In 2011, the Committee on Nutrition of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) systematically reviewed published evidence related to the safety and health effects of the administration of formulae supplemented with pro- and/or prebiotics compared with unsupplemented formulae( Reference Braegger, Chmielewska and Decsi 1 ).
With regard to probiotics, in line with the 2011 ESPGHAN document( Reference Braegger, Chmielewska and Decsi 1 ), our recent updated systematic review( Reference Skórka, Pieścik-Lech and Kołodziej 2 ) concluded that the available scientific data suggest that the administration of currently evaluated probiotic-supplemented formulae to healthy infants does not raise safety concerns with regard to growth and adverse effects. Some beneficial clinical effects are possible; however, there is no existing robust evidence to recommend their routine use. The latter conclusion may reflect the small amount of data on a specific probiotic strain(s) and outcomes, rather than a genuine lack of an effect. The efficacy and safety should be considered for each probiotic(s)-supplemented formula.
With regard to prebiotic-supplemented formulae, in 2011 the ESPGHAN Committee concluded that the administration of currently evaluated prebiotic-supplemented formulae to healthy infants does not raise safety concerns with regard to growth and adverse effects. The Committee did not support the routine use of prebiotic-supplemented formulae in infants( Reference Braegger, Chmielewska and Decsi 1 ). Subsequent to the Committee review, new evidence on the effects of supplementation of infant formulae with prebiotics was published. Here, we aimed to update the 2011 evidence on the effects of the administration of prebiotic-supplemented infant formulae to find out whether there is a need to revise current recommendations.
Methods
Criteria for considering studies for this review
The same methodology that has been already presented in two previous reviews( Reference Braegger, Chmielewska and Decsi 1 , Reference Skórka, Pieścik-Lech and Kołodziej 2 ) was followed. In brief, only randomised controlled trials (RCT) were eligible for inclusion. Participants had to be healthy term infants. Only studies that compared use of infant formula or follow-on formula supplemented with prebiotics (with prebiotic specification) during the manufacturing process compared with unsupplemented formula were included. Studies in which prebiotics were not administered during the manufacturing process, but thereafter, for example in capsules, the contents of which were supplemented to infant formula or feeds, were excluded. Studies in which synthetic human milk oligosaccharides were used were excluded. We also excluded trials evaluating fermented, acidified, and partially or extensively hydrolysed formulae supplemented with prebiotics. We focused on growth and such clinical outcomes as gastrointestinal infections, respiratory infections, tolerance, etc. However, no firm definitions of any of these clinical outcomes were applied. Of note, in some trials, these outcomes were reported as the primary or secondary outcomes; in others, these were reported as adverse events. In this review, we evaluated them as referred to in the original publications.
Search methods for identification of studies
Five databases were searched up to March 2017 to identify potential studies: Cochrane Library, MEDLINE, EMBASE, Web of Science and CINAHL. No restrictions by either date or language were used in the search strategies. The search results were imported into Endnote bibliographic software, providing a total of 3035 records to be screened by the research team. The search strategy for one of the databases (EMBASE) is provided in the online Supplementary Table S1. We also searched reference lists of identified studies, key review articles and previous systematic reviews. Certain publication types (i.e. letters to the editor, abstracts, proceedings from scientific meetings) were excluded, unless a full set of data were obtained from the authors. Three registers for clinical trials (www.clinicaltrials.gov, www.clinicaltrialsregister.eu; www.anzctr.org.au) were screened.
Data collection, analysis, extraction and management
Titles and abstracts of the papers identified by the search strategy were independently screened by three researchers (A. S., M. K., M. P.-L.). Full texts of the potentially relevant publications were obtained and assessed. Any disagreements were discussed within the study team until a consensus was reached. Data extraction was performed using standard data-extraction forms. In addition to data such as methods, participants, interventions and outcomes, we collected information about sample size calculation and the funding of each study.
Assessment of risk of bias in included studies
The Cochrane Collaboration’s tool for assessing risk of bias was used to establish the risk of bias. Type of randomisation method (selection bias), allocation concealment (selection bias), blinding of participants, personnel, intervention (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other forms of bias were considered( Reference Higgins and Green 3 ) (Fig. 1).
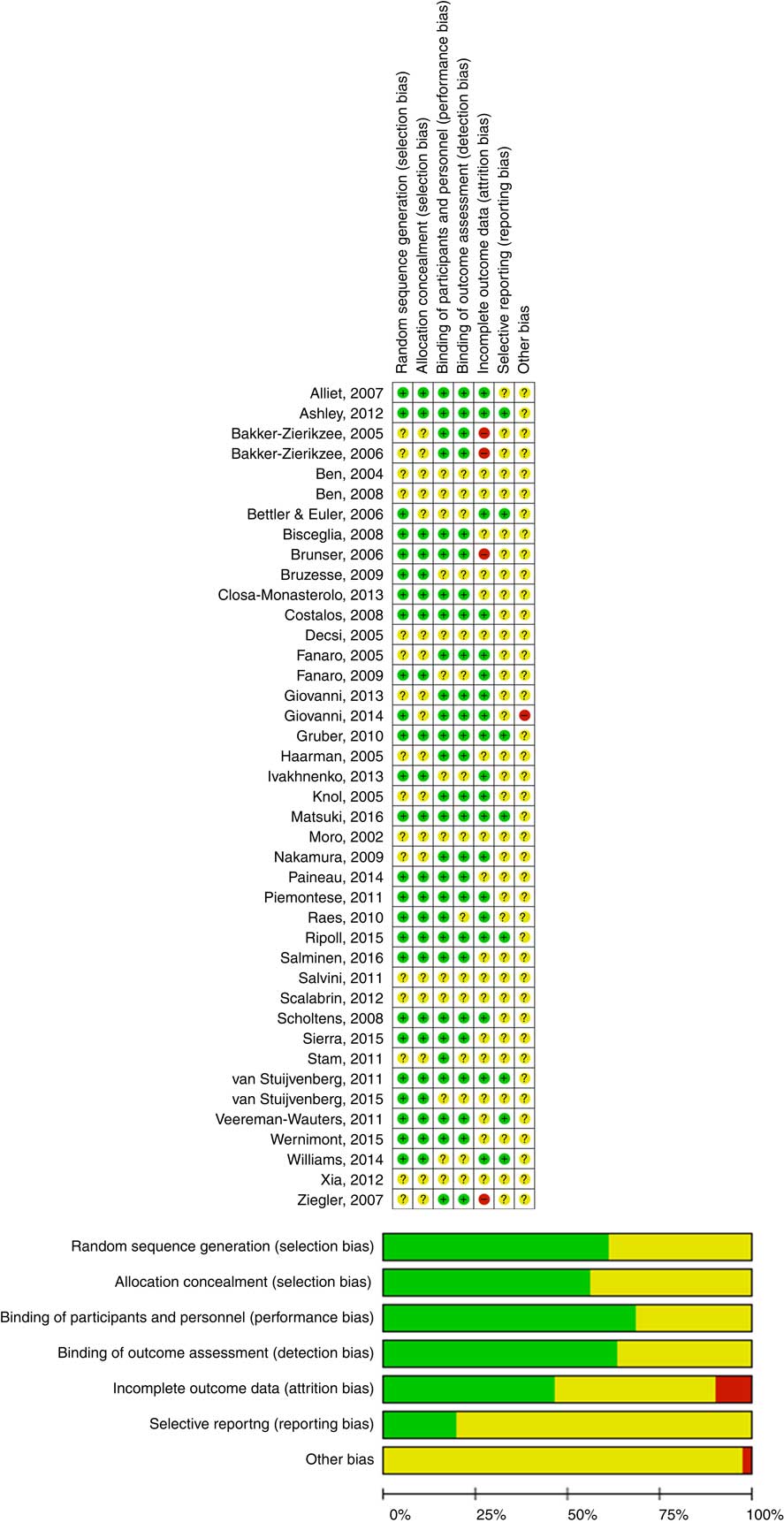
Fig. 1 Assessment of the risk of bias in included trials and the review authors’ judgements about each risk of bias item presented as percentages across all included studies. ![]() , Low risk of bias;
, Low risk of bias; ![]() , unclear risk of bias;
, unclear risk of bias; ![]() , high risk of bias. For a colour figure, see the online version of the paper.
, high risk of bias. For a colour figure, see the online version of the paper.
Measures of treatment effect
For reporting the effect, the results for individual studies and pooled statistics (if applicable) are reported as the risk ratio (RR), or mean difference (MD), between the experimental and control groups with 95 % CI. In other circumstances, we report the findings as reported by the authors of the included studies.
Data synthesis (statistical methods)
If appropriate, the data were analysed using Review Manager (RevMan) (Computer program; version 5.3, 2014; The Cochrane Collaboration). As various prebiotics differ in their effects, we did not perform pooling data (meta-analysis) of all prebiotic trials. Instead, we report evidence related to a specific prebiotic or their combinations separately. Compared with the 2011 report, no new meta-analyses were performed.
Results
Description of studies
For a flow diagram documenting the identification process for eligible trials (online Supplementary Fig. S1). Overall, forty-one publications met our inclusion criteria. In addition to the previously identified sixteen publications, twenty-five new publications were identified. In addition, four registered trials were identified: still recruiting at the time of the writing of this manuscript: NCT01143233, NCT03090360 and NCT02948114; active but not recruiting at the time of the writing of this manuscript: ACTRN12616001571460.
Table 1 summarises the characteristics of all included RCT evaluating the effects of prebiotic-supplemented formulae. Among new studies identified, there were three studies that were not included in the previous Committee systematic review despite having been published before the review( Reference Fanaro, Marten and Bagna 10 , Reference Bisceglia, Indrio and Riezzo 16 , Reference Bruzzese, Volpicelli and Squeglia 18 ). A number of RCT described the same study population but reported different outcome measures( Reference Ben, Zhou and Zhao 6 , Reference Ben, Li and Feng 7 , Reference Giovannini, Verduci and Zuccotti 11 , Reference Piemontese, Giannì and Braegger 24 , Reference Scalabrin, Mitmesser and Welling 26 , Reference Knol, Scholtens and Kafka 30 , Reference van Stuijvenberg, Stam and Grüber 31 , Reference van Stuijvenberg, Eisses and Grüber 34 – Reference Haarman and Knol 42 ). The online Supplementary Table S2 summarises the characteristics of the excluded trials, with reasons for exclusion. All of the included studies were carried out in healthy term infants. The vast majority of trials were conducted in Westernised countries and the majority were industry-funded trials.
Table 1 Characteristics of included randomised controlled trials in healthy term infants fed formulae supplemented with pro- and/or prebiotics compared with unsupplemented formulae (search up to March 2017). Overview of the findings: clinical results (Mean values with their standard errors; medians amd 25–75 percentiles; risk ratios (RR), mean differences (MD) and 95 % confidence intervals)

GOS, short-chain galactooligosaccharides; FOS, long-chain fructo-oligosaccharides; AOS, acidic oligosaccharides; IF, infant formula; BF, breast-feeding; BAE, adverse event; FF, follow-on formula; SYN1, 50:50 mixture of long chain inulin (Orafti HP) and oligofructose (b(2-1)-linked fructo-oligosaccharides with less than nine fructose moieties and partially containing a terminal glucose unit; PDX, polydextrose; LOS, lactulose; CFU, colony-forming units; GI, gastrointestinal; SAE, serious adverse event; URTI, upper respiratory tract infection; UTI, urinary tract infection.
* The effects as reported in a 2011 review (for details, see 1).
† Newly identified evidence (for details, see text).
The studies varied in the types of prebiotics used. As previously reported by the ESPGHAN Committee on Nutrition, the most commonly studied prebiotic was a 9:1 mixture of short-chain galacto-oligosaccharides (GOS) and long-chain fructo-oligosaccharides (FOS) (GOS/FOS)( Reference Bisceglia, Indrio and Riezzo 16 – Reference Veereman-Wauters, Staelens and Van de Broek 23 , Reference Knol, Scholtens and Kafka 30 , Reference Scholtens, Alliet and Raes 36 – Reference Bakker-Zierikzee, Tol and Kroes 38 , Reference Alliet, Scholtens and Raes 40 , Reference Haarman and Knol 42 , Reference Raes, Scholtens and Alliet 43 ).
Other prebiotics studied were as follows:
-
(1) GOS( Reference Ashley, Johnston and Harris 5 – Reference Ben, Li and Feng 7 , Reference Fanaro, Marten and Bagna 10 , Reference Giovannini, Verduci and Zuccotti 11 , Reference Sierra, Bernal and Blasco 14 , Reference Williams, Choe and price 15 , Reference Matsuki, Tajima and Hara 33 , Reference Giovannini, Verduci and Gregori 39 );
-
(2) acidic oligosaccharides (AOS)( Reference Fanaro, Jelinek and Stahl 4 );
-
(3) FOS( Reference Bettler and Euler 8 , Reference Brunser, Figueroa and Gotteland 9 , Reference Paineau, Respondek and Menet 12 , Reference Ripoll, Chappui and Respondek 13 , Reference Xia, Williams and Hustead 29 , Reference Wernimont, Northington and Kullen 32 );
-
(4) GOS/FOS/AOS( Reference Fanaro, Jelinek and Stahl 4 , Reference Piemontese, Giannì and Braegger 24 , Reference van Stuijvenberg, Stam and Grüber 31 , Reference van Stuijvenberg, Eisses and Grüber 34 , Reference Grüber, van Stuijvenberg and Mosca 35 , Reference Stam, van Stuijvenberg and Garssen 44 );
-
(5) oligofructose plus inulin (SYN-1)( Reference Veereman-Wauters, Staelens and Van de Broek 23 , Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ); and
-
(6) polydextrose (PDX) plus GOS with lactulose (PDX+GOS+ LOS)( Reference Nakamura, Gaskins and Collier 25 , Reference Ziegler, Vanderhoof and Petschow 27 ) or without lactulose (PDX+GOS)( Reference Ashley, Johnston and Harris 5 , Reference Nakamura, Gaskins and Collier 25 – Reference Ziegler, Vanderhoof and Petschow 27 , Reference Salminen, Endo and Isolauri 41 ).
The doses of prebiotics ranged from 0·1 to 0·8 g/100 ml, and the duration of the intervention ranged from 2 weeks to 12 months. All but five RCT( Reference Brunser, Figueroa and Gotteland 9 , Reference Fanaro, Marten and Bagna 10 , Reference Sierra, Bernal and Blasco 14 , Reference van Stuijvenberg, Stam and Grüber 31 , Reference van Stuijvenberg, Eisses and Grüber 34 ) reported the prebiotic supplementation of an infant formula. In these five trials, prebiotics were used to supplement follow-on formula.
Risk of bias in included trials
The quality of the included RCT varied (Fig. 1). Almost all of the included trials had a number of methodological limitations. The most common problems were a lack of description of randomisation procedures and/or allocation concealment and/or blinding.
Clinical effects
Below, we summarise evidence from trials reporting clinical outcomes that have not been included in the earlier report by the ESPGHAN Committee on Nutrition( Reference Braegger, Chmielewska and Decsi 1 ).
Growth
A number of trials assessed the effects of prebiotic supplementation on growth, including FOS supplementation of infant formula, either 0·4 g/100 ml( Reference Paineau, Respondek and Menet 12 ) or 0·5 g/100 ml,( Reference Ripoll, Chappui and Respondek 13 ) and GOS supplementation (with various GOS contents)( Reference Ashley, Johnston and Harris 5 , Reference Fanaro, Marten and Bagna 10 , Reference Giovannini, Verduci and Zuccotti 11 , Reference Sierra, Bernal and Blasco 14 , Reference Williams, Choe and price 15 ). In both cases, there were no significant differences between the prebiotic-supplemented and the unsupplemented formula groups. In 4 studies investigating the effects of the administration of formula supplemented with 0·8 g/100 ml of GOS/FOS, no significant differences between the prebiotic and control groups in anthropometric parameters (weight, length and head circumference) were found at any time( Reference Bisceglia, Indrio and Riezzo 16 , Reference Ivakhnenko and Nyankovskyy 20 , Reference Salvini, Riva and Salvatici 22 , Reference Veereman-Wauters, Staelens and Van de Broek 23 ). However, in a study by Bruzzese et al. the authors observed a transient increase in body weight. In the GOS/FOS-supplemented formula group compared with the control formula group, there was a significant increase in the mean body weight at 3 and 6 months of follow-up (P<0·01), whereas it was similar in the two groups at 9 and 12 months of follow-up. Mean body length was significantly greater in the GOS/FOS-supplemented formula group at all-time intervals (P<0·05), whereas mean head circumference was similar in the two groups at 3, 6, 9 and 12 months of follow-up (data not shown)( Reference Bruzzese, Volpicelli and Squeglia 18 ). The supplementation with FOS/GOS+AOS( Reference Piemontese, Giannì and Braegger 24 ) and the supplementation with two different doses of oligofructose-enriched inulin (0·4 g/100 ml and 0·8 g/100 ml supplementation)( Reference Veereman-Wauters, Staelens and Van de Broek 23 , Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ) had no significant effects on growth. Similarly, the supplementation with PDX/GOS( Reference Ashley, Johnston and Harris 5 , Reference Scalabrin, Mitmesser and Welling 26 ) resulted in no differences in anthropometric measures between infants receiving a control formula or a formula supplemented with 0·4 g/100 ml of a prebiotic blend of PDX and GOS from 14 to 60 d of age( Reference Scalabrin, Mitmesser and Welling 26 ) or from 14 to 120 d of age( Reference Ashley, Johnston and Harris 5 ).
Tolerance
Several new studies evaluated the influence of prebiotic-supplemented formulae on tolerance. Although there were different definitions of tolerance, there were no significant differences in tolerance between infants who received unsupplemented and prebiotic-supplemented formulae in the majority of studies. There were no differences in the number of days of abdominal pain (with crying) (0·22 (se 0·28) v. 0·23 (se 0·23), respectively; P value not shown) or nausea (0·05 (se 0·12) v. 0·03 (se 0·07), respectively, P value not shown) between infants who received unsupplemented or FOS-supplemented (0·4 g/100 ml) formula( Reference Paineau, Respondek and Menet 12 ), no significant differences between infants fed FOS-supplemented formulae (0·2 g/100 ml or 0·3 g/100 ml) or unsupplemented formula in the frequency of spitting up or vomiting (no exact data shown)( Reference Xia, Williams and Hustead 29 ), and no significant differences between infants fed FOS-supplemented formula (0·5 g/100 ml) or unsupplemented formula in the mean number of days with regurgitation (30·0 (se 5·8) v. 27·7 (se 5·0), respectively; P=0·79), crying (20·7 (se 6·0) v. 18·67 (se 5·2), respectively; P=0·85), and constipation (18·2 (se 4·7) v. 9·8 (se 2·4), respectively; P=0·23). However, there was a significantly reduced number of days with vomiting (4·1 (se 0·7) v. 7·7 (se 1·3); P=0·05) in the FOS-supplemented formula group( Reference Ripoll, Chappui and Respondek 13 ). No significant differences were found in tolerance symptoms, such as fussiness and gassiness, between an unsupplemented formula group and either a GOS-supplemented formula (0·4 g/100 ml) group( Reference Ashley, Johnston and Harris 5 ) or a PDX/GOS-supplemented formula group( Reference Ashley, Johnston and Harris 5 , Reference Scalabrin, Mitmesser and Welling 26 ). Regurgitation and vomiting scores were evaluated in infants receiving GOS/FOS- or oligofructose-enriched inulin-supplemented formulae( Reference Veereman-Wauters, Staelens and Van de Broek 23 ), with no significant differences found between the supplemented and unsupplemented formula groups. Tolerance was also defined as the incidence of episodes of crying, regurgitation, vomiting and flatulence. No differences between groups were found in a studies comparing GOS-( Reference Fanaro, Marten and Bagna 10 ) (no data shown), GOS/FOS- and AOS- (0·8 g/100 ml) supplemented formulae with unsupplemented formula (P>0·05)( Reference Piemontese, Giannì and Braegger 24 ). There were also no differences in either the duration of vomiting (median 2·0 (25–75 centile 0·0–4·0) v. 2·0 (25–75 centile 0·0–5·0), respectively; P=0·13) or the number of episodes of vomiting (median 0·87 (25–75 centile 0·4–1·56) v. 0·91 (25–75 centile 0·9–1·55), respectively; P=0·46) in the supplemented formula group compared with the unsupplemented formula group( Reference van Stuijvenberg, Stam and Grüber 31 ).
Stool frequency
Stool frequency was evaluated in twelve new trials. Although different prebiotics were used in the majority of studies, there were no significant effects on stool frequency between prebiotic-supplemented and unsupplemented formula groups, including FOS supplementation (two RCT)( Reference Xia, Williams and Hustead 29 , Reference Wernimont, Northington and Kullen 32 ), GOS supplementation (five RCT)( Reference Fanaro, Marten and Bagna 10 , Reference Williams, Choe and price 15 , Reference Matsuki, Tajima and Hara 33 ), FOS/GOS supplementation( Reference Veereman-Wauters, Staelens and Van de Broek 23 ), FOS/GOS/AOS supplementation( Reference Piemontese, Giannì and Braegger 24 ), oligofructose-enriched inulin supplementation( Reference Veereman-Wauters, Staelens and Van de Broek 23 ) and PDX/GOS supplementation( Reference Scalabrin, Mitmesser and Welling 26 ). Only four studies reported a higher frequency of stools in the prebiotic-supplemented formula group, either at all study time points (FOS/GOS)( Reference Bisceglia, Indrio and Riezzo 16 ) and (oligofructose-enriched inulin)( Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ) or at some time intervals (GOS at 7, 14 and 60 d),( Reference Ashley, Johnston and Harris 5 ) (GOS at 3 and 4 months)( Reference Sierra, Bernal and Blasco 14 ) and (PDX/GOS at 7, 14 and 60 d)( Reference Ashley, Johnston and Harris 5 ).
Stool consistency
A number of new trials assessed the effects of prebiotic supplementation on stool consistency, including FOS supplementation (three RCT)( Reference Ripoll, Chappui and Respondek 13 , Reference Xia, Williams and Hustead 29 , Reference Wernimont, Northington and Kullen 32 ), GOS supplementation (four RCT)( Reference Ashley, Johnston and Harris 5 , Reference Fanaro, Marten and Bagna 10 , Reference Sierra, Bernal and Blasco 14 , Reference Williams, Choe and price 15 ), FOS/GOS supplementation (two RCT)( Reference Bruzzese, Volpicelli and Squeglia 18 , Reference Veereman-Wauters, Staelens and Van de Broek 23 ), FOS/GOS/AOS supplementation (one RCT)( Reference Piemontese, Giannì and Braegger 24 ), PDX/GOS supplementation (two RCT)( Reference Ashley, Johnston and Harris 5 , Reference Scalabrin, Mitmesser and Welling 26 ) and oligofructose-enriched inulin supplementation (two RCT)( Reference Veereman-Wauters, Staelens and Van de Broek 23 , Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ). These trials documented a softening effect of prebiotic supplementation of formula on stool consistency (Table 1).
Gastrointestinal infections/diarrhoea
Six new studies evaluated the effects of prebiotic supplementation on the incidence of episodes of diarrhoea/gastrointestinal infections. Neither addition of FOS, GOS, nor FOS/GOS/AOS had an influence on the frequency of these episodes, with no differences between the supplemented and unsupplemented formula groups. Paineau et al. ( Reference Paineau, Respondek and Menet 12 ) found no difference between control and FOS-supplemented groups in the number of days with diarrhoea, defined as the number of days with liquid stools (0·10 (se 0·16) v. 0·18 (se 0·24), respectively; P value not shown). There were no significant differences between unsupplemented and GOS-supplemented formula groups in either the number of episodes of diarrhoea (defined as semiliquid or liquid faeces in three or more depositions per day for at least 3 d) per infant (0·20 (se 0·52) v. 0·27 (se 0·67); respectively; MD 0·07, 95 % CI −0·05, 0·19; P=0·36) or the number of infants with at least 1 episode of diarrhoea/year (15·9 v. 18·2 %, respectively; P=0·8)( Reference Sierra, Bernal and Blasco 14 ). There were also no significant differences in the number of episodes of gastroenteritis (diagnosed as a fever episode accompanied by vomiting and diarrhoea, or only diarrhoea) between infants receiving either standard or follow-up formula supplemented with 0·8 g/10 ml of GOS/FOS/AOS or control formula during the first 12 months of the intervention( Reference van Stuijvenberg, Eisses and Grüber 34 ), as well as in the adjusted frequency of febrile episodes due to diarrhoea analysed prospectively at 3–5 years (0·51 (25–75 centile 0·03–1·07) v. 0·53 (25–75 centile 0·03–1·51), respectively; P=0·22); however, the authors reported a shorter duration (d) of diarrhoea in the experimental group ((1·0 (25–75 centile 0·0–4·0) v. 2·0 (25–75 centile 0·0–7·0), respectively; P=0·01)( Reference van Stuijvenberg, Stam and Grüber 31 ). Only studies investigating formulae supplemented with GOS/FOS reported a beneficial effect of prebiotic supplementation. Ivakhnenko & Nyankovskyy( Reference Ivakhnenko and Nyankovskyy 20 ) found a significant reduction in the incidence of gastrointestinal tract infections at the age of 18 months in the GOS/FOS-supplemented (0·8 g/100 ml) formula group compared with the unsupplemented formula group (0·28 (se 0·05) v. 0·78 (se 0·12) episodes/child/18 months, respectively; MD −0·5; 95 % CI −0·53, −0·47; P<0·001). Bruzzese et al.( Reference Bruzzese, Volpicelli and Squeglia 18 ) reported, in the GOS/FOS-supplemented formula group compared with the unsupplemented formula group, a reduced rate of diarrhoeal episodes (defined as 3 or more loose or watery stools/d lasting for at least 3 d) (0·12 (se 0·04) v. 0·29 (se 0·05) episodes/child per 12 months, respectively; MD −0·17; 95 % CI −0·18, −0·16) and a reduced number of children with at least one episode of acute diarrhoea (10/96 v. 26/109, respectively; RR 0·37; 95 % CI 0·17, 0·82).
Respiratory tract infections
Only five studies reported the effects of prebiotic supplementation on the incidence of respiratory tract infections. No significant differences between groups in the number of episodes of upper respiratory tract infections per infant (1·65 (se 1·8) v. 1·84 (se 2·0), respectively; P=0·44) or in the number of infants with at least three episodes of upper respiratory tract infections per year (15·9 v. 16·7 %, respectively; P=0·9)( Reference Sierra, Bernal and Blasco 14 ) were reported when GOS-supplemented formula and unsupplemented formula were compared. No significant differences between groups were reported in the median adjusted number of upper respiratory tract infections as a suspected cause of fever as well as the number of episodes or duration in days of coughing (episodes 2·55 (25–75 centile 1·09–4·07) v. 2·63 (25–75 centile 1·09–4·56), respectively, P=038; duration: 22·5 (25–75 centile 8·0–42·5) v. 24·0 (25–75 centile 9·0–50·0), respectively, P=0·27) or runny or blocked nose (episodes: 2·59 (25–75 centile 1·02–5·26) v. 2·91 (25–75 centile 0·99–4·95), respectively, P=0·93; duration: 21·0 (25–75 centile 5·0–61·0) v. 24·0 (25–75 centile 6·0–60·0), respectively, P=0·66) between the prebiotics (FOS/GOS) and control groups during 12 months and at 3–5 years( Reference van Stuijvenberg, Stam and Grüber 31 , Reference van Stuijvenberg, Eisses and Grüber 34 ). Similar results were reported by Bruzzese et al.( Reference Bruzzese, Volpicelli and Squeglia 18 ). These authors found no significant differences between the FOS/GOS-supplemented formula group and the unsupplemented formula group in the number of patients with at least 1 episode of upper respiratory tract infections (60/94 v. 65/109, respectively; RR 1·07; 95 % CI 0·86, 1·33) and >3 episodes of upper respiratory tract infections (17/60 v. 29·65, respectively; RR 0·64; 95 % CI 0·39, 1·03). Also, Closa-Monasterolo et al. ( Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ) reported no significant differences in the mean number of infections between control and oligofructose-enriched inulin formula-fed groups. However, a significant reduction in the incidence of upper respiratory tract infections at the age of 18 months in the FOS/GOS-supplemented (0·8 g/100 ml) formula group compared with the control formula group (2·81 (se 0·51) v. 5·78 (se 0·97), respectively; MD −2·97; 95 % CI −3·26, −2·68) was reported by Ivakhnenko & Nyankovskyy( Reference Ivakhnenko and Nyankovskyy 20 ).
Allergic manifestations
Only two studies evaluated the effects of prebiotic supplementation on allergic manifestation. Sierra et al. ( Reference Sierra, Bernal and Blasco 14 ) reported no significant difference between the unsupplemented and GOS-supplemented formula groups in the number of allergic manifestations (atopic dermatitis, wheezing, food allergy) (28/132 v. 39/172, respectively; RR 1·39; 95 % CI 0·91, 2·12; P=0·12)( Reference Sierra, Bernal and Blasco 14 ). Whereas Ivakhnenko & Nyankovskyy( Reference Ivakhnenko and Nyankovskyy 20 ) found, in the GOS/FOS-supplemented formula group compared with the unsupplemented formula group, a significant reduction in the number of infants with allergic reactions to food (3/62 v. 9/53, respectively; RR 0·28; 95 % CI 0·09, 0·9), allergic reactions to cows’ milk protein (2/62 v. 8/53, respectively; RR 0·2; 95 % CI 0·05, 0·84), atopic dermatitis (3/62 v. 9/53, respectively; RR 0·28; 95 % CI 0·09, 0·9), and gastrointestinal symptoms of food allergy (2/62 v. 7/53, respectively; RR 0·24; 95 % CI 0·06, 0·98); however, there was no effect of formula supplementation on respiratory system allergic symptoms (3/62 v. 7/53, respectively; RR 0·36; 95 % CI 0·1, 1·2).
Antibiotic treatment
Five studies reported on the effects of prebiotic supplementation on frequency of antibiotic treatment. Four of them reported no significant difference between the unsupplemented and supplemented formula groups. Ripoll et al. ( Reference Ripoll, Chappui and Respondek 13 ) evaluated FOS-supplemented formula and reported no significant difference (P=0·12) in the number of infants with concomitant treatments (including antibiotics) between study groups. Sierra et al. ( Reference Sierra, Bernal and Blasco 14 ) reported no significant difference between the unsupplemented and GOS-supplemented formula groups in the percentage of patients with antibiotic treatment (19·8 v. 17·8 %, respectively; RR 0·88; 95 % CI 0·5, 1·45; P=0·48). In the studies by van Stuijvenberg et al. ( Reference van Stuijvenberg, Eisses and Grüber 34 ), there were no significant differences between the experimental (FOS/GOS-supplemented formula) and control groups in the median adjusted numbers of fever episodes for which systemic antibiotics were used during the 1st year of life as well as during follow-up of that group at 3–5 years( Reference van Stuijvenberg, Stam and Grüber 31 ). However, Bruzzese et al.( Reference Bruzzese, Volpicelli and Squeglia 18 ) found, in the FOS/GOS-supplemented formula group compared with unsupplemented formula group, a significantly reduced rate of antibiotic courses prescribed for children (1·03 (se 0·15) v. 1·48 (se 0·16), respectively; MD 0·45; 95 % CI −0·49, −0·4, P=0·038) and a significantly reduced number of children receiving more than two antibiotic courses/year (24/60 v. 43/65, respectively; RR 0·6; 95 % CI 0·42, 0·86); however, there was only a borderline significant difference between groups in the number of children receiving at least 1 antibiotic course (46/60 v. 58/65, respectively; RR 0·86; 95 % CI 0·73, 1·01).
Adverse events
A number of studies reported data on adverse events (Table 1), but none of them reported serious adverse events related to the tested formulae. Some authors defined adverse events as concomitant infections (nasopharyngitis, bronchitis, gastroenteritis and diarrhoea), whereas others defined adverse events as feeding-related gastrointestinal symptoms or symptoms of intolerance. No significant differences in the number of adverse events between supplemented and unsupplemented formula groups were reported by Ripoll et al. ( Reference Ripoll, Chappui and Respondek 13 ) and Wernimont et al. ( Reference Wernimont, Northington and Kullen 32 ) evaluating FOS supplementation, by Piemontese et al. ( Reference Piemontese, Giannì and Braegger 24 ) evaluating the FOS/GOS/AOS supplementation, and by Scalabrin et al. ( Reference Scalabrin, Mitmesser and Welling 26 ) evaluating PDX/GOS supplementation. Ashley et al. ( Reference Ashley, Johnston and Harris 5 ) evaluated GOS supplementation and PDX/GOS supplementation and found no differences in the overall incidence of at least one medically confirmed adverse event between supplemented and unsupplemented formula groups. However, these authors reported that the frequency of excessive spitting was significantly higher in the GOS-supplemented formula group (7·5 v. 0 %, P<0·05) and gas was significantly less frequent in the PDX/GOS-supplemented group (P<0·05) compared with the unsupplemented group. Also, Williams et al. ( Reference Williams, Choe and price 15 ) reported no significant differences among feeding groups in the proportions of subjects with specific adverse events, with one exception; there was a significantly higher proportion of serious adverse events, primarily respiratory tract infections, in the group that received a higher concentration of GOS (0·8 g/100 ml v. 0·4 g/100 ml; P<0·05). In two studies by Giovannini et al. ( Reference Giovannini, Verduci and Zuccotti 11 , Reference Giovannini, Verduci and Gregori 39 ), there were no differences in crying episodes, vomiting, or diarrhoeal episodes between the groups. However, the authors reported significantly lower regurgitation rates and episodes of infantile colic in the GOS-supplemented formula group (P<0·05), and lower stool frequency in the unsupplemented formula group (P<0·05). In a study evaluating oligofructose-enriched inulin supplementation, the frequencies of the majority of adverse events were generally equal in both groups; exceptions were the amount of time spent crying (min/d), which was significantly longer in the prebiotic group compared with the control group (P<0·05), as well as the number of episodes of loose stools, which was slightly fewer in the prebiotic group compared with the control group (2 v. 8 %; P<0·05)( Reference Closa-Monasterolo, Gispert-Llaurado and Luque 28 ). In some studies, no adverse events were observed throughout the study period in the control and experimental groups (GOS( Reference Matsuki, Tajima and Hara 33 ), FOS/GOS)( Reference Bisceglia, Indrio and Riezzo 16 , Reference Bruzzese, Volpicelli and Squeglia 18 , Reference Salvini, Riva and Salvatici 22 , Reference Veereman-Wauters, Staelens and Van de Broek 23 ).
Non-clinical outcomes
The online Supplementary Table S3 provides an overview of the non-clinical findings in all RCT that evaluated the effects of prebiotic-supplemented formulae. The findings were not consistent. However, differences differences in microbiota composition and immune parameters in infants fed prebiotic-supplemented formula v. unsupplemented formula (e.g. higher stool colony counts of bifidobacteria; increased SCFA concentrations; reduce faecal pH) were found.
Discussion
Summary of findings
We updated the 2011 evidence on the effects of the administration of prebiotic-supplemented infant formulae compared with unsupplemented formulae. Forty-one eligible trials were identified, described in twenty-five new publications.
Considering all of the evidence available (i.e. included in the earlier report by the Committee on Nutrition and the current up-date), supplementation of infant formula with FOS alone had no effect on growth, tolerance (assessed as some of following: abdominal pain, crying, nausea, vomiting, regurgitation), gastrointestinal infections, and stool frequency. However, it was associated with a softer stool consistency. Supplementation of infant formula with GOS alone had no effect on growth, tolerance (assessed as some of following: crying, regurgitation, fussiness, gassiness, vomiting, flatulence), gastrointestinal infections, respiratory tract infections, and allergic manifestations. However, it was associated with an increased stool frequency with a softer stool consistency in some trials. Supplementation of infant formula with FOS/GOS had no effect on growth or tolerance (assessed as some of following: crying, regurgitation, fussiness, gassiness, vomiting, flatulence, cramps). The effects on gastrointestinal and respiratory tract infections remain unclear, as this was documented in only some trials. Furthermore, the methodological quality of some them was poor. There was no effect of supplementation of infant formula with FOS/GOS on stool frequency. However, it was associated with a softer stool consistency. Supplementation of infant formula with AOS alone had no effect on growth, tolerance (assessed as crying, regurgitation, or vomiting), and stool frequency. However, it was associated with a softer stool consistency. Supplementation of infant formula with FOS/GOS/AOS had no effect on growth or tolerance (assessed as crying, regurgitation, vomiting). The effects on gastrointestinal and respiratory tract infections remain unclear, as this was documented in only some trials. Furthermore, the methodological quality of some them was poor. Supplementation of infant formula with FOS/GOS/AOS was associated with an increased stool frequency, with a softer/lower stool consistency. Supplementation of infant formula with PDX/GOS had no effect on growth or tolerance (as assessed by fussiness, gassiness). However, it was associated with a softer stool consistency. Supplementation of infant formula with oligofructose-enriched inulin had no effect on growth or tolerance (assessed as fussiness, regurgitation, or vomiting). However, it was associated with a softer stool consistency. Overall, adverse events were frequently reported; however, with minor exceptions, no differences were found between the prebiotic-supplemented and unsupplemented formula groups. None of the trials reported serious adverse events related to use of the study products.
Based on clinical outcomes, a number of studies analysed the effects of different prebiotics on growth, an essential outcome measure for evaluating the safety of infant formulae. All of these trials concluded that prebiotic supplementation of infant formulae did not have any significant effects on growth. All trials consistently showed that prebiotic supplementation of infant formulae has the potential to soften stools. However, the clinical significance of these findings remains unclear, although it may be beneficial in small infants with hard stools. Many, albeit not all, studies showed that prebiotic supplementation of infant formulae significantly increased stool frequency. Again, the clinical significance of these findings remains unclear. There is some inconsistent evidence suggesting that supplementation of infant formula with GOS/FOS may be associated with a reduced risk of gastrointestinal infections. However, the effect size was small and the confidence intervals were wide, so these results should be interpreted with caution. The supplementation of infant formula with GOS/FOS/AOS may be associated with a reduction in the duration of diarrhoea. With one exception, the available data showed no effect of prebiotic supplementation of infant formulae on respiratory tract infections. The only trial suggesting that supplementation of infant formula with GOS/FOS may be associated with a reduced risk of upper respiratory tract infections needs to be interpreted with caution, as the confidence intervals were wide. The effects of prebiotic supplementation of infant formulae on allergic diseases were inconclusive. One study with methodological limitations demonstrated that supplementation of infant formula with GOS/FOS resulted in a significant decrease in some allergic reactions. Supplementation with GOS/FOS/AOS reduced the risk of eczema. Supplementation with GOS had no effect on the rates of allergic manifestations and sensitisation. Thus, there is still too much uncertainty to draw reliable conclusions from the available data. The effects of prebiotic supplementation of infant formulae on antibiotic use were inconclusive. One study demonstrated that supplementation of infant formula with GOS/FOS resulted in a significant decrease in the number of fever episodes for which systemic antibiotics were used; the authors of other studies reported no significant decrease in antibiotic use with prebiotic supplementation. The reporting of symptoms such as crying, fussiness, regurgitation, and vomiting was not consistent. In trials in which these symptoms were evaluated as tolerance, no significant differences were found between the prebiotic-supplemented and unsupplemented formula groups. In trials in which these symptoms were evaluated as adverse events, differences were observed in some of the trials, but the effects were not consistent.
Strengths and limitations
An important strength of this systematic review is the use of rigorous methodology developed by the Cochrane Collaboration. The review addressed a clear question that was defined in terms of the study design, participants and interventions; however, the primary outcomes were not determined in advance.
We employed several methods to reduce bias (i.e. comprehensive literature search, pre-specified criteria for methodological assessment and analysis, no restrictions by language or year of publication). However, we cannot exclude the risk of publication bias, which was not formally addressed due to the limited number of included studies. The methodological quality of the included studies was generally moderate to low, which increases the risk of bias. Some of the prebiotics were evaluated in single trials only. The included studies were likely to be underpowered for addressing some outcomes (also adverse events).
The majority of included studies were industry-supported trials. There is bias associated with study funding sources. Compared with non–industry-sponsored studies, industry-sponsored studies tended to have more favourable effectiveness and harm findings and more favourable conclusions( Reference Lundh, Sismondo and Lexchin 45 ). Funding of research by manufacturers of infant formulae may be considered even more controversial because of the need for protection and promotion of breast-feeding. However, in the case of studies involving infant formulae, industry involvement is unavoidable, as investigators lack the means to manufacture quality infant products.
Comparison with other studies
Overall, even as new data have become available, the conclusions made previously did not change. With regard to growth, the 2011 systematic review by the ESPGHAN concluded that prebiotic-supplemented formulae do not raise safety concerns, that is, they do not have adverse effects on growth( Reference Braegger, Chmielewska and Decsi 1 ). Our review does not confirm the findings of a 2012 systematic review by Mugambi et al., which concluded that prebiotic-supplemented formula increased weight gain; however, currently, more data are available. Similar to our review, Mugambi et al. ( Reference Mugambi, Musekiwa and Lombard 46 ) concluded that use of prebiotic-supplemented formula increased stool frequency but, in contrast to our findings, it had no impact on stool consistency.
Our review found that all of the prebiotic-supplemented formulae were well tolerated; no serious adverse effects related to use of the study products were observed in any of the studies, regardless of the dose of prebiotics used. The dose is important. As recently concluded by Gibson et al. ( Reference Gibson, Hutkins and Sanders 47 ), ‘an appropriate dose must be sufficient to generate a prebiotic effect, but not too high to induce unwanted or adverse effects such as excessive gas formation or non-selective utilisation.’
Previously, the ESPGHAN Committee on Nutrition concluded that prebiotic supplementation of infant formulae has the potential to affect a number of non-clinical outcomes( Reference Braegger, Chmielewska and Decsi 1 ). Overall, one of the most consistent findings was that the use of prebiotic-supplemented formulae resulted in significantly higher stool colony counts of bifidobacteria. It is generally accepted that the establishment of gut microbiota is of great importance to gastrointestinal physiology and appears to modulate the health and well-being of the host organism. The lower incidence of gastrointestinal and other infections found in breast-fed infants may, in part, be related to a predominance of Bifidobacterium and Lactobacillus in their gut microbiota. Therefore, the establishment of a gut microbiota closer to that of breast-fed infants in formula-fed infants after supplementation with prebiotics might be considered in the context of the current hypothesis on the role of the gut microbiota in health and disease. The development of not only infectious diseases but also non-communicable diseases (e.g. allergy, diabetes, obesity) may be related to aberrant gut microbiota early in life. At least in some studies, it has been documented that prebiotic supplementation stimulates the production of SCFA, primarily acetic acid. SCFAs are measurable products of bacterial fermentation and play a role in normal colonic functions. However, whether the increase in SCFA concentrations per se is of benefit is currently not well established.
The same applies to other stool parameters, such as the reduced faecal pH values in infants who have received prebiotic-supplemented formulae. The effects of prebiotic-supplemented formulae on some immunologic parameters were not consistent. However, some effects may be potentially important. For example, prebiotic supplementation increased faecal IgA secretion. Considering that IgA is an antibody that plays a critical role in mucosal immunity, prebiotic supplementation may have an impact on the development of the immune system in infants. However, taken together, the interpretation of the non-clinical findings was difficult. Whether a change in any of these parameters per se is of benefit to the infants is currently not established.
Conclusions
In line with the 2011 ESPGHAN document, the available scientific data suggest that the administration of currently evaluated prebiotic-supplemented formulae to healthy infants does not raise safety concerns with regard to growth and adverse effects. Some favourable clinical effects are possible, primarily stool softening, which may be beneficial in some infants. Currently, there is no existing robust evidence to recommend the routine use of prebiotic-supplemented formulae. The latter conclusion may reflect the small amount of data on specific prebiotics and outcomes, rather than a genuine lack of an effect. The efficacy and safety should be considered for each prebiotic(s)-supplemented formula.
Acknowledgements
M. K. and M. P.-L. received travel funds to attend scientific meetings from companies manufacturing infant formulae. This study was funded in full by The Medical University of Warsaw.
All authors contributed to the initial protocol of the study. A. S., M. P.-L. and M. K. were responsible for data collection, data analysis and data interpretation. A. S. assumed the main responsibility for the writing of the first draft of the manuscript. All authors contributed to (and agreed upon) the final version.
H. S. had academic-associated speaking engagements and/or received research funding from companies manufacturing infant formulae. The remaining authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000120




