Tomatoes are the main source of lycopene in the human diet. The claimed ability of lycopene to prevent human diseases is to a certain extent related to the antioxidant properties, which probably reduce oxidative damage to macromolecules, such as lipids, proteins and DNAReference Rao and Rao1. Epidemiological studies describe an inverse relationship between a diet rich in tomatoes and tomato products and the incidence of CVD, which has been related to the presence of this carotenoid. The cardiopreventive effect of lycopene has been mainly associated with lipid peroxidation, including LDL oxidation, which was reduced significantly by a daily intake of lycopene (20–40 mg) obtained from tomato productsReference Agarwal and Rao2, Reference Hadley, Clinton and Schwartz3. Further meaningful markers of lipid oxidative damage are lipid peroxidation end products, such as malondialdehyde (MDA) and isoprostanes. Levels of MDA, commonly measured as thiobarbituric acid reactive substances (TBARS) are considered to be a predictive biomarker for the development of CVD and are also correlated with the severity of diseaseReference Sakuma, Hibino, Sato, Ohte, Akita, Tamai, Sasai, Yoshimata and Fujinami4, Reference Walter, Jacob, Jeffers, Ghadanfar, Preston, Buch and Mason5.
Little is known about the role of antioxidants, such as lycopene in the prevention of oxidative damage to proteins, which are among the major cell constituents, and any damage could result in loss of functionality of enzymes, receptors and membrane transportersReference Salvi, Carrupt, Tillement and Testa6. The formation of carbonyl groups (aldehydes and ketones) on protein side chains (carbonyl proteins; PCO) is considered to be a marker of severe oxidative stress and the severity of disease. The PCO content is deemed to be the most general and most commonly used marker of protein oxidationReference Dalle-Donne, Giustarini, Colombo, Rossi and Milzani7, although mechanisms of PCO generation are unspecific.
The inflammatory response also plays a key role in atherogenesis. In general, circulating cytokines and C-reactive protein (CRP) are found high in diseased states and can therefore be used as biomarkers for diseases that involve early endothelial activation and inflammation, such as CVDReference Albers, Antoine and Bourdet-Sicard8–Reference Ridker, Hennekens, Buring and Rifai10. In addition, CRP has been shown to be inversely related to frequency of fruit and vegetable consumptionReference Gao, Bermudez and Tucker11, while Sanchez-Moreno et al. Reference Sánchez-Moreno, Cano, De Ancos, Plaza, Olmedilla, Granado and Martin12 reported that the consumption of ‘gazpacho’, a Mediterranean vegetable soup made mainly with tomatoes, increases plasma vitamin C and decreases some biomarkers of oxidative stress and inflammation. These authors attributed this effect to the level of vitamin C, although this product also contains high levels of other antioxidants, mainly lycopene, which could also interact.
Bearing in mind the afore-mentioned, the aim of the present study was to ascertain the synergistic effect of vitamin C and tomato juice components, such as lycopene, on biomarkers of oxidative stress and inflammation. For this, a randomized intervention study was carried out, in which the subjects consumed tomato juice, which provided the same intake of lycopene but different intakes of vitamin C.
Subjects and methods
Subjects and study design
Twenty-four healthy volunteers (twenty females and four males) were recruited through advertisements in the University of Jena, Germany. Exclusion criteria were smoking, allergies and use of vitamin or mineral supplements and medication. Participants were aged between 19 and 27 (23 (sd 2)) years and had a BMI of 21·5 (sd 2·8) kg/m2. Lipid status as well as baseline concentrations of antioxidants (tocopherols, vitamin C and carotenoids) in plasma were comparable between groups. The study was approved (ethical vote no. 1526-/04/05) by the Ethics Committee of the University of Jena and complied with the Helsinki guidelines for clinical studies. All participants received verbal and written information and gave their written consent.
The study was a randomized 4-week trial, involving 2 weeks' depletion and 2 weeks' intervention and was conducted in June and July 2005. Participants were asked to follow some precise modifications of their normal diet for the whole study time. Fruit and vegetable intake was restricted to three servings per d and recorded daily. One portion was defined as a piece or a handful, as recommended by the European ‘five a day’ campaign.
Lycopene-containing foods were avoided totally for the whole study period, as were fruits rich in vitamin C, such as citrus fruits and strawberries. Fruit and vegetable intake was recorded daily in a dietary record. Otherwise, subjects were not restricted in what they ate.
After 2 weeks of depletion, participants were randomly divided into two groups. Group L consumed 250 ml tomato juice (41·8 mg lycopene/l and 90 mg vitamin C/l) twice daily with breakfast and dinner, thus ensuring consumption of lipids at the same time, for two consecutive weeks. Group LC consumed the same tomato juice but enriched with 870 mg/l vitamin C.
Collection of blood and urine samples
Blood samples (15 ml) were collected after overnight fasting between 07.00 and 08.00 hours into lithium-heparin tubes. Blood was withdrawn at the beginning of the study (T − 2), prior to intervention time (T0) and at the end of the study (T+2). Urine samples (24 h) were collected during the 24 h prior to blood sample collection. Blood samples were allowed to rest for 1 h at 6°C before being centrifuged (centrifuge model Universal 30 RF; Hettich, Tuttlingen, Germany), 1000 g, 10 min, 4°C to separate plasma from erythrocytes. Plasma and urine were transferred to 1·5 ml plastic tubes and kept at − 80°C until analysis. To stabilize vitamin C in plasma and urine, samples (200 μl) were transferred to tubes containing 300 μl TCA (5 g/100 ml) prior to sample freezing. To avoid systematic errors, analyses were not started until all the samples had been collected. All analyses of plasma and urine parameters were carried out in triplicate, except those of IL-1β, TNFα, CRP and 8-epi-PGF2α, which were carried out in duplicate.
Tomato juices
Tomato juice was prepared and delivered by Juver Alimentación, S.L.U. (El Churra, Murcia, Spain). After washing and cutting tomatoes, the hot break technique was used for rupture of cell structures. Part of the juice (juice L) was delivered in its natural form and another part was enriched with vitamin C in the form of l-ascorbic acid (juice LC) prior to pasteurization. Both juices were pasteurized (108°C, 30 s) and packaged in tetrapacks. The proximate composition of the juice (energy, protein, carbohydrate and fat) and folate was analysed following the methods recommended by Association of Analytical Chemists13 in an external laboratory. The bioactive compounds of the juices (vitamin C, carotenoids, tocopherols and total phenolic compounds) and antioxidant activity were analysed as described later. All analyses were carried out in triplicate.
Vitamin C
Vitamin C concentrations in plasma, urine and juice were analysed photometrically after oxidation (catalysed by Cu ions) of ascorbic acid to dehydroascorbic acid, which reacts with 2,4-dinitrophenylhydrazine to form a red complex. The absorbance was measured at 520 nmReference Speitling, Hüppe, Kohlmeier, Matiaske, Stelte, Thefeld, Wetzel, Kübler, Anders, Heeschen and Kohlmeier14. The vitamin C in tomato juice was analysed after extracting it from the food matrix with meta-phosphoric acid as described previouslyReference Klopotek, Otto and Böhm15.
Carotenoids
Carotenoids were extracted from plasma according to Fröhlich et al. Reference Fröhlich, Kaufmann, Bitsch and Böhm16. Samples were analysed on a C30-column (250 × 4·6 mm, 5 μm; Trentec, Gerlingen, Germany) at 17°C with the diode array detector set on 450 nmReference Böhm17. The carotenoid content of the samples was quantified comparing peak areas with those of authentic standards ((all-E)-lutein, (all-E)-zeaxanthin, (all-E)-cantaxanthin, (all-E)-β-cryptoxanthin, (all-E)-, (9Z)-, (13Z)- and (15Z)-β-carotene, (all-E)-α-carotene and (all-E)-lycopene). As standards of lycopene isomers were not available, (Z)-lycopene isomer peaks were quantified by comparing with peaks of (all-E)-lycopene. The carotenoid content of the tomato juices was analysed as described previouslyReference Seybold, Fröhlich, Bitsch, Otto and Böhm18.
Tocopherols
Tocopherols were extracted from plasma according to Fröhlich et al. Reference Fröhlich, Kaufmann, Bitsch and Böhm16 and analysed by HPLCReference Gahler, Otto and Böhm19 on a diol-column with fluorescence detection (λex = 292 nm, λem = 330 nm). Plasma tocopherol concentration was calculated from the peak areas of the respective standards, α-, β-, γ-, δ-tocopherols (Calbiochem, Darmstadt, Germany) and expressed as their sum. The tocopherol content of the tomato juices was analysed as described previouslyReference Gahler, Otto and Böhm19.
Total phenolic compounds
Phenolic compounds in tomato juice were determined photometrically after acid and alkaline hydrolysis as described previouslyReference Balz, Schulte and Thier20. The total phenolic content was expressed as gallic acid equivalents.
Cholesterol and TAG
Total cholesterol in plasma was determined enzymatically using the CHOD-PAP-methodReference Allain, Poon, Chan, Richmond and Fu21 (no. 12016630; Roche/Hitachi, Mannheim, Germany) and TAG was measured by using the GPO-PAP methodReference Wahlefeld, Wahlefeld and Bergmeyer22 (no. 2016648; Roche/Hitachi).
Antioxidant capacity
The antioxidant capacity (AC) of plasma and urine was determined by Trolox equivalent antioxidant capacity (TEAC)Reference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans23 and ferric-reducing ability of plasma (FRAP)Reference Schlesier, Harwat, Böhm and Bitsch24. The AC of the tomato juices was determined in aqueous extracts, weighting the sample (1–2 g) into 10 ml graduated flasks, which were made up to volume with distilled water and centrifuged (2000 g, 5 min, room temperature). The supernatant was assayed with the TEAC and FRAP.
Uric acid
Uric acid was measured in plasma and urine by an enzymatic colorimetric test using a commercially available uricase/POD kitReference Domagk and Schlicke25 (no. 130019990314; VWR International, Darmstadt, Germany).
Urinary creatinine
To normalize all urine determinations, creatinine was determined by the Jaffe picric acid spectrophotometric methodReference Helger, Rindfrei and Hilgenfeldt26. The creatinine present in the sample directly reacts with alkaline picrate resulting in the formation of a red colour, which is measured at 510 nm.
Carbonyl proteins
PCO was measured by forming labelled protein hydrazone derivatives, using 2,4-dinitrophenylhydrazine C6H3(NO2)2NHNH2(DNPH), which were quantified spectrophotometricallyReference Levine, Garland, Oliver, Amici, Climent, Lenz, Ahn, Shaltiel and Stadtman27. The PCO content was determined from the absorbance at 370 nm using a molar absorption coefficient of 22 000 M− 1cm− 1. The total protein content of the sample was determined according to Reznick and PackerReference Reznick and Packer28.
Thiobarbituric acid reactive substances
To measure MDA and related aldehydes, serum samples (100 μl) were mixed with 1 ml thiobarbituric acid (0·67 g/100 ml) and 500 μl TCA (20 g/100 ml) before being incubated at 100°C for 20 min. After centrifugation (Eppendorf, Hamburg, Germany), 2000 g, 10 min, room temperature, the absorbance of the supernatants was measured at 532 nm. The total content of aldehydes capable of reacting with thiobarbituric acid to form chromophores absorbing at 532 nm was estimated using a molar absorption coefficient for the MDA–thiobarbituric acid complex of 1·56 × 105 M− 1cm− 1 (Reference Buege and Aust29, Reference Nourooz-Zadeh, Tajaddini-Sarmadi and Wolf30).
IL-1β and TNF-α
Both parameters were determined with ELISA kits (cat. no: 583311; Cayman Chemical Co., Ann Arbor, MI, USA (IL-1β) and 589201 (TNF-α)). Plasma samples were prepared according to the manufacturer's instructions. The kits recommend an incubation time of between 15 min and 6 h Reference Beutler, Greenwald and Hulmes31, Reference Grassi, Roberge and Frobert32. As the colour of the samples developed very slowly, maximum incubation time was chosen to obtain better results.
C-reactive protein
An instant ELISA-Kit (BMS288INSTCE; Bender MedSystems GmbH, Vienna, Austria) was usedReference Powell, Roantree and Rantz33, preparing the plasma samples according to the manufacturer's instructions.
Urinary isoprostane 8-epi-PGF2α
An enzyme immunoassay from Oxford Biomedical Research (EA 85; Oxford Biomedical Research, Oxford, MI, USA) was appliedReference Roberts and Morrow34, preparing the urine samples according to the manufacturer's instructions.
Statistical analysis
All values are presented as means and standard deviations of triplicate or duplicate analyses, depending on the method. Baseline values were compared for normality of distribution and equality of variance with the Komogorov and χ2 tests. For each group, the parameters measured at the different time points (T − 2, T0 and T+2) were compared using the general linear model for repeated measurements. Differences between both groups for each time point were tested by using the one-way ANOVA. Bilateral correlations were determined using Pearson's correlation. The level of significance was P < 0·05 for all tests. Analysis was performed using SPSS 13.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Juice composition
The two tomato juice batches showed similar values of protein, carbohydrates, fat total dietary fibre, lycopene, β-carotene, lutein, tocopherols and folate. However, their respective vitamin C and phenol contents and antioxidant capacity differed significantly (Table 1). The lycopene content of both tomato juice samples was very similar with a mean value of around 41 mg lycopene/l. HPLC analysis showed that 84 % of the lycopene was in (all-E)-configuration, whereas the other 16 % was in (Z)-configuration. However, no (5Z)-lycopene was found. The vitamin C content of the fortified tomato juice (LC) was ten-fold higher than juice L, which was reflected in its significantly higher AC. The same raw material was used for the preparation of both juices and the only difference in the manufacturing process was the addition of l-ascorbic acid to juice LC. Thus, the higher content in phenolic compounds in that juice is due to the overestimation assumed for the Folin-Ciocalteu method, which is caused by interferences with vitamin C and other reducing substancesReference Georgé, Bart, Alter and Amiot35.
Table 1 Proximate composition, carotenoids, vitamin C and E and antioxidant capacity (FRAP and TEAC) of both juices‡
(Values are means and standard deviations per 100 ml)

FRAP, ferric-reducing ability of plasma; TEAC, Trolox equivalent antioxidant capacity; GAE, gallic acid equivalents.
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05) (ANOVA).
* In 500 ml juice L.
† In 500 ml juice LC.
‡ For details of subjects and procedures, see Subjects and methods.
Lycopene and total carotenoids
Table 2 shows the plasma concentrations of lycopene isomers and their percentages. Lycopene concentrations in plasma measured as the sum of all isomers detected did not differ between groups at the baseline time (T − 2). The concentration decreased significantly (P < 0·001) during depletion to levels below 0·5 μmol/l (Table 2) in both groups and showed a significant increase (P < 0·001) during the intervention period as lycopene was absorbed from the juice, surpassing initial levels in both groups (P < 0·001).
Table 2 Plasma concentration of lycopene isomers (μmol/l) and the (all-E)-/(5Z)-lycopene ratio, measured at the beginning (T−2) and end (T0) of depletion and at the end of intervention (T+2) in groups L and LC†
(Values are means and standard deviations for twelve subjects per group)

a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05), comparing baseline values with all subsequent values for one parameter and group.
Values in parentheses show percentual distribution.
P-values for statistical differences between the groups in T+2 (ANOVA) are given in the last column (P).
* Tentatively identified.
† For details of subjects and procedures, see Subjects and methods.
The most abundant isomers were (all-E)- and (5Z)-lycopene, although other isomers, recently identified as (13Z)-lycopene, (5Z,9Z')-lycopene, (9Z)-lycopene and (5Z,9Z)-lycopeneReference Fröhlich, Conrad, Schmid, Bitsch, Breithaupt and Böhm36 were also found. The (all-E)-:(5Z)-lycopene ratio (Table 2) was used to assess isomeric changes of lycopene taking place in plasma and Fig. 1 expresses the changes of this ratio during the study time, calculated by subtracting the baseline ratio from each value. The ratio shifted to absolute values of < 1, indicating that at low lycopene concentrations in plasma the (5Z)-isomer was the most frequent form (Table 2). As the total lycopene concentration increased (T+2), the ratio again shifted in favour of the (all-E)-form. After 2 weeks of intervention, (all-E)-lycopene was the most abundant isomer in plasma, the ratio being even higher than at the beginning of the study (Fig. 1).
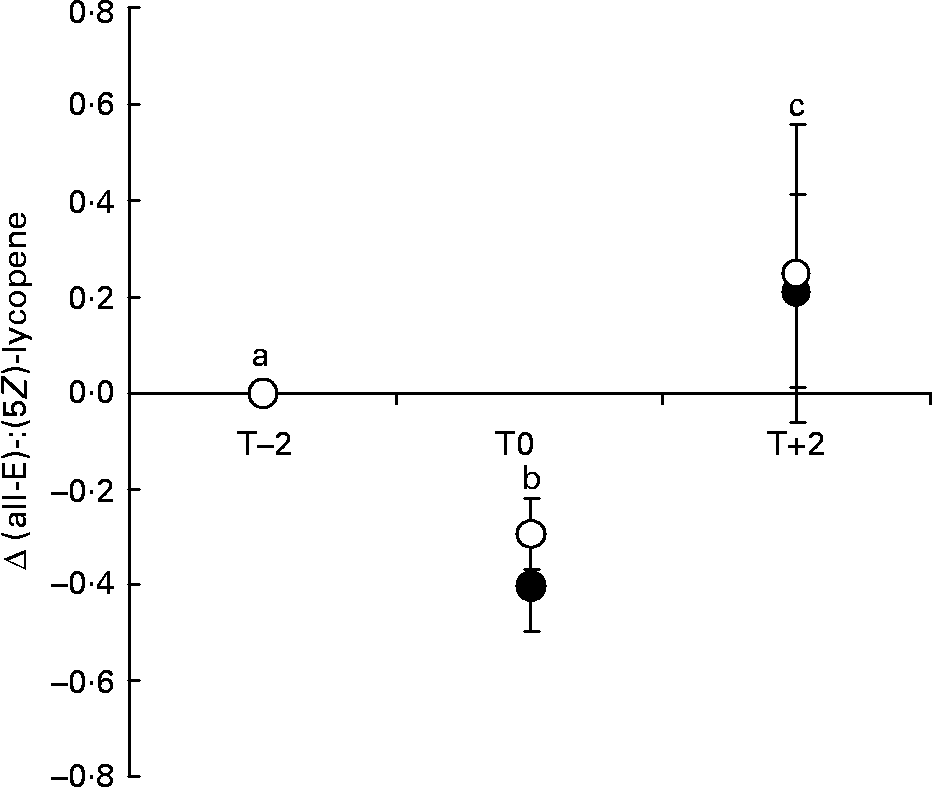
Fig. 1 Changes in (all-E)-:(5Z)-lycopene ratio in plasma for group L (●) and group LC (○), measured at the beginning (T-2) and end (T0) of depletion and at the end of intervention (T+2). Data are expressed as means and standard deviations per group. Changes were calculated for each subject by subtracting the baseline value from each value. Significant difference (P < 0·05) between measuring points is expressed by different letters (linear model). There were no differences between groups (ANOVA). For details of subjects and procedures, see Subjects and methods.
Total carotenoids in plasma (measured as the sum of all detected carotenoids) were significantly depleted in T0 (P < 0·001) mainly due to changes in total lycopene (Table 2), total β-carotene and (all-E)-lutein plasma concentrations (Table 3). After 2 weeks of juice intake, the concentrations in plasma rose significantly (P < 0·001), leading to a significant increase of total carotenoids. As these three carotenoids are the major carotenoids found in tomato, the changes in their plasmatic levels would reflect the consumption of tomato juice.
Table 3 Plasma vitamin C, total carotenoids, tocopherols, cholesterol, TAG, antioxidant capacity (TEAC, FRAP), carbonyl proteins (PCO), thiobarbituric acid reactive substances (TBARS), cytokines (IL-1β and TNF-α) and C-reactive protein (CRP), measured at the beginning (T−2) and end (T0) of depletion and at the end of intervention (T+2) in groups L and LC*
(Values are means and standard deviations for twelve subjects per group)

TEAC, Trolox equivalent antioxidant capacity; FRAP, ferric-reducing ability of plasma; MDA, malondialdehyde.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05), comparing baseline values with all subsequent values for one parameter and group.
P-values for statistical differences between the groups in T+2 (ANOVA) are given in the last column (P ).
* For details of subjects and procedures, see Subjects and methods.
Vitamin C
Vitamin C in plasma showed no statistical difference between the groups at T − 2 and levels decreased significantly during depletion in both groups. After 2 weeks of juice consumption, the plasma vitamin C level in group L remained unchanged, but increased significantly in group LC (Table 3). This behaviour was also observed in urine (Table 4).
Table 4 Urinary excretion of vitamin C, 8-epi-PGF2α and TBARS as well as antioxidant capacity (TEAC, FRAP), measured at the beginning (T−2) and end (T0) of depletion and at the end of intervention (T+2) in group L and LC*
(Values are means and standard deviations for twelve subjects per group)

TBARS, thiobarbituric acid reactive substances; TEAC, trolox equivalent antioxidant capacity; FRAP, ferric-reducing ability of plasma.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05), comparing baseline values with all subsequent values for one parameter and group.
P-values for statistical differences between the groups in T+2 (ANOVA) are given in the last column (P ).
* For details of subjects and procedures, see Subjects and methods.
Cholesterol and TAG
At the beginning of the present study, plasma cholesterol in participants ranged from 101 to 241 mg/dl (Table 3), with a mean value around 150 mg/dl. A significant decrease was observed for both tomato juice treatments (P = 0·008 and P = 0·002 for groups L and LC, respectively), the effect being correlated with the plasma concentration of total lycopene (in T+2: r 0·53, P = 0·003).
TAG differed significantly between groups at T − 2 due to the high levels (291·27 and 233·23 mg/dl) of two individuals in group L. If these values were excluded from the statistical analysis, no difference was observed between both groups at any of the measurement times.
Plasma TAG levels decreased significantly in subjects with initial values ≥ 80 mg/dl, regardless of the group. This effect was strongly correlated with the lycopene concentration in plasma (in T+2: r 0·80, P = 0·009).
Antioxidant capacity
Plasma AC did not statistically differ between groups during the study time and was not altered during depletion or intervention (Table 3), whether measured by FRAP or TEAC. No correlation was found with any plasma antioxidants, such as uric acid, lycopene and vitamin C.
AC in urine was also measured since it might reflect the overall antioxidant environment of the body. When measured by TEAC and FRAP, urinary AC had increased significantly after 2 weeks consumption of the juice fortified with vitamin C (TEAC P = 0·009; FRAP P < 0·001; Table 4), but was not enhanced after consumption of the normal tomato juice (group L). At the end of the present study, the results obtained by FRAP and TEAC correlated with vitamin C excretion in urine (FRAP r 0·65, P < 0·001); TEAC r 0·35, P = 0·003). In addition, they were inversely correlated with TBARS excreted in urine (FRAP r − 0·496, P < 0·001; TEAC r − 0·297, P = 0·011).
Biomarker of oxidative stress
After 2 weeks of juice consumption, TBARS were significantly reduced in the plasma of group LC (P = 0·002, Table 3) and in the urine of both groups (P < 0·001, Table 4). Although urinary excretion of isoprostane metabolite 8-epi-PGF2α was not altered during the study time in any of the groups, a significant correlation was observed between the TBARS and 8-epi-PGF2α (r 0·42, P = 0·003) at the end of the study. PCO concentrations also remained unaltered during depletion and intervention times (Table 3). No correlation was found with the ingested antioxidants.
Biomarker of inflammation
Juice consumption significantly reduced CRP concentration in the plasma of both groups (P = 0·017 and P = 0·001 for groups L and LC, respectively, Table 3). There was no statistical difference in the final concentrations between the groups. High variability was observed for the basal TNFα plasma levels, which ranged from 0·76 to 20·87 ng/l (Table 3), with the concentrations significantly higher in group L at the beginning of the study and at the end of the depletion phase (Table 3). After juice consumption, a significant decrease in this parameter was observed in group L, but not in group LC. At the end of the study, the levels of TNFα did not differ between groups. Basal IL-1β plasma concentration also varied strongly between participants, ranging from 0·29 to 44·29 ng/l (Table 3). The significantly higher concentration observed in group LC at T − 2 was reduced significantly during the intervention period. However, values were not correlated with plasmatic levels of lycopene or vitamin C.
Discussion
In the present study, the impact of tomato juice on human health was investigated by studying the relationship of lycopene and vitamin C with oxidative stress and inflammatory response in healthy young adults.
Volunteers were asked to avoid any source of lycopene in their diet, which was comparatively easy because lycopene is mainly provided by tomatoes and tomato products and a few other sources (watermelon, guava, apricots, chilli, sea buckthorn and rosehips) that do not form part of the regular diet. Vitamin C consumption could not be restricted in the same way, as this would have meant cutting out almost all plant food during the study time. Obviously, this could not be considered a healthy diet and could itself have caused oxidative stress and alteration in biomarkers.
Tomato juice was chosen as the source of lycopene, as homogenization and heat treatment of tomatoes is considered to increase bioavailability of this compoundReference Gärtner, Stahl and Sies37, Reference van het Hof, West, Weststrate and Hautvast38. Vitamin C was added as l-ascorbic acid, as is custom in the beverage industry. In addition, the juice contained low amounts of other micronutrients, such as folate, vitamin E and phenolic compounds.
A period of 2 weeks was chosen for intervention as previous studies have shown this to be sufficient to increase lycopene plasma concentrations significantlyReference Fröhlich, Kaufmann, Bitsch and Böhm16, Reference Böhm and Bitsch39. After 2 weeks' juice consumption, lycopene plasma concentrations had increased by 150 % and 162 % in groups L and LC, respectively, which indicates that the lycopene provided by the juice was bioavailable.
The same period of intervention was also chosen to investigate the effect of tomato products on various biomarkers of oxidative stress and inflammationReference Hadley, Clinton and Schwartz3, Reference Sánchez-Moreno, Cano, De Ancos, Plaza, Olmedilla, Granado and Martin12, Reference Rao and Shen40 and has been found to be sufficient to reduce lipoprotein sensitivity to oxidative damageReference Hadley, Clinton and Schwartz3, to decrease plasma F2-isoprostanes, PGE2 and monocyte chemotactic protein-1Reference Sánchez-Moreno, Cano, De Ancos, Plaza, Olmedilla, Granado and Martin12 and to reduce TBARS and protein oxidationReference Rao and Shen40.
Recently, the isomeric pattern of lycopene has come to the fore of investigation. Lycopene from tomatoes and other plant foods is predominantly in the form of (all-E)-lycopene, with a percentage of more than 85. However, plasma and tissue lycopene consists of more than 50 % (Z)-isomers. It is still unclear whether (all-E)-lycopene is converted in the human body into (Z)-isomers. Boileau et al. Reference Boileau, Merchen, Wasson, Atkinson and Erdman41 reported that (Z)-isomers are preferably adsorbed by the body because of their better solubility in bile acids and micelles and therefore may be preferentially incorporated in chylomicrons. On the other hand, van Breemen et al. Reference Van Breemen, Xu, Viana, Chen, Stacewicz-Sapuntzakis, Duncan, Bowen and Sharifi42 hypothesized that (all-E)-lycopene is released from the stabilizing food matrix and begins to equilibrate to a thermodynamic mixture of isomers. However, it remains unclear if the isomerization takes place in the intestinal lumen or at the enterocyte or post-enterocyte level. A recent human intervention study showed that there was no significant (all-E)-(Z)-isomerization of lycopene in the human stomach and the fact that lycopene (Z)-isomers are poorly absorbed strongly suggests that isomerization of lycopene occurs in the human body at a post-enterocyte levelReference Tyssandier, Reboul, Dumas, Bouteloup-Demange, Armand, Marcand, Sallas and Borel43. We observed that the ratio of (all-E)-:(Z)-lycopene changed according to the total plasma lycopene concentration. At low plasma concentrations (e.g. during the depletion phase or in a control diet low in lycopene) (Z)-isomers increased proportionally, but with increasing plasma lycopene concentrations the ratio shifted towards (all-E)-lycopeneReference Hadley, Clinton and Schwartz3, Reference Fröhlich, Kaufmann, Bitsch and Böhm16, Reference Edwards, Vinyard, Wiley, Brown, Collins, Perkins-Veazie, Baker and Clevidence44. Dietary lycopene is mainly in (all-E) configuration and if lycopene is omitted from the diet, the (all-E)- lycopene plasma level decreases and, as a result, the (Z)-isomers increase proportionally. This and the fact that (5Z)-lycopene is not present in tomato but is the predominant (Z)-isomer in plasma strongly suggests in vivo isomerization.
Drinking two glasses of tomato juice (500 ml) daily for 2 weeks reduced the inflammation marker CRP. This effect was not correlated with plasmatic levels of lycopene or vitamin C, although the reduction was higher in group LC (approximately 23 %), which also showed higher vitamin C plasma levels, than in group L (approximately 17 %). Other authors report an inverse correlation between the frequency of fruit and vegetable intake in general and CRP plasma concentrationsReference Gao, Bermudez and Tucker11, while reduced CRP levels after 4 weeks' intervention with a high intake of fruits and vegetables were correlated with plasma β- and α-carotene, but not with other carotenoidsReference Watzl, Kling, Möseneder, Barth and Bub45.
The cytokines TNFα and IL-1β showed no clear behaviour and could not be related to the consumption of lycopene and vitamin C.
Furthermore, 2 weeks' intervention with tomato juice reduced total cholesterol levels. Hypercholesterolaemia is a major risk factor for atherosclerosis. The reduction of the plasma cholesterol concentration by consumption of tomato juice may therefore reduce the risk for CVD. The effect was strongly correlated with lycopene plasma concentration but not with vitamin C. Lycopene has been shown to suppress the cholesterol synthesis and to augment the LDL receptor activity in macrophages in vitro Reference Fuhrman, Elis and Aviram46, two mechanisms that reduce the plasma total cholesterol concentration. However, human intervention studies do not consistently evidence this hypocholesterolaemic effect of lycopeneReference Hadley, Clinton and Schwartz3, Reference Böhm and Bitsch39. One reason for the inconsistent results, in addition to factors such as the study population (age, BMI) and the source of lycopene, might be the lycopene doses applied and the resulting plasmatic lycopene response. In the present study, volunteers consumed approximately 21 mg lycopene daily derived from tomato juice for two consecutive weeks. A reduction of total plasma cholesterol levels was also found after a 2-week intervention with 23 to 35 mg provided by tomato soup and vegetable juiceReference Hadley, Clinton and Schwartz3. In contrast, the daily consumption of only 5 mg (source fresh tomatoes, tomato juice and oleoresin capsules) for 6 weeks did not alter the plasma cholesterol levelsReference Böhm and Bitsch39. The results of these studies suggest that the hypocholesterolaemic effect is more related to the amount of intake of tomato products consumed than with the duration of intake.
In addition to lycopene and vitamin C, other antioxidant components such as vitamin E, Se, folate and phenolic compounds are present in tomatoes and could also add to the beneficial effects observed. However, the contribution of tomatoes and tomato products to the total intake of these compounds is considered to be low, as other plant food provides much higher amountsReference Willcox, Catignani and Lazarus47.
Lipid peroxidation in plasma, as measured by TBARS, was reduced by consuming tomato juice fortified with vitamin C for 2 weeks but not by consuming tomato juice alone, whereas the urinary excretion of TBARS decreased after consumption of both juices by an average of 17 %. The present data suggest that the very high intake of vitamin C was involved, at least partially, in the beneficial effect of tomato juice on this risk factor for atherosclerosisReference Sakuma, Hibino, Sato, Ohte, Akita, Tamai, Sasai, Yoshimata and Fujinami4 and a synergic action of vitamin C with tomato compounds such as lycopene or polyphenolic compounds seems likely. On the other hand, TBARS were also found to have fallen by 10 % after the consumption of 5–20 mg lycopene provided by ketchup and oleoresin capsulesReference Rao and Shen40, neither of which contains high amounts of vitamin C or any other bioactive compound present in tomatoes.
The second marker of lipid peroxidation measured in the present study was the urinary excretion of 8-epi-PGF2α. Studies have demonstrated that isoprostanes are found in plaque and are associated with increased risk for CVD, since they occur, for example, in increased amounts in human artherosclerotic lesionsReference Walter, Jacob, Jeffers, Ghadanfar, Preston, Buch and Mason5. Isoprostanes are a very specific marker of free radical-induced peroxidation of arachidonic acid and the consumption of 500 ml vegetable soup (gazpacho) daily for 2 weeks reduced this marker significantly in the plasma of healthy volunteersReference Sánchez-Moreno, Cano, De Ancos, Plaza, Olmedilla, Granado and Martin12. Measurement of the urinary excretion of 8-epi-PGF2α is considered to be an accurate tool for determining endogenous isoprostane productionReference Roberts and Morrow34. However, the present results do not agree with those obtained by Sánchez-Moreno et al. Reference Sánchez-Moreno, Cano, De Ancos, Plaza, Olmedilla, Granado and Martin12, as no change could be observed.
Total AC is increasingly used to monitor the redox status in vivo in intervention, bioavailability and epidemiological studies, although several intervention studies found no alterations in plasma AC after the ingestion of various lycopene-containing food items, as determined by different assaysReference Böhm and Bitsch39, Reference Pellegrini, Riso and Porrini48. This might be because antioxidant capacity is the sum of many different antioxidants, both endogenous, such as uric acid and albumin, and dietary, all of which contribute either to hydrophilic or lipophilic AC. In the present study, AC in plasma was not altered in both groups, but urinary AC was boosted by the treatment with the juice fortified with vitamin C. Many antioxidants are excreted in urine, such as vitamin C and phenolic compounds. Thus, urinary AC could reflect the antioxidant status and stress levels in the bodyReference Kirschbaum49 and urine is also a body fluid that can be obtained by non-invasive methods.
Conclusions
The consumption of tomato juice (500 ml) for 2 weeks reduced total cholesterol and that of CRP levels. The vitamin C-fortified juice slightly raised AC of plasma and urine and decreased TBARS. Thus, tomato juice decreased several risk factors associated with CVD, but the effect was stronger when vitamin C was added in a high dose. These results show that the protective properties of tomatoes cannot be made up only to lycopene. A synergic effect between vitamin C, lycopene and other tomato micronutrients seems likely to be responsible for the beneficial effects of tomato juice on oxidative stress and inflammation.
Acknowledgements
The authors gratefully acknowledge Fundación Seneca/Fondo Social Europeo of the Government of Murcia for awarding K. Jacob a predoctoral grant and Juver Alimentación, S.L.U. for its financial support. The authors are indebted to N. Knoll for her skilful analytical assistance, to F. Stein and colleagues for blood withdrawal, to K. Fröhlich for her advice and to all study participants for contributing continuously to this study.







