I deficiency (ID) is recognised as a public health problem across the world. Severe ID throughout pregnancy increases the risk of mental retardation, may harm brain development and intelligence development. ID may also lead to goitre formation during pregnancy( Reference Lazarus 1 , Reference Burrow, Fisher and Larsen 2 ).
I requirements increase during pregnancy and breast-feeding. The fetus is highly dependent on maternal thyroxine sources for its neurodevelopment until its own thyroid gland begins to function. After formation of the fetal thyroid gland, fetal thyroid hormone production increases, contributing to increased maternal I requirements. As the maternal glomerular filtration rate increases from the beginning of early pregnancy, iodide filtration increases, resulting in a reduced circulating pool of plasma I. Thyroid hormone demand thus increases from the early weeks of gestation, and this necessitates an adequate I supply( Reference Lazarus 1 – Reference Leung, Pearce and Braverman 3 ).
The WHO recommends a daily I intake of 150 µg/d for non-pregnant women and 250 µg/d for pregnant and lactating women( 4 ). Low median urinary I concentration (UIC) values have been reported in the last few years in pregnant American women( Reference Stagnaro-Green, Abalovich and Alexander 5 ). The American Thyroid Association, the Endocrine Society and the Neurobehavioral Teratology Society have recommended that women planning a pregnancy or who are pregnant or lactating should receive 150 µg/d I-containing supplements( Reference Caldwell, Pan and Mortensen 6 – Reference Obican, Jahnke and Soldin 8 ). However, data from the National Health and Nutrition Examination Survey (2001–2006) showed that only a small percentage (20 %) of pregnant American women routinely took an I-containing supplement( Reference Gregory, Serdula and Sullivan 9 ). Routine I supplementation is also recommended in Europe, but this is not systematically applied in many European countries( Reference Zimmermann and Delange 10 ).
Goitre prevalence below 5 % in a population indicates that salt iodisation in that region is sufficient( 4 ). Goitre regression usually lags behind urinary I increment for about 1 or more years; the measurement of UIC is recommended in order to demonstrate the success of such programmes( 4 ). Although many urine samples (twelve samples or more) are required for individual measurements, studies have reported that one sample is sufficient for population surveys. In all, 500 spot urine samples are reported to be adequate to describe a population’s median I excretion with approximately 5 % precision( Reference Andersen, Karmisholt and Pedersen 11 ). This is clarified in a subsequent study by the same group. They state that the median I concentration of spot urinary samples obtained from at least 500 subjects is a reliable measure of the I intake of the population( Reference Vejbjerg, Knudsen and Perrild 12 ).
Erdoğan et al.( Reference Erdoğan, Erdogan and Emral 13 ) identified ID in various regions of Turkey by conducting surveys among school-age children (SAC) between 1997 and 1999. Monitoring studies performed after the mandatory iodisation of table salt in 2007 showed that ID had been eradicated in most regions of the country( Reference Erdogan, Ağbaht and Altınsu 14 ). Trabzon was defined as an I-sufficient area in 2007 with a median UIC of 145 µg/l among SAC( Reference Erdoğan, Demir and Emral 15 ).
The number of studies regarding I status in pregnant women is limited. Studies carried out in Turkey to date have been mainly local with small samples. The purposes of this study were to investigate I status in pregnant women in Trabzon, a severely I-deficient area in 1997, which is now reported to be I sufficient, according to SAC I surveys conducted in 2007, following mandatory iodisation of table salt in 1999, and to propose relevant I supplementation for pregnant women who are prone to ID( Reference Erdoğan, Erdogan and Emral 13 , Reference Erdogan, Ağbaht and Altınsu 14 ).
Methods
The present study was carried out in Trabzon province, which has a pregnant women population of approximately 4500/year, between September 2011 and September 2012. The sample size was calculated using the formula n=(DEFF×Np(1−p))/((d²/Z²1−a/2×(N−1)+p×(1−p)), where the number calculated was 843, the number planned was 1000 and the number included was 864 (DEFF=design effect=1; p=50 %; d=0·05; z=1·96; N 4500; α=0·05; power=99 %)( 16 ). Lists of pregnant women registered with family health physicians were obtained, and 1000 women were selected by systematic sampling. Their names were given to family health physicians and these women were then invited to participate in the study. Registered pregnant women were referred by family health physicians. Family health clinics were visited in cases where pregnant women were unable to attend our clinic. Mean age of the pregnant women was 28 (25th–75th percentile 17–47) years; 29·5 % were in the 1st trimester, 35·5 % were in the 2nd and 35 % were in the 3rd trimester (n 255, 307 and 302, respectively). Women in weeks 1–13 of gestation represented the 1st trimester, women in weeks 14–27 the 2nd trimester and women in weeks 27–40 represented the 3rd trimester. None of the pregnant women were using I-containing supplements. Fasting blood and urine samples were collected, and levels of thyroid-stimulating hormone (TSH), free triiodothyronine (T3), free thyroxine (T4), anti-thyroglobulin (anti-Tg) and anti-thyroid peroxidase (anti-TPO) were determined from the serum. Immunochemiluminescent assays performed on an automated analyzer (Advia Centaur XP; Siemens( Reference Owen, Gantzer and Lyons 17 )) were used to measure levels of TSH, free T3, free T4, anti-Tg and anti-TPO. Thyroid ultrasonography (TUSG) was performed on 820 women. Ultrasonography (USG) was performed using a Hitachi 12 MHz USG device (Hitachi) on the 247 pregnant women who attended the hospital and using a portable Chison 7·5 MHz USG device (Chison) for the 573 women examined at family health clinics, by the same observers on all occasions (I. A., O. I.). The inter-observer variability among the two sonographers was assessed in twenty patients and it was <15 %. Urine samples collected at family health clinics were transferred to the hospital daily. Samples collected at family health clinics and at the hospital were all stored in de-iodised I tubes at −40°C. A single spot urine sample was collected in the morning (between 09.00 and 12.00 hours) from each participant. UIC was measured using the spectrophotometric method described by Sandell & Kolthoff( Reference Sandell and Kolthoff 18 ). The I laboratory is a part of the Ensuring the Quality of Iodine Procedures (EQUIP) programme conducted by the Center for Disease Control (CDC), USA, since 2009. The I laboratory is externally controlled by the 2013–2014 EQUIP programme, three times per year, and our average success as decided by the CDC is 75–80 %/year. A standard questionnaire was administered by either a training nurse or the relevant physician (I. A.). Demographic data, monthly income, iodised salt usage (i.e. consumption without cooking) and storage conditions (protecting the salt against humidity or light) were recorded. Pregnant women with a known history of any thyroid disease (nodular goitre, thyroiditis, hypothyroidism, hyperthyroidism, history of any thyroid operation or use of any medication for any thyroid disease) were excluded.
Statistical Package for the Social Sciences (SPSS) software (SPSS version 16.0; SPSS Inc.) was used for the statistical analyses. Standard descriptive analysis was used. Data normality was assessed using the Kolmogorov–Smirnov test and ANOVA (Bonferroni test as post hoc) for normally distributed data and the Mann–Whitney U test and the Kruskal–Wallis test (Mann–Whitney U test with Bonferroni correction as post hoc) for non-normally distributed data. Results are shown as arithmetic means and standard deviations for normally distributed data and medians and 25th–75th percentile for non-normally distributed data. P<0·05 was considered to be significant.
All women invited to take part were provided with written information about the study, and written consent was obtained from all the participants. The study was approved by the local ethics committe of Trabzon Kanuni Education and Research Hospital (Approval No. 27.12.2010-8/2010).
Results
The median UIC was 102 (25th–75th percentile=62–143) µg/l. Table 1 summarises the results of UIC in pregnant women including distribution of UIC by trimesters. UIC was >150 µg/l in only 191/864 (22·1 %) women and <150 µg/l in 673/864 (77·9 %) women. UIC in the 1st trimester was higher compared with the 2nd and 3rd trimesters. The median UIC was 122 µg/l at the 1st, 97 µg/l at the 2nd and 87 µg/l at the 3rd trimester (P<0·017) (Table 1). Thyroid function tests in terms of trimester are given in Table 2. Mean TSH values (reference range: 0·35–5·5 µIU/ml( Reference Sandell and Kolthoff 18 )) were 1·7 (sd 2·9), 1·7 (sd 1·2) and 2·1 (sd 1·31) µIU/ml, respectively, in the 1st, 2nd and 3rd trimesters (P<0·001; for all trimesters). Free T4 and T3 values decreased significantly during pregnancy (P<0·001; for all trimesters).
Table 1 Urinary iodine concentrations (UIC)Footnote * of pregnant women according to trimesters (Medians and 25th–75th percentiles)

* Morning spot urine samples.
† The median UIC of the 1st trimester is higher than the median UIC of the 2nd and 3rd trimesters.
‡ The difference is derived from the 1st trimester (P=0·017).
Table 2 Thyroid function tests of pregnant women with regard to trimesters (Mean values and standard deviations)

TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine.
* Normal reference range; not specific for trimesters.
† ANOVA.
The mean thyroid volume was 12·9 (sd 7·5) ml. Of the study population, 14·8 % (n 121) had thyroid volumes exceeding 18 ml. There was no correlation between thyroid volume and trimester.
Thyroid nodules were present in 17·7 % of pregnant women (n 153), and thyroid autoantibody positivity was observed in 14·5 % (n 119). Thyroid autoantibody positivity was significantly higher in those with heterogeneous thyroid parenchyma (n 186, 22·7 %) (P<0·001) reported at TUSG.
A total of 248 subjects (28·4 %) stated that they were aware of the importance of ID and its consequences. Of the 864 subjects, 784 (90·7 %) reported that they were using iodised salt, whereas thirty subjects (3·5 %) reported that they were not using it, and others (5·8 %) stated that they did not know about the importance of using iodised salt. When asked whether they stored or added the salt to food appropriately – that is, adding towards the end of the cooking process – 303 (64·9 %) subjects were found to be using it inappropriately by adding iodised salt at the beginning of the cooking process. In terms of storage, 216 (25 %) subjects used appropriate containers, and UIC levels were not different when stratified by the use of iodised salt, history of I-rich diet, awareness of the relationship between I and pregnancy, education level, monthly income level or history of thyroid disease in the family (Table 3) (P>0·5).
Table 3 Analysis of urinary iodine concentrations according to related factors (Medians and 25th–75th percentiles)
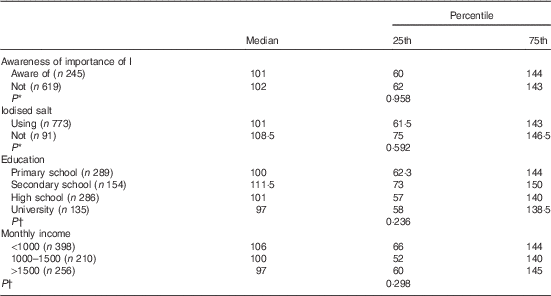
* Mann–Whitney U test.
† Kruskal–Wallis analysis.
Discussion
UIC measurement in spot urine samples is recommended for determination of I status among SAC by the WHO and International Council for Control of Iodine Deficiency Disorders (ICCIDD) for population I nutrition monitoring. It has also been reported to be useful for pregnant and lactating women( 4 ).
In order for a population to be considered I sufficient, the median UIC of SAC in that community must be >100 µg/l. Median UIC of 50–99 µg/l indicates mild, 20–49 µg/l indicates moderate and <20 µg/l indicates severe ID( 4 ). However, pregnant women have greater I requirements for the reasons discussed above. For pregnant women, median UIC >150 µg/l is insufficient, and it should be between 150 and 249 µg/l for this population( 4 ).
I status of SAC in Trabzon was reported to be severely deficient (median UIC of 14 µg/l) in a national survey conducted between 1997 and 1999( Reference Erdoğan, Erdogan and Emral 13 ). Following the mandatory iodisation of table salt with 25–40 mg/kg KIO3 or 50–70 mg/kg KI, monitoring studies in 2002 and 2007 determined median UIC of 113 and 145 µg/l, respectively, among SAC in Trabzon, showing that the province was I sufficient( Reference Erdogan, Ağbaht and Altınsu 14 , Reference Erdoğan, Demir and Emral 15 ).
These nationwide monitoring studies of SAC by Erdoğan et al.( Reference Erdoğan, Erdogan and Emral 13 , Reference Erdogan, Ağbaht and Altınsu 14 , Reference Erdogan, Erdogan and Delange 19 ) showed a significant correction of ID, especially in urban areas. However, previous studies among the pregnant population were limited and involved small sample sizes( Reference Mocan, Erem and Telatar 20 – Reference Kutlu and Kara 26 ). These small-sized studies among pregnant women from different regions of Turkey reported median UIC between 77·4 and 149·7 µg/l and that 49·6–100 % of pregnant women were at risk of ID( Reference Mocan, Erem and Telatar 20 – Reference Kutlu and Kara 26 ). Although the median UIC of SAC from most of these areas was sufficient or mildly deficient, ID was still a common problem among pregnant women( Reference Mocan, Erem and Telatar 20 – Reference Kutlu and Kara 26 ).
Current data from the Trabzon provincial centre, formerly endemic but currently an I-sufficient region in the light of SAC surveys, show that the population median UIC is below the WHO threshold of adequacy in all three trimesters of pregnancy. The use of iodised salt in the region among the pregnant population needs additional I support from the 1st trimester onwards. Large-scale monitoring studies recently conducted among pregnant women from the cities of Ankara and İstanbul also support our results (A Aktaş, Ü Aydınn, Bİ Aydoğan, K Erkenekli, G Koç, Z Berberoğlu, Y Aral, MF Erdoğan, unpublished results, and E Oral, AS Açıkgöz, B Aydoğan, Bİ Aydoğan, HÇ Acıoğlu, GA İlhan, B Dane, A Özel, H Erenel, B Tandoğan, E Çakar, H İşçi, B Kayan, H Aslan, A Ekiz, S Sancak, A Çelik, T Yoldemir, Ö Uzun, MF Erdoğan, unpublished results). The results of the 1st study performed among 312 pregnant women showed that 76, 82 and 91 % of women from Ankara – a borderline I-sufficient city( Reference Erdogan, Ağbaht and Altınsu 14 ) – were I deficient during the 1st, 2nd and 3rd trimesters of pregnancy, respectively (A Aktaş, Ü Aydın & Bİ Aydoğan, unpublished results). Smilarly, although İstanbul is described as an I-sufficent city in SAC surveys( Reference Erdogan, Ağbaht and Altınsu 14 ), one multicentre study involving 3543 pregnant women from nine different gynaecology and obstetrics clinics from the city of İstanbul, conducted in 2013, showed that 90·7 % of pregnant women were I deficient during pregnancy (E Oral, AS Açıkgöz & B Dane, unpublished results). UIC measured from single samples from pregnant women was not associated with history of stillbirth, congenital anomalies or spontaneous abortus, in agreement with our study results (E Oral, AS Açıkgöz & B Dane, unpublished results).
The consequences of severe ID during pregnancy and lactation are well known, whereas studies concerning the effects of mild-to-moderate ID are inconsistent( Reference Zimmermann, Jooste and Pandav 27 ). A recent observational study of 1040 mothers and their children showed that even mild ID in utero is linked to lower intelligence quotient and suboptimal reading ability in 8-year-old children( Reference Bath, Steer and Golding 28 ). Children’s scores worsened when the deficient group (UIC<150 µg/l) was subdivided into groups with UIC between 50 and 150 µg/l and those with UIC<50 µg/l( Reference Bath, Steer and Golding 28 ). Similarly, a study from Tasmania showed that children whose mothers had UIC<150 µg/l during pregnancy scored 10 % lower in spelling, 7·6 % in grammar and 5·7 % in English literacy performance compared with children whose mothers had UIC>150 µg/l( Reference Hynes, Otahal and Hay 29 ). Another study from the Netherlands showed that low maternal UIC during pregnancy is associated with impaired executive functioning in children( Reference van Mil, Tiemeier and Bongers-Schokking 30 ).
Among intervention studies examining the impact of maternal I supplementation on the neurodevelopment of children, Velasco et al.( Reference Velasco, Carreira and Santiago 31 ) observed that children of I-supplemented mothers exhibited better behavioural and psychomotor performance than those of untreated mothers. In a study of development quotient from Spain, delayed neurobehavioural performance was reported to be higher in children whose mothers did not receive supplementation from early pregnancy( Reference Berbel, Mestre and Santamaria 32 ).
Murcia et al.( Reference Murcia, Rebagliato and Iniguez 33 ) reported lower psychomotor development indices among infants whose mothers received >150 µg/d of I supplementation than those of mothers receiving <100 µg/d. In contrast, Santiago et al.( Reference Santiago, Velasco and Muela 34 ) determined no difference in terms of mental development or psychomotor scales, between mothers using iodised salt or I supplementation. A recently published study from the Netherlands also detected no association between maternal low UIC and children’s cognition. In that study, the authors stated that the research was not powerful enough to detect a significant association because the number of women with ID was very low( Reference Ghassabian, Steenweg-de and Peeters 35 ). Finally, a large meta-analysis investigating the effects of maternal I supplementation concluded that large-scale controlled, prospective trials are needed to clarify the situation( Reference Taylor, Okosieme and Dayan 36 ).
We found no difference when our results were stratified by the use of iodised salt, awareness of the importance of I in pregnancy, education level or monthly income. This may be due to the use of spot and single urine examples, as this was a population survey study. More samples per participant (e.g. ten to twelve independent urine samples per participant) could yield I status at the individual level. More participants, with only single spot urine per participant, may not appreciably add to the interpretation of population median values as conducted in the present study and would not increase the interpretive value for individuals. As described in two different reviews, analyses of as few as two independent urine samples per participant, followed by appropriate statistical modelling to remove within-individual variation, could yield fully interpretable population distributions of habitual UIC, which could be effectively used to evaluate proportion of the study below a threshold( Reference Zimmermann and Andersson 37 , Reference Rohner, Zimmermann and Jooste 38 ).
ID levels as well as TSH levels increased by trimester in our study. Median UIC were lower in the 2nd and 3rd trimesters. Free T3 and free T4 levels in the serum decreased parallel to the increase in TSH and decrease in median UIC. These findings may partly be explained by physiological changes in thyroid hormone metabolism in pregnancy (i.e. dilution or an increasing glomerular filtration rate and increasing I demand), as well as the worsening of I status during the trimesters( Reference Lazarus 1 – 4 ).
In terms of thyroid nodularity (the prevalence of nodule in the general population), the 35·2 % prevalence rate reported by a prevalence study performed in our region by Koçak et al.( Reference Koçak, Erem and Deger 39 ) was considerably higher than that in our population. However, the prevalence of nodules in the young population (20–29 years) in that study was 12·5 %, rising to 38·4 % in individuals aged over 70 years( Reference Koçak, Erem and Deger 39 ). Similarly, overall goitre prevalence was higher than that in our population (14·8 v. 28·4 %), and there was a significant increase in volume with advancing age. In this formerly I-deficient area, prevalence of goitre was 12·5 % in the 20–29 years age group and 38·4 % in the over 70 years age group( Reference Koçak, Erem and Deger 39 ).
Iodised salt stored in open containers may lose 50–100 % of its I content due to humidity and other factors when KI is used for iodisation( Reference Diosady, Alberti and Venkatesh 40 ). Similarly, Wang et al.( Reference Wang, Zhou and Wang 41 ) reported that iodised salt loses 2–63 % of its I content when salt is added before or early during cooking. In all, 25 % of the women in our study stored iodised salt appropriately, and 65 % added iodised salt properly after cooking. Fortunately KIO3, which is much more stable than KI, has been used for salt iodisation in Turkey since 2010(42).
In conclusion, despite the global I supplementation programme, which has improved I status worldwide, ID is still a serious health problem, especially among pregnant women. Current knowledge is in favour of I supplementation in this risk group. Until the effects of maternal I supplementation, in mild ID, are clarified by large-scale prospective controlled trials, pregnant women living in borderline deficient and I-sufficient areas, such as Trabzon city, should receive 100–200 µg/d I-containing supplements in addition to iodised salt, depending on the severity of defficiency among SAC.
Acknowledgements
The authors thank the family physicians for providing lists of registered pregnant women and inviting them to participate in the study. The authors also thank the nurse Ebru Güneykaya and the secretaries Hatice Konaş and Sevgi Özer for their secretarial assistance.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors’ contributions are as follows: I. A., E. A. and M. F. E. designed the study; I. A. and O. I. collected data; I. A. drafted the paper; I. A. and M. F. E. wrote the manuscript; and I. A., M. F. E. and M. T. were responsible for data analyses and data interpretation. M. F. E. critically read and revised the paper. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.






