Traditionally, mink rations have been supplemented with relatively large amounts of fat-soluble vitamins( Reference Lassén 1 ), compared with rations for other species, owing to the risk of vitamin losses in the perishable ingredients of mink diets (e.g. offal from the fishing industry and abattoirs). Improved chemical stability of commercial vitamin mixtures, improved mixing, transport and storage conditions of feed rations as well as the inclusion of large amounts of pig and poultry slaughter offal in mink rations during recent years have increased the risk of over-supplying mink with fat-soluble vitamins. Over-supply of fat-soluble vitamins, particularly with retinol (RET), increases the risk of liver damage and has in several species been shown to negatively affect the bioavailability of cholecalciferol (vitamin D3 (VD3)) and α-tocopherol (TOC)( Reference Aburto and Britton 2 – Reference Linday, Umhau and Schindledecker 5 ).
A sufficient and balanced supplementation with fat-soluble vitamins is vital to secure satisfactory growth and an effective immune system in mink and other species( Reference Kono and Arai 6 ). The fat-soluble vitamins RET, VD3 and TOC are particularly sensitive to over- or under-supply in the diet, because these vitamins have been shown to interact heavily in vivo and in vitro with respect to dose–response and chemical properties( Reference Aburto and Britton 2 , Reference Aburto and Britton 3 , Reference Goncalves, Roi and Nowicki 7 ).
RET, VD3 and TOC are complex substances involved in many physiological functions in the body. RET is required for various biological processes including vision, function of epithelial surfaces and the immune system, reproduction and normal embryonic development( Reference Ross and Harrison 8 ). VD3 is involved in processes ranging from maintaining Ca homoeostasis in plasma and bones to modulating the function of the immune system( Reference Braidman and Anderson 9 , Reference Lips 10 ). However, to become physiologically active, VD3 must be hydroxylated first in the liver to 25-hydroxycholecalciferol (25VD3), which is the main VD3 metabolite in plasma and the form measured as an indicator of physiological VD3 status, and later in the kidneys to the physiologically active metabolite 1,25-dihydroxycholecalciferol. TOC acts as an antioxidant in the body and is very important for the development and functionality of the immune system( Reference Nakamura and Omaye 11 ). It exists in eight different isomeric configurations: four (RSS, RRS, RRR, RSR) with 2R configuration and four (SRR, SRS, SSR, SSS) with 2S configuration. The RRR isomer is the form of TOC that occurs in nature( Reference Kono and Arai 6 ), whereas TOC used for feed additives consists of a racemic mixture (all-rac) of all eight stereoisomers. In commercial vitamin mixtures, all-rac-TOC is typically acetylated, in order to stabilise its functional phenol group during storage and feed processing (pelleting, etc.), and therefore is added to rations as all-rac-α-tocopheryl acetate. The acetate group must be hydrolysed before the TOC can be absorbed from the gastro-intestinal tract( Reference Jensen, Engberg and Hedemann 12 ). According to the officially accepted biopotency factor observed in rats, RRR-TOC is 1·49 times more active than all-rac-α-tocopheryl acetate( 13 ). However, in humans, only TOC with 2R configuration is considered biologically active( 14 ). Jensen & Lauridsen( Reference Jensen and Lauridsen 15 ) showed that mink, like humans, almost completely exclude TOC with the 2S configuration from circulation, and Blatt et al. ( Reference Blatt, Pryor and Mata 16 ) showed that the relative biopotency of RRR-TOC and all-rac-TOC varied with dosage. To counterbalance these discrepancies in the present experiment, it was decided to add the same amount of natural and synthetic TOC on molecular basis.
Interactions between RET, VD3 and the different stereoisomers of TOC are described in several species. For instance, supplementation with large doses of RET has been shown to negatively affect the bioavailability of TOC in growing pigs( Reference Ching, Mahan and Wiseman 17 , Reference Olivares, Rey and Daza 18 ) and calves( Reference Ametaj, Nonnecke and Franklin 4 ). RET supplementation has been shown to affect the bioavailability of VD3 in both humans( Reference Linday, Umhau and Schindledecker 5 , Reference Spiesman 19 ) and poultry( Reference Aburto and Britton 2 , Reference Aburto and Britton 3 ), which has led to the conclusion that the ratio of RET:VD3 seems to be more important than the actual concentrations of the vitamins in the diet( Reference Linday, Umhau and Schindledecker 5 , Reference Spiesman 19 , Reference Metz, Walser and Olson 20 ). The aim of the present study was to elucidate the risk of over-supplementing mink with RET and study the interactions between RET, VD3 and TOC in this species.
Methods
The present study complied with the Danish Ministry of Justice Law no. 1306 (23 November 2007) concerning experiments with animals and care of animals used for experimental purposes, and was conducted with the approval of the Danish Veterinary and Food Administration under the Danish Ministry of Food, Agriculture and Fisheries. The study was carried out at the Copenhagen Fur Research Centre in Holstebro, Denmark, between 1 July and 15 September 2010.
Animals, housing and feeding
A total of 110 male and female brown mink kits (Mustela vison) were used for the study. Littermates were housed pairwise (one male and one female) in cages from 2 weeks before the start of the study. All animal facilities were sheltered under permanent outdoor sheds with no sunlight access. The basic ration was a wet mink feed ration. The composition of this ration as fed is shown in Table 1. For each treatment group, 180 kg of the basic ration was supplemented with TOC, RET and VD3 according to the treatment plan and stored at −18°C in 5-kg bags. One bag per day was thawed and fed to the respective treatment group during the study period. The mink were fed once a day and had ad libitum access to water.
Table 1 Composition of basic ration as fed

* Vitral B-mink super (Agro Korn A/S). B1 3000 mg/kg; B2 6000 mg/kg; B6 5000 mg/kg; folic acid 1000 mg/kg; B12 20 mg/kg; d-pantothenic acid 15 000 mg/kg; biotin 200 mg/kg; choline chloride 100 000 mg/kg.
† Protein:fat:carbohydrates.
Treatments and design
The study was carried out as a longitudinal dose–response study with 100 mg/kg feed per d of either RRR-TOC or all-rac-TOC and different amounts of RET added to the feed between treatment groups. Before the study period, mink were placed in pairs (one male and one female) in cages for 2 weeks to habituate them to each other and to their housing facilities. At the beginning of the study, mink were weighed and started on the basic ration without added RET, TOC and VD3, and were maintained on this diet for 4 weeks to minimise their stores of fat-soluble vitamins and decrease the variability between animals. The natural concentrations of RET and TOC in the diets were analysed three times in July in duplicate each time and averaged to 0·29 (SE 0·12) mg/kg feed (972 (SE 402) IU/kg) for RET and 22 (se 3) mg/kg feed for TOC. VD3 was not analysed in the feed. Subsequently, the mink were weighed and randomly assigned to eleven treatment groups with ten mink per treatment. One group – group 0 – was euthanised immediately using CO2, which was considered the control group. The remaining groups 1–10 were treated as follows: groups 1–5 received 100 mg all-rac-TOC/kg feed and groups 6–10 received 100 mg RRR-TOC/kg feed daily. In addition to the added TOC, groups 1 and 6 received 0 IU RET/kg feed, groups 2 and 7 received 1500 IU RET/kg feed, groups 3 and 8 received 3000 IU RET/kg feed, groups 4 and 9 received 4500 IU RET/kg feed and groups 5 and 10 received 6000 IU RET/kg feed daily. All feed rations were supplemented with 700 IU VD3/kg feed. RET was added as all-trans-retinyl acetate, all-rac-TOC was added as all-rac-α-tocopheryl acetate, RRR-TOC was added as ImmunE® Natur (Agro Korn A/S) and VD3 was added as pure cholecalciferol (Agro Korn A/S). All vitamins were kindly provided by Agro Korn A/S. Mink in treatment groups 1–10 were weighed and euthanised using CO2 after 4 weeks of study. The TOC concentration of the experimental diets was analysed in duplicate for each of the ten experimental groups and presented as the mean concentration of each source (n 5). RET concentrations in the two diets not supplemented with RET were analysed in duplicate, and the results are presented as the mean concentration of these two diets.
Sample material
Feed intakes were measured by weighing refusals, and feed samples were collected once per study period and on the last day of the study. After euthanasia, blood samples were extracted from all mink using a syringe by heart puncture and placed in sodium heparin-coated sample tubes and left on ice to cool. Blood samples were centrifuged at 2000 g for 10 min to isolate the plasma. Liver and kidneys from all mink were removed, weighed and placed on ice. The gall bladder was punctured, and its contents were removed before weighing the liver. All plasma and organ samples were subsequently stored at −18°C until analysis.
Chemical analyses
Chemical analyses were performed at the Department of Animal Science, Aarhus University. All the samples and standard vitamin solutions were protected from light during preparation. Plasma, feed and organ concentrations of RET and TOC were determined as described by Jensen et al.( Reference Jensen, Jensen and Jakobsen 21 ). The stereochemical composition of TOC in plasma and liver was determined after methylation of TOC stereoisomers into their methyl esters and subsequent separation by chiral HPLC as described by Jensen et al.( Reference Jensen, Nørgaard and Lauridsen 22 ). Plasma concentrations of 25VD3 and VD3 were determined as described by Hymøller & Jensen( Reference Hymøller and Jensen 23 ). In summary, after precipitation of proteins in ethanol and methanol, saponification of TAG in potassium hydroxide and extraction of fat-soluble vitamins in heptane, samples were dried using N2 and the residues were re-dissolved in 85 % methanol. Separation and quantification of the vitamins were carried out by reverse-phase gradient HPLC and UV-detection at 265 nm, using 1α-hydroxycholecalciferol (Sigma-Aldrich) as the internal standard. Organ samples were homogenised by Ultra Turrax (IKA Labortechnik) in an ice bath and, as plasma and feed samples, were precipitated in ethanol and methanol, saponified with potassium hydroxide and extracted in heptane. Separation and quantification were carried out by HPLC as described by Jensen et al.( Reference Jensen, Jensen and Jakobsen 21 ).
Statistical analyses
ANOVA was performed using the MIXED models procedure of SAS® (SAS Institute). The statistical model used was Y
ijkl
=μ+α
i
+β
j
+(αβ)
ij
+λ
k
+g
ijkl
+e
ikjl
, where Y
ijkl
is the dependent variable, μ the overall mean, α
i
the fixed effect of RET concentration i in the feed (0, 1500, 3000, 4500, 6000 IU/kg feed), β
j
the fixed effect of TOC source j in the feed (RRR-TOC, all-rac-TOC), (αβ)
ij
the effect of the interaction between RET concentration i and TOC source j in the feed, λ
k
the fixed effect of animal sex k (male, female), g
ijkl
the random effect of group l and e
ijkl
the random residual error. To test for any effect of interaction between RET level in the feed, TOC source in the feed and sex of the mink, this interaction was initially included in the model but subsequently removed because of there being no significant effects. When comparing male and female mink in the control group 0, only sex was included in the model. The linear relation between liver concentrations of RET and feed concentrations of RET and plasma concentrations of TOC and feed concentrations of RET was estimated using the REQ procedure of the SAS® system. Random effects were assumed to be normally distributed with a mean value of 0 and constant variance of
![]() $$g_{{ijkl}} \sim N\left( {0,\sigma _{g}^{2} } \right)$$
and e
ikjl
~N(0, σ
2). Differences were considered statistically significant when P≤0·05 and as tendencies if 0·05<P≤0·10. Results of the statistical analysis are presented as least squares mean with their standard error of the mean.
$$g_{{ijkl}} \sim N\left( {0,\sigma _{g}^{2} } \right)$$
and e
ikjl
~N(0, σ
2). Differences were considered statistically significant when P≤0·05 and as tendencies if 0·05<P≤0·10. Results of the statistical analysis are presented as least squares mean with their standard error of the mean.
Results
Male mink in the control group 0 weighed 1768 (SE 135) g and female mink weighed 1200 (SE 121) g when euthanised before the study period. Plasma and liver concentrations of RET, 25VD3, TOC and stereoisomers of TOC in control group 0 are shown in Table 2. Plasma RET concentrations were higher in female mink than in male mink (P=0·03), but no other significant differences were found at this point. At euthanasia, after the study period, male mink weighed on average 2577 (SE 37) g and female mink 1500 (SE 37) g. Male mink grew on average 769 (SE 74) g and female mink 319 (SE 48) g during the study period. The natural RET concentration in the basic ration during the study period was 3525 (SE 554) IU/kg feed. The concentration of TOC in the diets given to the all-rac-TOC group was 91 (SE 8) mg/kg feed and that in the diets given to the RRR-TOC group was 93 (SE 33) mg/kg feed. Feed consumption, weight of the mink and growth rate were not significantly affected by adding RET and TOC to the ration. The average feed consumption was 6·3 (se 1·9) kg/mink during the study period.
Table 2 Plasma, liver and kidney concentrations of retinol (RET), 25-hydroxycholecalciferol (25VD3) and α-tocopherol (TOC) and TOC stereoisomers in plasma and liver in male and female mink in the control group 0 (Least square mean values with their standard errors of means; n 5)
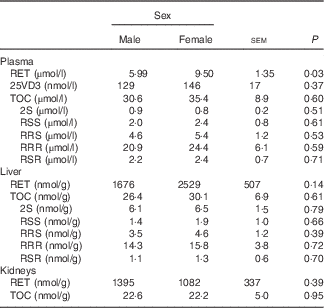
The RET concentration in plasma was higher in female mink than in male mink (P≤0·001), and plasma concentrations of RET tended to increase with increasing amounts of added RET to the feed (P=0·06). However, the plasma RET concentration was not significantly affected by whether RRR-TOC or all-rac-TOC was added to the feed along with RET (P=0·12) (Table 3). The plasma concentration of 25VD3 was lower when the RET concentration in the feed was high (P≤0·001), and was higher in mink receiving all-rac-TOC in the feed than in mink receiving RRR-TOC in the feed (P≤0·001). Therefore, the plasma 25VD3 concentration was the highest when RET in the feed was low and all-rac-TOC was added to the feed, and was the lowest when RET in the feed was high and RRR-TOC was added to the feed (Table 3). Plasma concentrations of 25VD3 were the highest (274 nmol/l; P≤0·05) at a RET:VD3 ratio of 4·29:1 in the feed, calculated on an IU basis. The corresponding RET concentration was 10·12 µmol/l (Table 4). Sex of the mink had no significant effect on plasma levels of 25VD3 (P=0·57).
Table 3 Plasma concentrations of retinol (RET), 25-hydroxycholecalciferol (25VD3), α-tocopherol (TOC) and TOC stereoisomers depending on added RET level and TOC type in the feed (Least square mean values with their standard errors of means; n 10)

* All-trans-RET acetate added to the feed.
† 100 mg/kg feed of all-rac-TOC or RRR-TOC.
Table 4 Plasma concentrations of 25-hydroxycholecalciferol (25VD3) and retinol (RET) in relation to the RET:cholecalciferol (VD3) ratio in the feed (Least square mean (LSM) values with their standard errors of means; n 10)

a,b,c LSM values within a row with unlike superscript letters were significantly different (P≤0·05).
* All-trans-RET acetate added to the feed.
† Added as pure VD3.
Overall, the concentration of TOC in plasma was unaffected by the RET concentration of the feed (P=0·63) and no interaction was observed, but plasma TOC concentrations were higher in mink receiving RRR-TOC in the feed than in those receiving all-rac-TOC (P≤0·01) (Table 3). However, a separate regression analysis of each of the two TOC sources showed a linear increase in TOC concentration for all-rac-TOC with increasing RET concentration in the feed (R 2 0·96; P≤0·01). This increase was mainly caused by a relative increase in RRR-TOC, whose relative contribution increased linearly from 43 to 48 % of all TOC stereoisomers with increasing RET concentration in the feed (P=0·03). Again female mink had a higher TOC concentration in plasma than male mink (P≤0·01). Concentrations of RRR-TOC in plasma were higher in mink receiving RRR-TOC in the feed than in mink receiving all-rac-TOC (P≤0·001), whereas concentrations of other stereoisomers of TOC were higher in mink receiving all-rac-TOC in the feed than in mink receiving RRR-TOC (P≤0·001) (Table 3).
Organ weights were similar among groups, and on average the weight of the liver was 3·3 (SE 0·6) g/100 g mink and the weight of both kidneys was 0·5 (SE 0·1) g/100 g mink. RET concentrations in the liver tended to be affected by the RET concentration of the feed (P=0·05) (Table 5) and as an average of all mink increased linearly with increasing feed RET concentrations (P≤0·001; R 2 0·98). There was no significant effect of TOC source in the feed on liver RET concentrations (Table 5), but female mink had higher liver RET concentrations than males (P≤0·05). RET concentrations of the kidneys were unaffected by the RET concentration in the feed, but were generally higher in mink receiving RRR-TOC than in those receiving all-rac-TOC (P≤0·01), except for the 1500 IU group, which resulted in a significant interaction between RET and TOC in the feed (P≤0·05). Male mink had higher RET concentrations in the kidneys compared with female mink (P≤0·001).
Table 5 Liver and kidney concentrations of retinol (RET) and α-tocopherol (TOC) and liver concentrations of TOC stereoisomers depending on added RET level and TOC type in the feed (Least square mean values with their standard errors; n 10)

* All-trans-RET acetate added to the feed.
† 100 mg/kg feed of all-rac-TOC or RRR-TOC.
The level of RET in the feed had no significant effect on TOC levels in the kidneys and liver (Table 5). TOC concentrations were higher in the kidneys of mink receiving RRR-TOC than in those receiving all-rac-TOC in the feed (P≤0·05). In contrast, TOC concentrations in the liver were higher in mink receiving all-rac-TOC compared with mink receiving RRR-TOC (P≤0·001). In the liver, 2S-TOC accounted for 45 % of total TOC, whereas RRR-TOC only accounted for 24 % of total TOC in the liver of mink fed all-rac-TOC. RRR-TOC accounted for 95 % of total TOC in livers from mink fed RRR-TOC. In general, female mink had higher TOC concentrations in the liver (P≤0·01) and tended to have higher TOC concentrations in the kidneys than male mink (P=0·07). Concentrations of stereoisomers of TOC in the liver were unaffected by the RET concentration in the feed, and no interactions between RET concentration and TOC source in the feed were observed.
Discussion
The natural concentration of RET in the feed was higher than that expected during the study period, probably due to the large amounts of poultry and fish offal used, which contain various amount of entrails including liver, and therefore a certain amount of RET in the form of retinyl palmitate. The TOC concentration corresponded to the added amounts. Adding RRR-TOC to the rations appeared to cause a larger variation in TOC concentrations of the rations than adding all-rac-TOC, probably due to mixing technicalities. Feed consumption and growth rate of the mink were similar among treatment groups, probably because none of the treatment groups experienced RET deficiency due to the high natural RET concentration of the feeds.
The lower variability in body weight between animals and sexes at the beginning of the study compared with the end of the study may be related to a relatively higher growth rate of the mink at the beginning of the study than by the end of the study. A high growth rate emphasises differences between animals in general, and between males and females, owing to a higher growth rate in males than in females. The differences between male and female mink in concentrations of fat-soluble vitamins in plasma and organs are most likely related to differences in feed consumption, growth rate and feed utilisation between sexes. However, no reliable data supporting this are available, because mink are typically housed one male and one female together during the growth season to avoid aggressive behaviour.
In species other than mink, supplementing diets with large doses of RET has been shown to negatively affect the bioavailability of TOC. In growing pigs, Ching et al. ( Reference Ching, Mahan and Wiseman 17 ) found a negative impact on serum and liver TOC concentrations from increasing dietary levels of RET from 2200 to 13 200 IU/kg, regardless of whether 15 or 90 IU/kg RRR-TOC or all-rac-TOC was supplemented in the feeds. In pigs fed a diet containing approximately 110 mg/kg TOC and either 100 000 IU RET/kg diet or 0 IU RET/kg diet, the liver concentrations of TOC were significantly lower in pigs fed the diet high in RET than in pigs fed the diet low in RET( Reference Olivares, Rey and Daza 18 ). Moreover, in calves, feeding large amounts of RET was associated with reduced plasma levels of RRR-TOC( Reference Ametaj, Nonnecke and Franklin 4 ). The physiological explanation behind this apparent antagonistic relation between RET and TOC is unknown; however, in rat liver, RET has been shown to suppress gene expression of proteins related to the formation of carriers of RRR-TOC (e.g. lipoproteins in plasma)( Reference Zolfaghari and Ross 24 , Reference Zolfaghari and Ross 25 ). In cell cultures modelling intestinal epithelium with Caco-2 cells, Goncalves et al.( Reference Goncalves, Roi and Nowicki 7 ) showed that both RET and VD3 added to the cell medium reduced the uptake of TOC in a dose-dependent manner. The enzyme (carboxyl ester hydrolase) responsible for hydrolysing the ester of TOC has been shown in in vitro studies to be non-competitively inhibited by RET-acetate( Reference Lauridsen, Hedemann and Jensen 26 ). In the present study, the RET concentration in the feed had no effect on TOC concentrations in plasma, liver or kidneys, regardless of the type of TOC supplied to the mink. There are several possible explanations for this finding: as a carnivore, mink may be biologically suited to handling large amounts of RET originating from their prey, or the carboxyl ester hydrolase enzyme activity may not be a limiting factor in mink. A similar lack of antagonistic relation between RET and TOC has been previously shown in humans, where the RET concentration in plasma – as an indicator of RET nutritional status – had no correlation to TOC levels in plasma in lactating women, whereas TOC levels in the colostrum correlated negatively to the plasma RET concentration( Reference Lira, Lima and Mendeiros 27 ).
Supplementing the mink in the present study with RRR-TOC gave rise to a higher concentration of TOC in plasma and kidneys than supplementing with all-rac-TOC, which on the other hand caused a higher TOC concentration in the liver. In growing male mink kits, it was previously shown that plasma and liver concentrations of RRR-TOC decreased throughout the entire weaning period in RRR-TOC-supplemented mink, whereas concentrations of 2S-isomers of TOC increased in all-rac-TOC-supplemented mink( Reference Hedemann, Clausen and Jensen 28 ). Furthermore, serum and liver TOC concentrations in growing pigs were found by Chung et al.( Reference Chung, Mahan and Lepine 29 ) to be higher when RRR-TOC was used as a feed additive than when all-rac-TOC was used in amounts between 16 and 96 IU/kg feed. This is probably because the synthetic isomers of all-rac-TOC are accumulated in the liver, owing to the preference of TOC transfer protein (α-TTP) for secreting the natural RRR-stereoisomer of TOC into lipoproteins( Reference Hosomi, Arita and Sato 30 , Reference Burton, Traber and Acuff 31 ). Il’ina et al.( Reference Il’ina, Roukolainen and Belkin 32 ) found that the TOC concentration was higher in the kidneys than in the liver in both wild and farmed mink. However, the TOC supplementation of the farmed mink in their study was not provided; therefore, the opposite relation between TOC concentrations in the liver and the kidneys in the present study could be a consequence of the dose of TOC supplied in the feed in the two studies, respectively. The distribution of stereoisomers in the liver in the present study shows that the generally accepted ratio of 1:1·49 between all-rac-TOC and RRR-TOC in not applicable in mink, but to establish the ratio in mink a dose–response study with the two different TOC forms must be carried out.
In the present study, plasma concentrations of 25VD3 were lower in the treatment groups with the highest plasma concentrations of RET compared with treatment groups with lower plasma concentrations of RET. In broiler chickens, different studies have indicated that high dietary RET levels interfered with the bioavailability of VD3 and 25VD3, particularly when VD3 was supplied in low levels in the feed. On the other hand, RET did not affect the VD3 bioavailability when VD3 was synthesised endogenously in the skin of the birds from exposure to UV light or supplied at recommended levels in the feed (2000–3000 IU/kg feed)( Reference Aburto and Britton 2 , Reference Aburto and Britton 3 , Reference Aburto, Edwards and Britton 33 ). However, direct plasma concentrations of VD3 and 25VD3 were not analysed in these studies, but were assessed as biological activity of VD3 and expressed in terms of bone ash concentration.
In turkey poults fed diets high (400 000 IU/kg) or low (4000 IU/kg) in RET and/or high (900 000 IU/kg) or low (900 IU/kg) in VD3, where low was equivalent to the National Research Council recommendations and double-low was used as control, the bones of the animals failed to develop normally when RET was high and VD3 was low. In all other treatment groups, including the control group, bones developed normally. Therefore, it was speculated that the ratio between RET:VD3 in the diet was more important than the actual level of RET and VD3 in the diet( Reference Linday, Umhau and Schindledecker 5 , Reference Spiesman 19 , Reference Metz, Walser and Olson 20 ). Studies in rats have shown severe antagonistic effects of RET on VD3 with respect to normal serum Ca levels; however, in these studies, calculated ratios between RET:VD3 were very high between 30:1 and 30 000:1( Reference Rhode, Manatt and Clagett-Dame 34 , Reference Rhode and DeLuca 35 ), when applying the following conversion factors to reported results: VD3 40 IU/µg and RET 3·3 IU/µg( Reference Linday, Umhau and Schindledecker 5 ). In many species, including humans, a RET:VD3 ratio between 4:1 and 7:1 is considered acceptable( Reference Linday, Umhau and Schindledecker 5 ). However, a ratio of approximately 10:1 is commonly applied in rations for most production animals. The rationale behind this is unknown, but results from the present study indicate that the RET:VD3 ratio needs further investigation. The physiological background for the antagonistic effect of RET on VD3 is unknown. It may be due to competition during uptake from the intestine, during incorporation into mixed micelles in lymph or during plasma transport of VD3 metabolites. Alternatively, it may be related to the liver’s double function as a RET storage site and site of hydroxylation of VD3, because excessive amounts of RET stored in the liver could negatively affect the hydroxylation process of VD3. However, this aspect needs further investigation( Reference Metz, Walser and Olson 20 ).
In the studies by Aburto & Britton( Reference Aburto and Britton 2 , Reference Aburto and Britton 3 ), increasing the supplementation of broiler chickens with all-rac-TOC had a negative impact on the biological activity of VD3, whereas in the present study the plasma concentrations of 25VD3 was lower when mink were supplemented with RRR-TOC than when supplemented with all-rac-TOC, regardless of the RET supplementation level in the feed. In cell cultures, Goncalves et al.( Reference Goncalves, Roi and Nowicki 7 ) showed that the VD3 uptake was reduced when TOC was added to the cell medium. Goncalves et al.( Reference Goncalves, Roi and Nowicki 7 ) argued that it was because VD3 and TOC share transport mechanisms in the intestine. This observation is supported by the present study where RRR-TOC supplementation had a more negative effect on plasma 25VD3 levels than supplementation with all-rac-TOC. RRR-TOC and all-rac-TOC are assumed to be absorbed equally in the intestines( Reference Borel, Preveraud and Desmarchelier 36 ), and thus the observed difference is most likely related to RRR-TOC binding more efficiently to α-TTP in the liver than synthetic stereoisomers of TOC because of its biologically favoured conformation.
In conclusion, supplementing with the fat-soluble vitamins RET, VD3 and TOC in diets of mink gives rise to pronounced physiological interactions between the three fat-soluble vitamins in the body of the mink. Particularly, RET has a negative dose-dependent effect on plasma levels of 25VD3, but the RRR-stereoisomer of TOC seems to be involved heavily in regulatory processes controlling plasma and organ levels of RET as well. Interactions between RET, VD3 and TOC in general need further investigation in all species.
Acknowledgements
The authors thank the technical staff of the Copenhagen Fur Research farm in Holstebro, Denmark, and the laboratories at the Department of Animal Science at Aarhus University in Tjele, Denmark. Agro Korn A/S is acknowledged for kindly providing the vitamins used for the feed rations.
The study was supported by Copenhagen Fur and Aarhus University.
The authors’ contributions are as follows: L. H. contributed to the laboratory analyses, data analysis, interpretation of the findings and writing the final manuscript. T. N. C. and S. K. J. contributed to formulating the research questions, study design, laboratory analyses, data analysis, interpretation of the findings and writing the final manuscript.
The authors have no conflicts of interest to declare.








