When creatine combines with P to form phosphocreatine, it acts as a reserve of high-energy phosphate that is used to rapidly convert ADP back to adenosine triphosphate(Reference Andres, Ducray and Schlattner1). In humans, although 95 % of the total creatine pool is found in skeletal muscle, high levels are also found in the heart, brain and testes. In particular, it is surprising that the influence of brain creatine status has not attracted more interest as the brain is the most metabolically active organ in the body, accounting for 20 % of BMR, although it represents only about 2 % of body weight(Reference Benton, Prasad, Lieberman and Kanarek2). The present study has therefore examined the influence of creatine supplementation on human cognition, in particular comparing the response of vegetarians and omnivores as there are reports that a vegetarian diet is associated with poorer creatine status(Reference Burke, Chilibeck and Parise3).
A survey of the literature found only a few studies of the impact of creatine supplementation on cognitive functioning that have produced inconsistent findings. Rawson et al. (Reference Rawson, Lieberman and Walsh4) gave a creatine supplement to young adults for 6 weeks and found no influence on a battery of cognitive tests. However, McMorris et al. (Reference McMorris, Mielcarz and Harris5) concluded that creatine supplementation aided cognition in the elderly when they found a significant effect of supplementation. Interestingly, Laakso et al. (Reference Laakso, Hiltunen and Kononen6) found that the level of creatine was significantly lower in the brain of those carrying apoE ɛ4, a genetic risk factor for Alzheimer's disease, and that the level of brain creatine was related to memory problems. The authors speculated that creatine supplementation may be beneficial in those with the apoE ɛ4 gene. Earlier McMorris et al. (Reference McMorris, Harris and Swain7) had reported in those who were sleep-deprived for 24 h that creatine supplementation had a positive effect on mood and cognition. A review(Reference Andres, Ducray and Schlattner1) concluded that in experimental paradigms of neurological diseases, supplementation with creatine can reduce neuronal loss. Adhihetty & Beal(Reference Adhihetty and Beal8) similarly noted that a disruption of the provision of cellular energy is a characteristic of many neurodegenerative disorders, and that the use of creatine supplementation is beginning to be considered. They concluded that it is ‘most efficacious as a treatment paradigm in Huntington's and Parkinson's disease’. Consistent with these findings, Rawson et al. (Reference Rawson, Lieberman and Walsh4) suggested that creatine supplementation might only be beneficial in those with impaired cognitive processing.
As creatine can be synthesised by the body from the amino acids glycine, arginine and l-methionine, it is not an essential nutrient, and it has been suggested that most of the creatine in the brain is synthesised in situ (Reference Wyss and Schulze9). There are, however, several reasons to suggest that neural activity may be susceptible to creatine supplementation. Using quantitative localised proton magnetic resonance spectroscopy, the consumption of 5 g of creatine monohydrate four times a day for a month resulted in an average increase of 8·7 % in brain creatine levels(Reference Dechent, Pouwels and Wilken10). Similarly the consumption of 8 g of creatine/d for 5 d resulted in decreased levels of oxygenated Hb in the brain: a response interpreted as evidence that increased oxygen utilisation had occurred while performing a demanding cognitive task for 15 min(Reference Watanabe, Kato and Kato11). Over time, the decrease in the performance of the task was less in those who had consumed a creatine supplement.
A question that has not been adequately considered is the influence of a vegetarian diet, as creatine is found mostly in meat, fish and other animal products, with only a trace in some plants. The levels of muscle creatine are lower in vegetarians, leading to the suggestion that in terms of exercise they are likely to benefit to a greater extent from loading with creatine(Reference Barr and Rideout12). For example, Burke et al. (Reference Burke, Chilibeck and Parise3) examined the levels of muscle creatine and exercise performance after supplementation. At baseline, muscle biopsy found lower levels of creatine in vegetarians. Following supplementation, the levels increased more in vegetarians than omnivores, a rise associated with an increased ability to exercise. Although there is an isolated suggestion that creatine improved the cognitive performance of vegetarians(Reference Rae, Digney and McEwan13), to date no study has systematically related the response to supplementation to the diet consumed. The present study therefore predicted a differential response to creatine supplementation in those who were and were not following a vegetarian diet.
Methods
Subjects
When they responded to a poster, potential subjects indicated whether they were vegan, vegetarian or if they ate meat. Female undergraduates (n 121), mean age 20·3 (se 2·1) years, were recruited. None were consuming a creatine supplement, and all reported that they were in good health. Two groups were established: meat-eaters (n 51) and those who were vegan or vegetarian (n 70). All subjects completed the trial. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of Swansea University. Written informed consent was obtained from all subjects.
Supplements
Randomly and under a double-blind procedure, the subjects consumed either a placebo (sixty subjects) or a creatine (sixty-one subjects) supplement. Creatine monohydrate, 20 g/d, was consumed as 5 g tablets (Isostar Creatine; Wander Limited, King's Langley, Hertfordshire, UK). The subjects consumed the tablets at home and were asked to space their consumption throughout the day, in an empty stomach to facilitate absorption. The placebo was a glucose tablet of a similar size and appearance. Although there are suggestions that there may be a cognitive benefit from consuming glucose(Reference Benton and Parker14), any response is short term and hence would not impact on the measures obtained under the present conditions. The tablets were consumed for a final time the evening before cognitive testing took place for a second occasion the following morning.
Procedure
A battery of cognitive tests was completed twice: before and after consuming the supplements. The supplements were consumed for 5 d. The subjects were asked to return any tablets that had not been consumed which established that compliance in this relatively short-term study did not appear to be a problem. Although diet was not specifically monitored over the 5 d of the study, before testing, it was again established that those who had initially reported that they were vegetarian had avoided meat during the study.
Cognitive tests
Memory – word recall
Two lists of thirty nouns were created; all words had six letters and two syllables, chosen to be high in imagery, concreteness and frequency of use(Reference Quinlan15). They were presented at the rate of one word per 2 s using a tape recorder. Immediately after presentation, the subjects wrote down as many words as they could recall in 2 min. The number correctly recalled is reported.
Reaction times
The reaction-time procedure was based on that of Jensen(Reference Jensen and Vernon16). On a panel, eight lamps were arranged in a semicircle 140 mm. from a central button (the home key). The index finger was placed on the home key. There was a 0·5 s delay followed by a warning tone after which there was a further random delay between 1 and 4 s, after which one of the eight lamps was illuminated. The subjects then, as quickly as possible, moved the hand from the home key to a button below the light, which when pressed extinguished it. The decision time is the delay between a light being illuminated and the hand being removed from the home key. Movement times were measured as the time taken for the hand to leave the home key and to press the button beneath the illuminated light.
Following a practice session of ten trials using all eight lamps, simple reactions were assessed when only one lamp could be illuminated. Choice reaction times were then recorded when two, four or eight lamps could be illuminated. In total, forty trials were run when each of one, two, four and eight lights were used. As recommended by Jensen(Reference Jensen and Vernon16), decision times were Winsorised to remove outliers; those scores outside the normal range of 170–990 ms and outside three standard deviations.
Vigilance – rapid information processing task
A computer generated a series of digits, at the rate of 100 digits/min for 5 min. The subjects pressed the space bar when they detected target sequences of three consecutive odd, or three consecutive even, digits. Eight of these sequences were presented every minute. Following the presentation of the third digit in a target sequence, 1500 ms was allowed for a correct response. A response under other circumstances was recorded as incorrect.
Verbal fluency
The Controlled Oral Word Association Test(Reference Benton and Hamsher17) requires that subjects name as many words as possible, within 1 min, beginning with a given letter of the alphabet, excluding proper nouns, numbers and the same word with a different suffix. Before consuming the supplements, the subjects were given the letters C, F and L and after consuming the supplements, they were given the letters P, R and W. The score analysed was the sum of all acceptable words.
Side effects
At the end of the study, the subjects were asked if they had experienced any side effects and to guess whether they had consumed creatine or the placebo.
Statistical analysis
The data were analysed with appropriate ANOVA designs. The basic approach when only one outcome measure was examined was a three-way ANOVA: supplement (placebo/creatine) × diet (vegetarian/meat eater) × time (before/after supplement), with the last factor as a repeated measure. Additional factors, such as the number of lights to which responses were made in the reaction-time test, were added when necessary. Where a significant interaction resulted, it was examined by calculating simple and simple–simple main effects. Preliminary analysis found that those who were vegetarian as opposed to meat-eaters, and those who subsequently consumed the placebo as opposed to creatine supplement, did not differ on the baseline performance of any test.
Results
Memory
Fig. 1 illustrates that creatine influenced the recall of the word lists: the interaction supplement × diet × time reached significance (F(1, 117) = 6·41; P < 0·01). Before the supplement was consumed, the memory of vegetarians and meat-eaters was similar, irrespective of whether they were about to consume the placebo (F(1, 118) = 0·93; NS) or the creatine supplement (F(1, 118) = 0·93; NS). After consuming the tablets for 4 d, the memory of vegetarians and meat-eaters was similar if they consumed the placebo (F(1, 118) = 0·01; NS). However, after 4 d of consuming the creatine supplement, memory was better in vegetarians rather than in those who consumed meat (F(1, 118) = 5·06; P < 0·03). However, in those who were meat-eaters, the consumption of the creatine supplement was associated with poorer memory compared with baseline values (F(1, 117) = 17·84; P < 0·001).

Fig. 1 The influence of creatine supplementation on memory. Values are the number of words recalled, before and after supplementation, as means with their standard errors represented by vertical bars. □, Vegetarian; ■, meat-eater.
Reaction times
Neither decision times (tablet × before/after, F(1, 117) = 0·11; NS) nor movement times (F(1, 117) = 0·16; NS) were influenced by the supplementation of creatine or by the dietary style (Table 1). However, variability in the speed of response is known to be a more sensitive measure, and therefore the standard deviations of the decision times were analysed: the interaction supplement × number of lamps monitored × time reached statistical significance (F(3, 351) = 2·59; P < 0·05). Fig. 2 illustrates the effect that reflected differences when eight but not fewer lamps were monitored. In those who were consuming the placebo, performance was more variable when tested a second time (F(1, 119) = 6·58; P < 0·01), although after consuming the creatine supplement it was associated with a similar performance on both occasions (F(1, 19) = 0·03; NS). This effect occurred irrespective of whether the subject was a vegetarian.
Table 1 Influence of creatine supplementation on measures of cognition (Mean values with their standard errors)
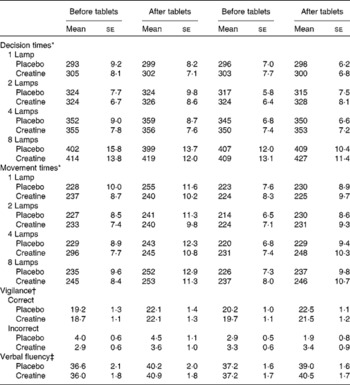
* With decision and movement times, the values are in ms.
† With vigilance, the number of correct sequences observed and inappropriate responses are reported.
‡ The total number of words reported is the measure of verbal fluency.

Fig. 2 The influence of creatine supplementation on the standard deviation of the decision times. Values are means of the standard deviations, with standard errors represented by vertical bars when various numbers of lamps were monitored. (a) Placebo (□, before tablets; ■, after tablets). (b) Creatine.
Vigilance
Supplementation did not influence the ability to sustain attention. Neither the number of correct (supplement × diet × time, F(1, 117) = 3·56; NS) nor the number of incorrect responses (supplement × diet × time, F(1, 117) = 0·60; NS) reached statistical significance (Table 1).
Word fluency
Similarly, supplementation did not influence word fluency, with the interaction supplement × diet × time being non-significant (F(1, 117) = 0·21; NS; Table 1).
Side effects
When asked to guess which supplement they had consumed, of those consuming the placebo, 18·3 % said creatine, 31·6 % placebo and 50 % did not know. These data were compared with those who received the active supplement of whom 14·8 % said creatine, 24·6 % placebo and 60 % did not know. The use of χ2 demonstrated that these distributions were not significantly different. When asked whether they experienced side effects, 85 % of those consuming the placebo and 73·8 % of those after taking creatine said that they had experienced none. The distributions obtained, depending on the supplement consumed, did not differ significantly. The side effects were in all cases minor, for example reporting feeling bloated or having a headache. These data suggested that the blind was maintained throughout the study.
Discussion
The present findings supported the view that brain creatine status can influence psychological functioning, as supplementation influenced memory (Fig. 1) and decision times (Fig. 2) in at least some of the sample. The finding that the supplementation of creatine was associated with better memory (Fig. 1) is consistent with Rae et al. (Reference Rae, Digney and McEwan13) who when considering only vegetarians found that supplementation improved memory. The present findings raise various questions concerning the effect on memory. Overall, memory was poorer when assessed on the second occasion irrespective of whether the placebo or creatine supplement had been consumed, raising the possibility that unexpectedly the second word list was more difficult than the first, although the way in which the two lists were matched argues against this as an explanation. Alternatively, there may have been a lack of motivation towards the end of the study, although the findings with the other cognitive measures do not support a generally more negative attitude. The major finding was that after supplementation, the memory of vegetarians was better than that of meat-eaters (Fig. 1). However, at baseline, memory did not differ depending on dietary style, so any hypothesised creatine deficiency in vegetarians did not influence memory, rather it was found that vegetarians were more sensitive to supplementation with creatine.
The finding that decision times were less variable after consuming the creatine supplement (Fig. 2) is consistent with the report(Reference Watanabe, Kato and Kato11) that it reduced the rate at which a decline in the performance of a demanding task occurred. Although mean reaction times are often reported, there is a long tradition of considering intra-individual variability; in fact, Jensen(Reference Jensen and Vernon16) considered this the most sensitive measure that can be derived from reaction times. Increased intra-individual variability is observed during ageing as well as traumatic brain injury, schizophrenia, attention-deficit hyperactivity disorder and dementia(Reference Benton and Hamsher17). Thus, intra-individual variability can be viewed as a behavioural marker of compromised neural functioning. MacDonald et al. (Reference MacDonald, Li and Bäckman18) concluded that intra-individual variability reflects multiple neural determinants including the functioning of the frontal lobes. They suggested that various neurotransmitters, in particular catecholamine and acetylcholine, contribute to greater intra-individual variability in cognitive performance(Reference MacDonald, Nyberg and Bäckman19).
The results can be viewed as creatine supplementation selectively influencing a demanding task that placed greater physiological demands on the brain, as it was only the monitoring of eight lamps that benefited from creatine (Fig. 2). The consumption of the placebo was associated with a poorer performance when tested for a second time, a decline prevented by consuming the creatine supplement. That the nature of the pre-existing diet did not influence the response to supplementation with the reaction time task suggested a more general effect.
It is possible that the dose used influences the response of the brain. Athletes wishing to benefit from creatine supplementation are recommended to consume 5 g doses, four times a day, for 5–6 d, after which a maintenance dose of 2–5 g a day is administered. Casey & Greenhaff(Reference Casey and Greenhaff20) found no evidence that increasing the dose beyond 20 g a day, during this initial period, further increased creatine uptake or athletic performance. The dose used in the present study reflected that used in the previous study that has considered increases in the creatine content of muscle. It is possible that the rate at which levels increase in the brain differ from muscle, but McMorris et al. (Reference McMorris, Harris and Swain7) reported that following 24 h of sleep deprivation, a dose similar to that used in the present study improved mood and the performance of tasks that placed a heavy demand on the prefrontal cortex.
One can speculate about the underlying mechanism. Phosphocreatine can be expected to play a role in supplying energy to metabolically active areas of the brain. There are several isoforms of creatine phosphokinase, the enzyme that converts creatine to phosphocreatine. The most common variety is found on the outer surface of the inner mitochondrial membrane and is coupled to the adenine nucleotide translocator. In the brain, this mitochondrial type is found, but there is also a brain-type creatine phosphokinase. Visualisation of the brain-type creatine phosphokinase shows that it is found in the ‘somata of Golgi type 1 neurons in the cerebellum, red nucleus and pons and is distributed throughout the cell body and within nuclei. Brain-phosphokinase immunostaining also appears in neuronal processes and is concentrated in the molecular layers of the cerebellum and the hippocampus and in cortical pyramidal cell dendrites’; the mitochondrial type is found ‘in the somata of all Golgi type 1 neurons in the cerebellum, pontine reticular formation, red nucleus, hippocampus and cerebral cortex… and appears throughout the cell body but not in nuclei’(Reference Friedman and Roberts21). As most of the brain-type creatine phosphokinase is found in neuronal processes, particularly in synaptic regions, a role in neurotransmission is suggested. The uneven distribution of creatine phosphokinase indicates that rather than having a general function, it is important in some aspects of functioning rather than others. The high concentration in the hippocampus is of particular interest, given the known importance of this area of the brain in memory. The previous report that creatine supplementation improves memory(Reference Rawson, Lieberman and Walsh4) and the present finding that memory benefited from supplementation (Fig. 1) are consistent with these physiological data.
It is, however, possible that the effect of supplementation may be indirect. For example, in vegetarians supplemented with creatine, Rooney et al. (Reference Rooney, Bryson and Digney22) found that glucose homeostasis was affected. In an oral glucose tolerance test, there was a greater glucose response, although it did not affect insulin levels. Given the repeated suggestions that a glucose drink and individual differences in glucose tolerance influence memory(Reference Benton and Owens23, Reference Donohoe and Benton24), this is a mechanism that should be examined.
To date, the findings are too preliminary to allow the role of creatine in the brain and the impact of its supplementation on cognition to be established. However, the present and previous reports(Reference Watanabe, Kato and Kato11, Reference Rae, Digney and McEwan13) that supplementation influences cognitive functioning, and the evidence that creatine supplementation influences basic brain physiology(Reference Dechent, Pouwels and Wilken10, Reference Watanabe, Kato and Kato11), suggest that the topic will repay further examination.
Acknowledgements
There are no conflicts of interests, and neither of the authors has any financial interest in the sale of creatine or creatine-containing products. The supplements were a gift from Wander Limited, King's Langley, Hertfordshire, UK, otherwise the study was not externally funded. The data were collected by R. D. who was responsible for the statistical analysis. D. B. designed the study and reported the findings.





