in the first 3 months of life is not only predictive of feeding problems but also of developmental deficits, thus making this period valuable for a range of early detection and intervention services(Reference McDougall, Drewett and Hungin11, Reference Skuse, Pickles and Wolke12). However, no study to the author's best knowledge has examined the impact of undernutrition on hearing loss in early childhood, particularly in a developing country. The present community-based study therefore set out to examine the nutritional status of infants not older than 3 months and determine the relationship between undernutrition and sensorineural hearing loss as a further basis for fostering integrated research and policy initiatives in developing countries for early childhood development(Reference Black, Walker and Wachs13).
Methods
Study population
All infants aged 0–3 months attending community-based health clinics for routine Bacille Calmette-Guérin (BCG) immunisation in inner-city Lagos, Nigeria from July 2005 to December 2006 were enlisted for the study as previously described(Reference Olusanya, Wirz and Luxon14). Although hearing screening was still offered until April 2008, the study period was limited to December 2006, as effective follow-up for diagnostic hearing evaluation for all referred infants could not extend beyond this date due to resource and logistical constraints. BCG immunisation is administered in the first week of life with coverage ranging from 82 % in sub-Saharan Africa to 96 % in Latin America, thus providing an effective platform for attracting a high proportion of infants born outside hospitals in developing countries, usually more than 50 % of annual births(15). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Lagos State Health Management Board, Nigeria and University College London, UK as part of a wider pilot study on universal infant hearing screening in Lagos, Nigeria(Reference Olusanya, Wirz and Luxon14). Written informed consent was obtained from the mothers of all participating infants.
Study variables
The primary outcome measure was sensorineural hearing loss (permanent congenital and early-onset hearing loss >30 dB hearing loss (HL)) in at least one ear classified as mild (30–40 dB HL), moderate (41–70 dB HL), severe (71–90 dB HL) or profound (>90 dB HL)(4). The hearing assessment protocol has been previously described(Reference Olusanya, Wirz and Luxon14). In summary, all infants received two-stage hearing screening tests consisting of transient evoked otoacoustic emissions (TEOAE) followed by automated auditory brainstem response (ABR) for those referred by TEOAE. TEOAE is a physiological test of the integrity of the outer hair cells in the cochlea while automated ABR is an electro-physiological measure of the function of the auditory pathway from the eighth cranial nerve through the brainstem. All those who failed automated ABR were scheduled for diagnostic evaluation with tympanometry, ABR and visual response audiometry based on guidelines by the UK National Newborn Hearing Screening Programme. Thus, the case definition of CESHL in the present study is based on failure of the diagnostic tests rather than referral by the initial screening tests.
The nutritional indices of interest were weight-for-age, BMI-for-age and length-for-age expressed as z-scores. Moderate-to-severe or ‘significant’ wasting, underweight and stunting were defined as BMI-for-age, weight-for-age and length-for-age with z-scores below − 2 sd, respectively. The terms ‘underweight’, ‘wasting’ and ‘stunting’ have been used as indices for ‘nutritional status’ to facilitate easy comparison with other studies based on the latest WHO Multicentre Growth Reference(16). However, it is pertinent to note that any of these undernourished physical states can also be related to non-nutritional factors. ‘Any undernourished physical state’ was included as a summary factor to reflect the co-morbidity of nutritional deficits in this population. Anthropometric measurements for each child were obtained at the time of enrolment by a trained research worker throughout the study period. Weight was measured with a digital scale (Tanita Baby Scale, model 1583; Tanita Corp, Tokyo, Japan). Length was measured supine using graduated polyurethane plastic mats (Child Growth Foundation, London, UK). Birth weight and length could not be ascertained, as birth records of the participants, particularly those born outside hospitals, were not available in this community-based setting.
Potential confounding variables that were studied were guided by existing evidence from the literature on factors that could be reliably elicited or determined from parental interview. These included maternal factors such as age, consanguinity, family history of deafness and social class, used as a proxy for socio-economic status. Social classes were determined based on mother's education and father's occupation. Social class I was termed as ‘high’, II and III as ‘middle’ and IV or V as ‘low’. Infant factors included sex, congenital anomalies (including syndromes), gestational age, chronological age at screening, gestational type, place of birth, type of attendant present at birth, feeding method and history of severe neonatal jaundice (requiring phototherapy and/or exchange blood transfusion).
Data management and statistical analysis
Sex-specific z-scores for the three nutritional indices were obtained from the WHO Multicentre Growth Reference package using the macro provided by the organisation(16). The default settings in the software regarding cut-offs for out-of-range or biologically improbable values were used in the data analysis and all such values were recorded as missing data. Strength of association for categorical variables was estimated by OR, the corresponding 95 % CI and the difference between mean values of continuous variables was determined with Student's t test. All tests of significance were two-sided. Maternal and infant factors with significance (P < 0·05) or borderline significance (0·10>P ≥ 0·05) in univariate analyses were considered as confounders for the nutritional factors. In arriving at the final model, three separate multivariable logistic regression models were built for nutritional factors adjusting for the confounding variables. The first model contained all the three individual nutritional indicators based on biological plausibility. The second model included only nutritional factors with significant univariate association with CESHL while the third model contained only the summary nutritional variable ‘any undernourished physical state’. In each model, non-significant factors (P ≥ 0·05) were eliminated by the backward stepwise method. Interaction effects of variables in each model were assessed with the likelihood ratio test. A crude estimate of model calibration was determined by the Hosmer–Lemeshow test while the predictive utility of the models was based on the c-statistic (as shown by the area under the receiver operating characteristic curve). SPSS for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
A total of 3676 infants were enrolled over the study period but 281 (7·6 %) who did not complete the required stage(s) of the hearing assessment protocol were excluded from this analysis. An additional nine infants with incomplete anthropometric records were also excluded. From the final population of 3386 infants, 195 infants failed TEOAE but ninety-five of this group subsequently passed automated ABR and were exited from the programme. Of the 100 infants who failed both TEOAE and automated ABR, seventy-one were confirmed with CESHL. Of these, twelve children (16·9 %) had unilateral hearing loss while bilateral hearing loss was mild in twelve (16·9 %), moderate in thirty (42·3 %), severe in fourteen (19·7 %) and profound in three (4·2 %). The median age of diagnosis of hearing loss was 51 d (interquartile range 26, 72 d). The characteristics of the study participants are shown in Table 1.
Table 1 Nutritional and other factors associated with early-onset sensorineural hearing loss (n 3386)
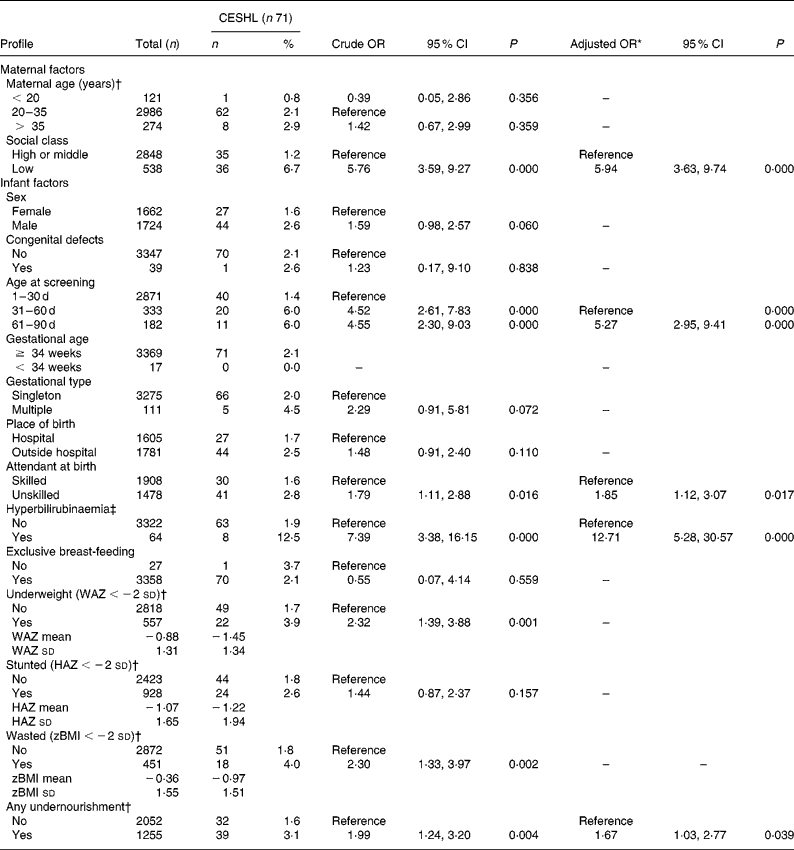
CESHL, congenital or early-onset sensorineural hearing loss; WAZ, weight-for-age z-score; HAZ, length-for-age z-score; zBMI, BMI-for-age z-score.
* Adjusted for the confounding effects of significant (P < 0·10) covariables from univariate analysis in a final model that excluded all individual nutritional indicators. Consanguinity and family history of deafness were not reported among infants with CESHL and were therefore not adjusted for.
† Missing data: maternal age, 5 (0·1 %); underweight, 11 (0·3 %); stunted, 35 (1·0 %); wasted, 63 (1·9 %); any undernourishment, 8 (0·2 %).
‡ Requiring phototherapy.
The majority (84·8 %) of the infants were enrolled in their first month of life and about 98·1 % (n 3323) had gestational age of at least 37 weeks (not shown in Table 1). Although over half (52·6 %) were born outside hospitals, more than half (56·3 %) had skilled attendants at birth. However, only 42·3 % of those with CESHL had skilled attendants. The mean z-score was − 0·88 (sd 1·31) for weight-for-age, − 1·07 (sd 1·65) for height-for-age, and − 0·36 (sd 1·55) for BMI-for-age. Stunting was the most prevalent nutritional deficit in the general population (27·7 %) as well as in those with hearing loss (35·3 %). The vast majority of the infants in both groups were exclusively breast-fed but more than one-third (37·9 %) were undernourished by at least one measure. Established risk factors for CESHL such as consanguinity, family history of deafness and prematurity ( < 34 weeks) were non-existent among infants with CESHL in the present study.
A total of nine factors including wasting, underweight and ‘any undernourished physical state’ were significantly associated with CESHL in the univariate analyses. After the multivariable logistic regression analyses, underweight emerged as the only nutritional predictor of CESHL in the first two models. However, only three infants with CESHL were underweight without being stunted or wasted, thus underscoring the significant co-morbidity of nutritional deficits in this population. This was corroborated by the significant association between ‘any undernourished physical state’ and CESHL in the third and final model. In this model, ‘any undernourished physical state’ was associated with 67 % increased odds for CESHL (OR 1·67; 95 % CI 1·03, 2·77). The model was well calibrated (Hosmer–Lemeshow test: χ2 = 3·67, df = 4, P = 0·595) and similar in discrimination (c-statistic = 0·820) to the first model (c-statistic = 0·816) and the second model (c-statistic = 0·819). Non-nutritional factors retained in the final model were low social class (OR 5·94; 95 % CI 3·63, 9·74), chronological age of more than 30 d (OR 5·17; 95 % CI 3·10, 8·61), the absence of a skilled attendant at birth (OR 1·85; 95 % CI 1·12, 3·07) and severe neonatal jaundice (OR 12·71; 95 % CI 5·28, 30·57). No significant interactions were found among the variables included in the regression models.
The difference in mean z-scores between infants with CESHL and those without was significant for weight-for-age (P = 0·001) and BMI-for-age (P = 0·001) but not for height-for-age (P = 0·535). The mean z-score among malnourished infants with CESHL compared with non-malnourished infants with CESHL showed significant differences for weight-for-age (P < 0·001) and height-for-age (P < 0·001) but not for BMI-for-age (P = 0·071). Table 2 provides an overview of the co-morbidity of nutritional status for all infants with and without CESHL. Although there were virtually no significant differences in the severity of hearing loss among undernourished infants with CESHL, infants with any undernourished physical state were significantly associated with severe-to-profound hearing loss (P = 0·010) compared with infants without any nutritional deficits. In fact, compared with those without CESHL, infants with severe-to-profound CESHL were associated with almost four-fold odds of an undernourished physical state (OR 3·92; 95 % CI 1·38, 11·17).
Table 2 Severity of sensorineural hearing loss in undernourished infants
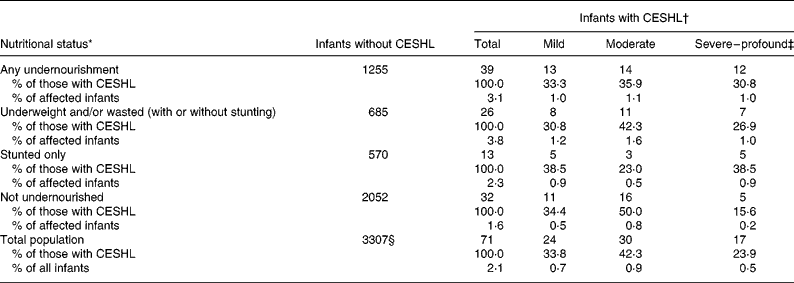
CESHL, congenital and/or early-onset sensorineural hearing loss.
* Comprising underweight, stunting and wasting based on z-scores for weight-for-age, length-for-age and BMI-for-age < − 2 sd using the WHO Child Growth Standards(16).
† Hearing loss classification: mild (30–40 dB hearing loss including all unilateral hearing loss); moderate (41–70 dB hearing loss); severe–profound (> 71 dB hearing loss).
‡ Comparison of all infants with and without any undernourishment showed a significant association between undernourishment and severe-to-profound CESHL (P = 0·010).
§ Missing data = 8 (0·2 %).
Discussion
The high prevalence of undernutrition in infants aged 0–3 months in a general population associated with high occurrence of maternal and childhood malnutrition has been demonstrated in the present study. The high prevalence of stunting in this age group is likely to be underpinned substantially by intra-uterine growth restriction among other factors. While more than half (55 %) of the infants with CESHL were undernourished by at least one measure of growth and development, evidence from the present study also suggest that infants who suffer from any undernourished physical state whether attributable to intra-uterine growth retardation, maternal problems including mother's nutritional status during fetal development or insults arising from infectious disease at or soon after birth are at significant risk of severe-to-profound sensorineural hearing loss very early in life. Thus, undernutrition leading to developmental deficit or co-existing with unrelated developmental deficit such as CESHL presents a double burden for the affected infants from such an early age and should be of concern for current global child health policy. As Black et al. aptly advocated, initiatives aimed at addressing undernutrition in the developing world need to recognise the multidimensional determinants and consequences of this condition transcending mortality and morbidity to the core domains of optimal early childhood development(Reference Black, Walker and Wachs13).
So far, most studies investigating the developmental trajectory of undernutrition or evaluating the nutritional status of children with disabilities have often focused on pre-school and school-aged children with little or no attention paid to the disease burden in the first year of life. Invariably, opportunities for effective intervention for developmental deficits such as CESHL are often missed. While undernutrition can be prevented or substantially curtailed by micronutrient supplementation, quality diet and improved breast-feeding practices it is still essential to provide safety nets for those who are unlikely to benefit adequately from such primary prevention initiatives. One study noted that malnutrition in early infancy is often not addressed in therapeutic feeding programmes as it conflicts with current guidelines for exclusive breast-feeding and because it requires the services of highly skilled medical personnel(Reference Prost, Jahn and Floyd17). As demonstrated in the present study existing well-established platforms for child health interventions offer opportunities for the early detection of undernourished infants and potential developmental consequences associated with this condition from early infancy.
The increased risk of CESHL associated with older infants is not unexpected considering the possible contribution of perinatally acquired causes of hearing loss from the prevailing adverse health and social conditions in resource-poor countries which would have caused some delay in attending well-child clinics for BCG. For example, the effects of the attack and treatment of common conditions such as septicaemia, meningitis and pneumonia are more likely to be detected when the affected infants are well enough after hospital discharge to attend the immunisation clinics usually after the first month of life. Although evidence from developed countries also point to an increasing rate of CESHL with age from early childhood, the underlying causes are likely to differ from the situation in developing countries(Reference Fortnum, Summerfield and Marshall18). As observed in the present study, the burden of CESHL is likely to be understated if hearing screening were limited to the first month of life. Hence, ongoing surveillance, particularly of infants with established risk factors for CESHL who passed the screening tests in the first month, is necessary. This is consistent with the recommended practice even in developed countries where the incidence of postnatally acquired CESHL is usually lower(4).
Studies associating malnutrition with deafness in infants within the first 3 months of life are rare. A systematic review of nutritional deficiencies in the pathogenesis of ear diseases also found limited evidence associating micronutrient and vitamin deficiency with middle-ear disease which is a significant cause of permanent hearing loss in young children(Reference Elemraid, Mackenzie and Fraser19). The review also noted the lack of studies on the possible influence of maternal nutritional deficits on the development of the auditory system of the fetus. Another study among apparently normal 7- to 11-year-old schoolchildren in a population with endemic cretinism, however, associated mild iodine deficiency with higher hearing thresholds particularly in children with high serum thyroglobulin concentrations(Reference van den Briel, West and Hautvast20). The evidence in the present report would therefore warrant a prospective large-scale study to establish the mechanism of the insult to the auditory system and the effects of specific micronutrient deficiencies in this pathway. Such studies are also needed to establish the potential contribution of intra-uterine growth retardation to the burden of CESHL in this age group which was not achievable in the present study due to the lack of anthropometric data at birth for the vast majority of the infants.
The findings on severe neonatal jaundice and the absence of skilled attendants at birth are consistent with our earlier observation during the first 10 months of this pilot project(Reference Olusanya, Wirz and Luxon21). Although the present study did not set out to determine the risk factors for undernutrition it is quite plausible for both of these factors to lie in the causal pathway for underweight in the first month of life. For instance, infants without skilled professionals present at birth, principally as a result of being born outside hospitals, may be more vulnerable to poor feeding practices than infants whose mothers benefited from health talks on newborn care in hospitals before discharge. Similarly, severe neonatal jaundice, particularly kernicterus, with the associated choreoathetoid cerebral palsy and the resultant motor and feeding difficulties is the commonest illness among newborns necessitating hospital (re)admission. The affected infants were likely to have experienced significant weight loss during this period due to suboptimal feeding. A detailed analysis of the feeding pattern of the participants was outside the scope of the present report but studies among older infants with developmental disabilities in developing countries such as India and Nigeria have found that feeding difficulties among these children predisposed them to a higher risk of undernutrition compared with their non-disabled peers(Reference Yousafzai, Filteau and Wirz22, Reference Tompsett, Yousafzai and Filteau23).
The observed association between CESHL and low social class accords with findings from other studies from both developed and developing countries and mirrors the broader impact of household poverty and economic deprivation on early childhood development(Reference Shonkoff and Phillips24). The symbiotic relationship between poverty or low socio-economic status and undernutrition is well documented in the literature and has been recognised as a major theme under the millennium development goals of the UN up to 2015(Reference Linnemayr, Alderman and Ka25, 26).
Despite the satisfactory performance of the final model, a number of limitations of the present retrospective cross-sectional study are worth noting. For example, an evaluation of the developmental status was limited because of the cross-sectional design of the study among infants not older than 3 months. The potential contribution of intra-uterine growth retardation to the observed association between nutritional status and CESHL could not be determined due to lack of data on birth weight and length. It was also not possible to ascertain causal relationships with hearing loss status as well as the degree of reversal or deterioration in the nutritional status of the participants for an extended period that would have been allowed by an ideal longitudinal study. In addition, the causative association between undernutrition and CESHL is still unclear and merits further investigation. It may also be useful to compare these outcomes with those based on other growth standards from the National Center for Health Statistics or the Centers for Disease Control. The potential impact of maternal nutritional status was also not explored. Nonetheless, the present study draws attention to a relatively uncharted course in our understanding of the full impact of malnutrition on early childhood development and underscores the need for well-designed prospective studies particularly in resource-poor communities.
Acknowledgements
During the study period, the author was affiliated to the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust (University College London, 30 Guilford Street, London WC1N 1EH, UK).
The support of Linda Luxon and Sheila Wirz of the Institute of Child Health, University College London on the substantive project that preceded this post-doctoral work is acknowledged. The author also received helpful statistical advice from three anonymous reviewers.
The hearing instruments for this project were provided by Natus Medical Inc., USA, Otodynamics (UK) Ltd and Oticon Foundation, Denmark. Training support was received from NHS Newborn Hearing Screening Programme/MRC Hearing and Communication Group, UK. Educational materials for parents and health professionals were sponsored by the Education Trust Fund, an agency of the Federal Government in Nigeria and Hearing International, Nigeria, a local charity, acted as financial guarantor. None of the sponsors was involved in the study design, collection, analysis and interpretation of data, the writing of the manuscript, or the decision to submit these results for publication.
The author has no conflict of interests to declare.




