The pathogenesis of inflammatory bowel disease (IBD) is clearly associated with microflora–host interactions that produce inflammatory mediators(Reference Fedorak and Madsen1, Reference Shanahan2). It has been demonstrated that some non-invasive organisms are capable of inducing epithelial cells to produce chemotactic cytokines (for example, IL-8) with resultant neutrophil migration(Reference Strober3). Further, experimental evidence suggests that the presence of human commensal bacteria in genetically susceptible individuals is essential in the pathogenesis of IBD(Reference Fedorak and Madsen1). Not surprisingly, therefore, therapies have been sought to modify the intestinal flora; one of these is the administration of probiotics.
Probiotics are live micro-organisms which, when ingested, can confer health benefits(Reference Petrof, Kojima, Ropeleski, Musch, Tao, De Simone and Chang4). Various probiotics are now moving into the mainstream of medical therapy for pouchitis(Reference Fedorak and Madsen1, Reference Shanahan2). Moreover, some evidence of probiotic efficacy has been reported in the treatment of IBD(Reference Fedorak and Madsen1, Reference Shanahan2). Typically, probiotics are various strains of Lactobacillus or Bifidobacteria species. They exist as either single entities or as combination products(Reference Fedorak and Madsen1, Reference Shanahan2).
Immunomodulatory actions, such as the reduction of pro-inflammatory cytokines (for example, TNF-α, interferon (IFN)-γ), and the increased secretion of regulatory cytokines (for example, IL-10), have been suggested as one of the mechanisms of action for probiotics(Reference Fedorak and Madsen1). The transcription factor NF-κB, once separated from its inhibitory protein (IκB), translocates into the nucleus where it activates genes encoding immunologically relevant proteins (for example, TNF-α, IL-1β and IL-6)(Reference Baeuerle and Henkel5–Reference Tak and Firestein7). NF-κB is thought to play a key role in the pathogenesis of intestinal inflammation. Evidence supporting the pro-inflammatory role of NF-κB comes from both animal models of enteric inflammation and from patients with IBD. It is not surprising, therefore, that the inhibition of NF-κB has been proposed as an important therapeutic target for IBD(Reference Jobin and Sartor8–Reference Schottelius and Baldwin11). Recently, it was reported that probiotics could inhibit the NF-κB signal transduction system(Reference Petrof, Kojima, Ropeleski, Musch, Tao, De Simone and Chang4, Reference Jijon, Backer, Diaz, Yeung, Thiel, McKaigney, De Simone and Madsen12).
Several different probiotic agents have demonstrated evidence of efficacy in the dextran sulfate sodium (DSS)-induced colitis model(Reference Osman, Adawi, Ahrne, Jeppsson and Molin13, Reference Arakai, Andoh, Takizawa, Takizawa and Fujiyama14). The pathogenesis of DSS-induced colitis involves a defect in epithelial barrier function, as related to the direct cytotoxic effect of DSS(Reference Egger, Bajaj-Elliott, MacDonald, Inglin, Eyesselein and Buchler15, Reference Strober, Fuss and Blumberg16). Changes in epithelial barrier function, as measured by the permeability to Evan's blue dye, can be found early during the time course of DSS-induced colitis(Reference Kitajima, Takuma and Morimoto17). This alteration in the colonic mucosal barrier subsequently leads to the influx of various inflammatory cells, macrophage activation and pro-inflammatory cytokine production(Reference Egger, Bajaj-Elliott, MacDonald, Inglin, Eyesselein and Buchler15, Reference Strober, Fuss and Blumberg16). Additionally, the nuclear expression of the p65 subunit of NF-κB has previously been shown to be up-regulated during DSS-induced colitis and is thought to play a critical role in promoting intestinal inflammation(Reference Spiik, Ridderstad, Axelsson, Midtvedt, Bkork and Pattersen18, Reference Murano, Maemura, Hirata, Toshina, Nishiwaka, Hammamoto, Sasaki, Saitoh and Katsu19).
Escherichia coli strain M-17 (EC-M17) is a novel probiotic agent with beneficial effects on the gastrointestinal tract(Reference Adler, Jacob and Eliakim20). EC-M17 is believed to be a direct descendant of the M17 strain first identified by the Russian bacteriologist L. G. Peretz in 1933. The original strain was maintained by the Tarasevich Institute of the Russian Ministry of Health and has been used extensively in humans as a therapy for gastrointestinal diseases and infections. The Russian literature documents the use of EC-M17 in thousands of individuals, mostly for the treatment of dysentery(Reference Bogomolov and Genina21, Reference Kurnosova, Zatsepin and Feklisova22).
The first goal of the present study was to examine the in vitro effects of EC-M17 on the NF-κB signalling system and on the secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α). A second goal of the present study was to evaluate the effects of EC-M17 on relevant clinical, histological and biochemical parameters in the murine DSS colitis model. In conjunction with the DSS colitis model, the effects of EC-M17 on nuclear p65 expression and relevant pro-inflammatory cytokines were also evaluated in vivo. A third goal was to compare the effects of EC-M17 with those produced by a known therapy for intestinal inflammation (i.e. pouchitis, IBD), metronidazole. Finally, a fourth goal of the present study was to evaluate the effects of EC-M17 plus metronidazole in DSS-induced colitis.
Experimental methods
Escherichia coli strain M-17 information
EC-M17 is a non-pathogenic bacterium of the family Enterobacteriaciae. It is a non-spore-forming gram-negative rod of the serotype O2 with flagellar antigen H type 41. EC-M17 is a facultative anaerobic bacillus that converts nitrates to nitrites, and is oxidase-negative and catalase-positive. Except for the presence of type 1 fimbriae, samples of EC-M17 are negative for all other virulence factors. American Type Culture Collection (ATCC) 202226 is the designation given to the parental seed stock of EC-M17 that was deposited with the American Type Culture Collection. EC-M17 is produced for research use, using a standard microbiological fermentation process.
Whole genome shotgun sequencing of a naturally occurring naladixic acid-resistant substrain of the parental EC-M17 strain has been successfully completed by the Pathogenomics Sequence Analysis Facility of the University of Minnesota to approximately an 8-fold coverage of the genome (V Kapur, unpublished results). To date, no direct comparison has been done to compare the sequence of EC-M17 with that of E. coli Nissle 1917. However, according to a published report, the Nissle strain differs from M17 in both serotype (O6 v. O2 for M17) and flagellar H antigen (H1 v. H41)(Reference Kokesova, Frolova, Kvberka, Sokol, Rossmann, Bartova and Tlaskalova-Hogenova23).
Reagents
DSS of molecular weight 36 000 to 50 000 Da was obtained from MP Biomedicals (Aurora, OH, USA). Key reagents for the myeloperoxidase (MPO) assay, which include 3, 3′, 5, 5′ tetramethylbenzidine, N, N dimethylformamide, H2O2 and hexadecyl-trimethyl-ammonium bromide, were obtained from Sigma Chemical Company (Saint Louis, MO, USA). Metronidazole was also obtained from Sigma. An NF-κB p65 antibody (sc-109) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Mouse cytokine ELISA kits (IL-1β, IL-6, IL-10, IL-4) were obtained via Pierce Endogen Inc. (Rockford, IL, USA). The IL-12 mouse ELISA kit was purchased from Biosource International (Camarillo, CA, USA). The TransAM™ NF-κB p65 assay kit was obtained from Active Motif (Carlsbad, CA, USA). The BioBalance Corporation (New York, NY, USA) provided the EC-M17 as a 5 × 1011 colony-forming units (cfu)/ml stock suspension in 0·6 % saline.
Effects of Escherichia coli strain M-17 in an nuclear factor-κB reporter gene cell line
A NF-κB reporter stable cell line, derived from human 293T embryonic kidney cells, was obtained from Panomics (Redwood City, CA, USA). Integrated into the cell line is a luciferase reporter construct that is regulated by six copies of the NF-κB response element. For these studies, the cells were plated into twenty-four-well culture plates and grown to confluence. The medium was then removed and replaced with serum-free medium for 16 h. Cells were treated with vehicle (0·6 % saline) or EC-M17 (1 × 108 cfu/ml), immediately before activating the NF-κB signalling pathway with the addition of TNF-α (100 ng/ml). All treatments were performed in triplicate. After 6 h, the cells were washed and lysed before the luciferase activity was quantified as relative light intensity. Light intensity was measured using an assay kit from Promega Corporation (Madison, WI, USA) and a Perkin Elmer HTS 7000+ Bioassay plate reader in the luminescence mode.
Effects of Escherichia coli strain M-17 in a RAW 264·7 macrophage cell line
The RAW 264·7 mouse macrophage cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). RAW 264·7 cells were grown in Dulbecco's modified Eagle's medium containing 10 % fetal bovine serum. The lipopolysaccharide (LPS) stimulation studies were conducted at a cell density of 2 × 106/ml. EC-M17 was added to the macrophage cell culture system, at a concentration of 1 × 108 cfu/ml, just before LPS (5 μg/ml). For the NF-κB p65 measurement, nuclear extracts were obtained from cells either immediately (0 h) or 3 h after LPS-stimulation. A protein determination of the nuclear extracts was done with the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). For the p65 analysis, 10 μg protein was utilised per sample. The nuclear binding of p65 was measured with the TransAM™ NF-κB p65 assay kit, according to the manufacturer's instructions.
For the cytokine secretion experiments, after 0 or 4 h of LPS exposure, the culture medium was collected for the measurement of cytokines (TNF-α, IL-1β and IL-6) by ELISA (Pierce-Endogen), according to the manufacturer's directions. Probiotic-conditioned culture media (CM) was prepared by adding EC-M17 to the macrophage culture media for 5 min or 2 h. The media was then collected and centrifuged at 10 000 rpm for 10 min. Next, the CM was passed through a 0·22 μm filter before use in cytokine secretion experiments. In order to heat kill EC-M17 (HK), the probiotic was boiled at 100°C for 20 min. Then, the heat-killed EC-M17 was centrifuged at 10 000 rpm. The cellular pellet was washed in PBS and re-centrifuged. The pellet was then re-suspended in a sufficient quantity of saline to achieve a final concentration of 1 × 108 cfu/ml. HK and CM were used in cytokine secretion studies with the RAW 264·7 cell line, as described above. CM was used at a final concentration of 10 % (v/v). In some experiments, we also evaluated whether metronidazole (50 μg/ml) could inhibit cytokine secretion.
Mice
Male C57 BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Three separate DSS-induced colitis studies were conducted, all using 9–11-week-old mice. The initial study compared the effects of three different dosages of EC-M17. The subsequent study compared the effects of EC-M17 alone, metronidazole alone, and the combination of EC-M17 and metronidazole. An additional study was conducted to analyse the NF-κB p65 subunit in murine colonic samples.
Preparation of Escherichia coli strain M-17 and metronidazole
A 0·6 % saline solution (vehicle) was prepared by diluting sterile 0·9 % saline (Hospira Inc., Lake Forest, IL, USA) with sterile water (Abbot Laboratories, North Chicago, IL, USA). Subsequently, the 1 × 1011 cfu/ml stock suspension of EC-M17 was diluted with 0·6 % saline so as to yield the final EC-M17 concentrations used (5 × 107 cfu/ml, 1 × 108 cfu/ml, 5 × 108 cfu/ml, and 5 × 109 cfu/ml). The 0·6 % saline solution was used as the vehicle treatment solution in the colitis studies. Metronidazole was suspended in 0·6 % saline and then heated with hot water for approximately 2 min. Using this method, metronidazole became completely soluble in the vehicle. Based on the relevant literature, a 40 mg/kg dose of metronidazole was used(Reference Rath, Schulz, Freitag, Dieleman, Li, Linde, Scholmerich and Sartor24).
Escherichia coli strain M-17 dosage assessment in dextran sulfate sodium-induced colitis in mice
During the first 7 d of the study, mice were given Millipore-filtered drinking water. Throughout this initial 7 d period, mice were dosed once daily with vehicle (at a dose volume of 5 ml/kg; n 8), with EC-M17 (5 ml/kg) at a 5 × 107 cfu/ml concentration (n 8), with EC-M17 (5 ml/kg) at a 5 × 108 cfu/ml concentration (n 8) or with EC-M17 (5 ml/kg) at a 5 × 109 cfu/ml concentration (n 8). The dosing was by orogastric administration. EC-M17 or vehicle dosing continued for the next 6 d while 2 % DSS was administered in the drinking water. One group of animals received water without DSS. The severity of colitis was assessed by disease activity index (DAI) measurements during the 13 d study period.
Disease activity index
A DAI was determined with a 0 to 4 point severity scale, based on stool consistency and evidence of intestinal bleeding. The method used (Table 1) was that of Murthy et al. (Reference Murthy, Cooper, Shim, Shah, Ibrahim and Sedergan25) with slight modifications(Reference Fitzpatrick, Wang and Le26). DAI were determined on days 0, 2, 4, 6, 8, 10, 12 and 13. The DAI was calculated as the sum of the scores for stool consistency and evidence of gastrointestinal bleeding divided by 2(Reference Murthy, Cooper, Shim, Shah, Ibrahim and Sedergan25). The presence of occult blood was determined on faecal smears by a hemoccult fecal occult blood kit (Beckman-Coulter Inc., Brea, CA, USA).
Table 1 Disease activity index scoring system

The comparative effects of Escherichia coli strain M-17, metronidazole, and Escherichia coli strain M-17 plus metronidazole in dextran sulfate sodium-induced colitis in mice
During the first 7 d of the study, mice were given Millipore-filtered drinking water. The water consumption by mice was determined during this period. During this initial 7 d period, mice were dosed once daily with vehicle (two groups n 6 and n 9; at a dose volume of 5 ml/kg), with EC-M17 (5 ml/kg) at a 5 × 109 cfu/ml concentration (n 10), with metronidazole (40 mg/kg; n 10) or with both EC-M17 (5 ml/kg) at a 5 × 109 cfu/ml concentration and metronidazole (40 mg/kg; n 10). The dosing was by orogastric administration. EC-M17, metronidazole, EC-M17 plus metronidazole, or vehicle dosing continued for the next 6 d, while 2 % DSS was administered in drinking water. One group of animals received water without DSS. Water consumption data were also collected during this phase of the study. DAI were determined on days 0, 2, 4, 6, 8, 10, 12 and 13.
Colonic histological evaluation
On day 13, these mice were euthanised with carbon dioxide. The colon was rapidly excised, and the colon length was measured. Subsequently, the colon was opened and faecal material removed by thorough rinsing in 0·9 % saline. A 2·5 cm segment of distal colon was immediately fixed in 10 % buffered formalin, and the tissue was processed for a histological evaluation.
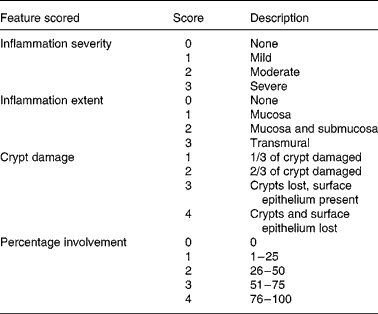
Histological damage was determined on a forty-point severity scale (Table 2). The percentage area of involvement was determined with a 25 mm ocular grid that was attached to an Olympus CH light microscope (Olympus, Center Valley, PA, USA). All histological evaluations were done at 400 × magnification and the calculation of histology scores involved the extent of inflammation, infiltration and crypt damage. For example, if there was a dense infiltrate of inflammatory cells that covered 90 % of the grid area, the inflammation severity score would be 12. This reflects the product of the severity of inflammation (sub-score = 3) and the percentage involvement (sub-score = 4). If the inflammation extended into the submucosa and was found in 80 % of the grid area, the inflammation extent score would be 8. This reflects the product of the inflammation extent (sub-score = 2) and the percentage involvement (sub-score = 4). If the crypts were entirely lost in 100 % of the area being evaluated, the crypt damage score would be 16. This reflects the product of the crypt damage (sub-score = 4) and the percentage involvement (sub-score = 4). Therefore, the total histology score for this area of the slide would be 36; 12 (inflammation severity)+8 (inflammation extent)+16 (crypt damage). Six areas on each histology slide were evaluated and a mean histology score was determined for each slide. The evaluation was done on coded slides so that the investigator (L. R. F.) was unaware of the treatment group. Other investigators have used this scoring system in conjunction with the DSS-induced colitis model(Reference Krieglstein, Cerwinka, Laroux, Grisham, Schurmann, Bruwer and Granger27, Reference Williams, Fuller, Dieleman, DaCosta, Haldeman, Sartor and Lund28).
Table 2 Histological scoring system
Measurement of myeloperoxidase and cytokines
A 2·5 cm segment of colon, adjacent to that obtained for histological evaluation, was used to measure MPO and various cytokines from colonic homogenates, as described previously by the principal investigator(Reference Fitzpatrick, Wang and Le26, Reference Fitzpatrick, Wang and Le29). Briefly, the homogenates were centrifuged at 10 000 rpm for 15 min. The pellet was retained for measurement of MPO by the 3, 3′, 5, 5′ tetramethylbenzidine method. The supernatant fraction was sampled and frozen at − 70°C for the subsequent determination of colonic cytokine levels. The following cytokines were measured in these colonic samples: IL-12, IFN-γ, IL-1β, IL-6, IL-10 and IL-4, using mouse cytokine ELISA kits.
Western blot analysis of the nuclear factor-κB p65 subunit in murine colonic samples
In a separate study, fourteen mice were treated, as described here: (1) vehicle for 13 d while given filtered drinking water for the entire time period (n 2), (2) EC-M17 (5 ml/kg of a 5 × 109 cfu/ml concentration) for 13 d while maintained on filtered water for the entire time period (n 3), (3) vehicle for 13 d while given 2 % DSS in drinking water for the final 6 d (n 5) or (4) EC-M17 (5 ml/kg of a 5 × 109 cfu/ml concentration) for 13 d, while maintained on 2 % DSS for the final 6 d (n 4). Mice were euthanised on study day 13 and colon samples were snap-frozen in liquid N2 before the collection of nuclear extracts from colonic homogenates. Briefly, for the preparation of nuclear extracts, the colonic tissue samples were cut into tiny pieces and washed with 500 μl cold PBS. The sample was centrifuged at 7000 rpm (i.e. for 6 min at 4°C). Then, lysis buffer (10 mm-HEPES, 10 mm-KCl, 1 mm-dithiothreitol, 0·1 mm-EDTA, 0·5 mm-PMSF (phenylmethanesulfonyl fluoride), 1 % IGEPAL® ((octylphenoxy)polyethoxyethanol, octylphenyl-polyethylene glycol), 1X Complete® protease inhibitor tablets (Roche Diagnostics, Manheim, Germany), aprotinin (2 μg/ml), leupeptin (2 μg/ml) and benzamidine (0·5 μg/ml)) was added to the tissue pellets. The samples were then incubated on ice for 30 min. The tissue pellets were then homogenised with a tissue miser (Thermo Fisher Scientific Inc., Waltham, MA, USA). These samples were further homogenised with a pestle tissue homogeniser (Thermo Fisher Scientific Inc.) and then centrifuged at 14 000 rpm (i.e. for 10 min at 4°C). The pellets were gently washed with lysis buffer without 1 % IGEPAL. The washes were discarded and the pellets re-suspended with nuclear lysis buffer (20 mm-HEPES, 0·4 m-NaCl, 1·5 mm-MgCl2, 25 % glycerol, 0·2 mm-dithiothreitol, 0·2 mm-EDTA, 0·5 mm-PMSF, 1 % IGEPAL, aprotinin (2 μg/ml), leupeptin (2 μg/ml) and benzamidine (0·5 μg/ml)). The samples were periodically vortexed throughout a 30 min period and then centrifuged at 14 000 rpm (15 min at 4°C). These supernatant fractions, which are the nuclear extracts, were retained for use in Western blot studies. A protein determination of these colonic extracts was done with the Bio-Rad protein assay (Bio-Rad Laboratories).
Western blots were performed using a standard technique that utilised 30 μg protein per colonic sample. Briefly, 4–15 % 2-amino-2-(hydroxymethyl)propane-1,3-diol-HCl ready gels (Bio-Rad Laboratories) were run at 100 V for about 1 h. Gels were then transferred onto PROTRAN® nitrocellulose membranes (Schleicher & Schuell Bioscience Inc., Keene, NH, USA) and the blots were blocked with PBS–Tween containing 5 % blotto (non-fat dried milk). Next, the blots were incubated in primary antibody (rabbit polyclonal antibody to p65), washed in PBS–Tween, and then exposed to an appropriate secondary antibody (goat-anti-rabbit). After another series of washes, equal amounts of an oxidising agent and a luminol reagent (Western Lightning; Perkin-Elmer Life Sciences Inc., Waltham, MA, USA) was applied for 1 min. Subsequently, the blots were dried and exposed to Kodak Scientific Imaging XB-1 film. NF-κB p65 was expressed as a 65 kDa protein as determined by internal molecular-weight standards (Bio-Rad Laboratories). For evaluating the levels of colonic p65 expression, a densitometry analysis was performed with a QuantiScan software program (Biosoft, Great Shelford, Cambs, UK). The p65 data were standardised to the actin levels in the nuclear samples, in order to account for any possible differences in the overall protein levels of the samples. The nuclear p65 expression data were normalised to the mean level found in vehicle- and water-treated mice, and it is reported as the fold increase compared with these data.
Statistical analyses
Statistical analyses were performed with GraphPad Prism computer software (GraphPad Software Inc., San Diego, CA, USA). Data are presented as means with their standard errors. Multiple treatment groups were analysed by one-way ANOVA, and then individual group comparisons were made by the Newman–Keuls multiple comparison test. To determine differences between two treatment groups, the Student's t test was utilised. For DAI, data were compared using the Mann–Whitney test. A P value < 0·05 was considered statistically significant.
Ethical considerations
The relevant mouse colitis studies were approved by the Institutional Animal Care and Use Committee at the Penn State College of Medicine.
Results
Effects of Escherichia coli strain M-17 on the nuclear factor-κB reporter gene cell line
An initial in vitro dose–response study demonstrated that the EC-M17 probiotic could dose dependently attenuate TNF-α-induced activation of a NF-κB-driven luciferase reporter gene system (Fig. 1 (a)). At 5 × 107 cfu/ml, some inhibition (29 %; P < 0·05) of NF-κB signalling was evident in human 293T embryonic kidney cells. However, more profound inhibition was seen in cells exposed to the 5 × 108 cfu/ml and 5 × 109 cfu/ml concentrations of EC-M17 (89 and 96 % inhibition, respectively; P < 0·001 in both cases). A 1 × 108 cfu/ml concentration of EC-M17 was selected for use in a follow-up study that included an untreated control group (vehicle). Even at this concentration, which was 50-fold less than the concentration of the solution used in the in vivo metronidazole comparative study in DSS-induced colitis (see below), TNF-α-induced activation of NF-κB was significantly reduced (65 %; P < 0·001) by the probiotic (Fig. 1 (b)). This reduction in NF-κB activation occurred without affecting cell viability, as determined by an MTS mitochondrial metabolism assay (Promega).

Fig. 1 The effects of Escherichia coli strain M-17 (EC-M17) on a NF-κB reporter gene assay in human 293T embryonic kidney cells that were activated by the addition of TNF-α (100 ng/ml). EC-M17 was added to the assay immediately before TNF-α treatment. After a 6 h incubation, the luciferase activity was measured as relative light intensity, using a plate reader in the luminescence mode. (a) Cells were treated with vehicle (0·6 % saline; □) or three different concentrations of EC-M17 (5 × 107 colony-forming units (cfu)/ml (![]() ), 5 × 108 cfu/ml (
), 5 × 108 cfu/ml (![]() ), 5 × 109 cfu/ml (■)). Mean value was significantly different from that of the vehicle-treated control group: *P < 0·05, ***P < 0·001. (b) EC-M17 was used at 1 × 108 cfu/ml (
), 5 × 109 cfu/ml (■)). Mean value was significantly different from that of the vehicle-treated control group: *P < 0·05, ***P < 0·001. (b) EC-M17 was used at 1 × 108 cfu/ml (![]() ) and two vehicle controls (□), one of which was a non-TNF-α control, were also included. *** Mean value was significantly different from that of the vehicle-treated, TNF-α-treated control group (P < 0·001). Values are means from three experiments with their standard errors represented by vertical bars.
) and two vehicle controls (□), one of which was a non-TNF-α control, were also included. *** Mean value was significantly different from that of the vehicle-treated, TNF-α-treated control group (P < 0·001). Values are means from three experiments with their standard errors represented by vertical bars.
Effects of Escherichia coli strain M-17 on the RAW 264·7 cell line
After 3 h of exposure, EC-M17 alone (without LPS) only mildly increased the nuclear binding of p65 compared with vehicle. However, the LPS-induced increase in p65 binding was significantly inhibited (78 %; P < 0·05) by EC-M17 at 1 × 108 cfu/ml (Fig. 2 (a)). A confirmation of assay specificity was demonstrated by complete inhibition of p65 binding by a wild-type oligonucleotide control.

Fig. 2 (a) The effects of Escherichia coli strain M-17 (EC-M17) on NF-κB p65 in a RAW 264·7 cell line. The NF-κB signalling pathway was activated by treating the cells with lipopolysaccharide (LPS; 5 μg/ml). Cells were treated with vehicle (0·6 % saline; □) or EC-M17 at 1 × 108 colony-forming units (cfu)/ml (![]() ). EC-M17 was added immediately before LPS treatment and cells were collected for assay 3 h after LPS treatment. Exposure of the nuclear extract to a wild-type oligonucleotide, which competitively blocks p65 nuclear binding, constituted a positive control (■). * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). Values are means from two experiments with their standard errors represented by vertical bars. (b–d) The effects of EC-M17, EC-M17-conditioned media (CM) and heat-killed EC-M17 on cytokine secretion in a RAW 264·7 cell line. The CM was prepared by adding EC-M17 to the macrophage culture medium for 5 min (
). EC-M17 was added immediately before LPS treatment and cells were collected for assay 3 h after LPS treatment. Exposure of the nuclear extract to a wild-type oligonucleotide, which competitively blocks p65 nuclear binding, constituted a positive control (■). * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). Values are means from two experiments with their standard errors represented by vertical bars. (b–d) The effects of EC-M17, EC-M17-conditioned media (CM) and heat-killed EC-M17 on cytokine secretion in a RAW 264·7 cell line. The CM was prepared by adding EC-M17 to the macrophage culture medium for 5 min (![]() ) or for 2 h (
) or for 2 h (![]() ). The cells were treated with vehicle (0·6 % saline;
). The cells were treated with vehicle (0·6 % saline; ![]() ), EC-M17 at 1 × 108 cfu/ml (
), EC-M17 at 1 × 108 cfu/ml (![]() ), heat-killed EC-M17 (■) prepared from a 1 × 108 cfu/ml concentration, or EC-M17 CM prepared from a 1 × 108 cfu/ml concentration. Except where indicated, macrophages were stimulated with the addition of LPS (5 μg/ml). Heat-killed EC-M17 and EC-M17 CM were added to the assay immediately before LPS treatment and culture media was collected for assay 4 h after LPS treatment. (b) TNF-α secretion in cells treated with and without LPS. * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). (c) IL-1β secretion in cells treated with and without LPS. * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). (d) IL-6 secretion in cells treated with and without LPS. The leftmost bar corresponds to vehicle-treated cells that were not exposed to LPS. Values are means from two or three experiments with their standard errors represented by vertical bars.
), heat-killed EC-M17 (■) prepared from a 1 × 108 cfu/ml concentration, or EC-M17 CM prepared from a 1 × 108 cfu/ml concentration. Except where indicated, macrophages were stimulated with the addition of LPS (5 μg/ml). Heat-killed EC-M17 and EC-M17 CM were added to the assay immediately before LPS treatment and culture media was collected for assay 4 h after LPS treatment. (b) TNF-α secretion in cells treated with and without LPS. * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). (c) IL-1β secretion in cells treated with and without LPS. * Mean value was significantly different from that of the vehicle-treated, LPS-treated control group (P < 0·05). (d) IL-6 secretion in cells treated with and without LPS. The leftmost bar corresponds to vehicle-treated cells that were not exposed to LPS. Values are means from two or three experiments with their standard errors represented by vertical bars.
Cytokine secretion in this macrophage cell line was essentially near zero at baseline. After 4 h, EC-M17 only mildly stimulated secretion (data not shown). By comparison, treatment with LPS resulted in substantial increases in the secretion of pro-inflammatory cytokines (Fig. 2 (b), (c) and (d)). Live EC-M17 significantly inhibited (>90 %; P < 0·05) the LPS-induced secretion of TNF-α, IL-1β and IL-6, when compared with LPS-treated cells exposed only to vehicle. Metronidazole (50 μg/ml), however, when exposed to RAW 264·7 cell cultures, did not reduce LPS-stimulated cytokine secretion (data not shown). Neither heat-killed EC-M17, nor EC-M17 conditioned media, effectively reduced cytokine secretion in LPS-treated macrophages (Fig. 2 (b), (c) and (d)). In these studies (Fig. 2), at 1 × 108 cfu/ml, EC-M17 did not affect macrophage viability as determined by the trypan blue exclusion method.
The effects of different doses of Escherichia coli strain M-17 in dextran sulfate sodium-induced colitis
The effects of three different dosages of EC-M17 on mean DAI scores were compared on the final day of the study (day 13). It was observed that 5 ml/kg of both 5 × 108 cfu/ml and 5 × 109 cfu/ml of EC-M17 significantly reduced mean DAI scores when compared with vehicle in DSS-treated mice (34·8 % (P < 0·05) and 43·5 % (P < 0·05), respectively). The lowest concentration of EC-M17 (5 × 107 cfu/ml) did not significantly reduce the mean DAI. Therefore, the 5 × 109 cfu/ml concentration of EC-M17 was selected for use in the comparative study with metronidazole.
Comparative effects of Escherichia coli strain M-17, metronidazole, and Escherichia coli strain M-17 plus metronidazole in dextran sulfate sodium-induced colitis
Before the administration of DSS (study day 7), there were no significant differences in the DAI scores among the treatment groups (Table 3). Also, during the pre-DSS phase of the study, only slight differences in water consumption were observed among all the treatment groups. Therefore, through this phase of the study, all mice tolerated the antibiotic and probiotic treatment regimens relatively well and no major overt effects were evident.
Table 3 Disease activity index (DAI) in C57 BL/6 mice over the 13 d course of the study*
(Mean values with their standard errors)

DSS, dextran sulfate sodium; EC-M17, Escherichia coli strain M-17.
* The DAI (0 to 40) was calculated as the sum of the scores for stool consistency and occult blood/gross bleeding divided by 2 (see Table 1).
† Excluding ‘Study day,’ column headers correspond to intervention/colitis induction. Colitis induction did not begin until day 7.
‡ Mean value was significantly different from that of vehicle-treated, water-treated control group (P < 0·01).
§ Mean value was significantly different from that of vehicle-treated, DSS-treated control group (P < 0·01).
The administration of 2 % DSS to otherwise untreated (vehicle/DSS) C57 BL/6 mice resulted in significant increases in DAI scores on days 12 and 13 when compared with vehicle-treated mice that did not receive DSS (vehicle/water; Table 3). However, the increased DAI that was found in vehicle-treated mice was less prominent in mice treated with EC-M17, metronidazole, and EC-M17 plus metronidazole. On day 13, the DSS-treated group that also received EC-M17 (EC-M17/DSS) had a mean DAI score 68·4 % less, when compared with DSS-treated mice that also received vehicle (P < 0·01). DSS-treated mice that received metronidazole (metronidazole/DSS) also had significantly reduced DAI (P < 0·01), but the reduction was not as large (47·4 %). DSS-treated mice that received both EC-M17 and metronidazole (EC-M17 plus metronidazole/DSS) had significantly reduced DAI which was reflected in a more profound reduction in the mean DAI score (78·9 %; P < 0·01). As on day 13, treatment with EC-M17, metronidazole, and EC-M17 plus metronidazole significantly reduced (P < 0·01) the DAI as compared with vehicle/DSS treatment on day 12 (Table 3).
Water consumption values were generally similar in all of the treatment groups from days 7 to 12. Water consumption ranged between 3·5 (sem 3) and 4·5 (sem 0·3) ml/d. However, on study day 13, water consumption in the vehicle/DSS treatment group (2·0 (sem 0·2) ml/d) was significantly less (P < 0·05) than in all the other treatment groups.
Treatment of mice with EC-M17 (alone, or in combination with metronidazole) resulted in less shortening in colon length, as compared with that found in the vehicle/DSS-treated mice. On day 13, the colon length values (cm) were: 7·1 (sem 0·2) for vehicle/water, 6·4 (sem 0·1) for vehicle/DSS, 7·2 (sem 0·1) for EC-M17/DSS, 6·8 (sem 0·2) for metronidazole/DSS and 7·3 (sem 0·1) for EC-M17 plus metronidazole/DSS. All of the colon length values in the probiotic and metronidazole treatment groups were significantly (P < 0·05) longer than the value found in the vehicle/DSS treatment group.
Effects of Escherichia coli strain M-17 on colonic cytokine levels in dextran sulfate sodium-induced colitis
The administration of 2 % DSS to C57 BL/6 mice led to a 2·6-fold increase in the mean colonic IL-12 level (Fig. 3 (a)). On study day 13, mice treated with EC-M17, metronidazole, or a combination of these agents, had significantly lower levels of colonic IL-12 as compared with vehicle-treated mice. In mice treated with both EC-M17 and metronidazole, there was a 52 % reduction in the colonic IL-12 content. In a similar fashion, following DSS administration, there was a 3·5-fold increase in the colonic IFN-γ content (Fig. 3 (b)). Animals treated with EC-M17, metronidazole, or both of these agents, had significantly lower levels of colonic IFN-γ (P < 0·05 v. vehicle/DSS). In mice treated with both the probiotic and the antibiotic, the level of colonic IFN-γ was virtually the same as in vehicle- and water-treated animals.

Fig. 3 The effects of oral administration of Escherichia coli strain M-17 (EC-M17) (5 mg/kg per d of a 5 × 109 concentration; n 10; ![]() ), metronidazole (40 mg/kg per d; n 10;
), metronidazole (40 mg/kg per d; n 10; ![]() ) and EC-M17 plus metronidazole (5 ml/kg per d of a 5 × 109 concentration and 40 mg/kg per d, respectively; n 10; ■) on colonic levels of pro-inflammatory cytokines in C57 BL/6 mice. Also, there were six vehicle-treated animals (□) that did not undergo colitis induction with dextran sulfate sodium (DSS) and nine vehicle-treated (
) and EC-M17 plus metronidazole (5 ml/kg per d of a 5 × 109 concentration and 40 mg/kg per d, respectively; n 10; ■) on colonic levels of pro-inflammatory cytokines in C57 BL/6 mice. Also, there were six vehicle-treated animals (□) that did not undergo colitis induction with dextran sulfate sodium (DSS) and nine vehicle-treated (![]() ) animals that underwent DSS colitis induction. (a) IL-12 content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. (b) Interferon (IFN)-γ level in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. (c) IL-1β content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the EC-M17-treated, DSS-treated control group (P < 0·05). (d) IL-6 level in DSS-treated and water-treated animals. The leftmost bar corresponds to the vehicle-treated group. Mean value was significantly different from that of vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. Values are means with their standard errors represented by vertical bars.
) animals that underwent DSS colitis induction. (a) IL-12 content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. (b) Interferon (IFN)-γ level in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. (c) IL-1β content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the EC-M17-treated, DSS-treated control group (P < 0·05). (d) IL-6 level in DSS-treated and water-treated animals. The leftmost bar corresponds to the vehicle-treated group. Mean value was significantly different from that of vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. Values are means with their standard errors represented by vertical bars.
The ingestion of DSS resulted in a 52-fold increase in the mean colonic IL-1β level (Fig. 3 (c)). Lower mean IL-1β contents were observed in mice treated with EC-M17 (18·5 %; NS), metronidazole (46·6 %; P < 0·05), or a combination of these agents (66·7 %; P < 0·05) when compared with vehicle-treated mice. Additionally, DSS-treated mice that received EC-M17 plus metronidazole had a significantly reduced colonic IL-1β (P < 0·05) when compared with DSS-treated mice that received EC-M17 only.
There was a very large increase (>200-fold) in colonic IL-6 with DSS treatment (Fig. 3 (d)). An overall reduction in IL-6 was noted in animals treated with EC-M17; however, a high IL-6 content in the colon of one of the probiotic-treated mice was responsible for the large standard error value and the failure to achieve statistical significance (compared with vehicle). Animals treated with metronidazole, or EC-M17 plus metronidazole, had significantly lower (P < 0·05) levels of colonic IL-6, as compared with vehicle-treated mice. In animals treated with both EC-M17 and metronidazole, there was a 78 % reduction in the colonic IL-6 content.
The colonic IL-10 content was attenuated in vehicle-treated mice that received DSS (114 (sem 10) pg/2·5 cm) as compared with vehicle-treated mice that did not receive DSS (179 (sem 20) pg/2·5 cm). However, there was no evidence that the probiotic and antibiotic treatment regimens increased the IL-10 level beyond that found in vehicle/DSS-treated mice. In a similar fashion to IL-10, the colonic IL-4 content was attenuated in vehicle/DSS-treated mice. Again, there was no evidence that the probiotic and antibiotic treatment regimens significantly increased the IL-4 level beyond that found in vehicle/DSS-treated mice (data not shown).
Effects of Escherichia coli strain M-17 on colonic myeloperoxidase and histology in dextran sulfate sodium-induced colitis
As shown in Fig. 4 (a), DSS-treated mice that received vehicle had a significant increase in the colonic MPO activity. These data indicate increased colonic neutrophil influx in these animals(Reference Fitzpatrick, Wang and Le26, Reference Fitzpatrick, Wang and Le29). Mice treated with EC-M17, metronidazole, or a combination of these two agents had reduced levels of MPO (Fig. 4 (a)). The combined treatment regimen significantly reduced colonic MPO by 55·3 % (P < 0·05) compared with vehicle. Interestingly, the combined treatment regimen also significantly reduced colonic MPO by 38·2 % (P < 0·05) compared with EC-M17 treatment alone.

Fig. 4 The effects of oral administration of Escherichia coli strain M-17 (EC-M17) (5 mg/kg per d of a 5 × 109 concentration; n 10; ![]() ), metronidazole (40 mg/kg per d; n 10;
), metronidazole (40 mg/kg per d; n 10; ![]() ) and EC-M17 plus metronidazole (5 ml/kg per d of a 5 × 109 concentration and 40 mg/kg per d, respectively; n 10; ■) on colonic levels of myeloperoxidase (MPO) and on histology scores in C57 BL/6 mice. Also, there were six vehicle-treated (□) animals that did not undergo colitis induction with dextran sulfate sodium (DSS) and nine vehicle-treated (
) and EC-M17 plus metronidazole (5 ml/kg per d of a 5 × 109 concentration and 40 mg/kg per d, respectively; n 10; ■) on colonic levels of myeloperoxidase (MPO) and on histology scores in C57 BL/6 mice. Also, there were six vehicle-treated (□) animals that did not undergo colitis induction with dextran sulfate sodium (DSS) and nine vehicle-treated (![]() ) animals that underwent DSS colitis induction. (a) MPO content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the EC-M17-treated, DSS-treated control group (P < 0·05). (b) Histology scores in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the metronidazole-treated, DSS-treated control group (P < 0·05). Values are means with their standard errors represented by vertical bars.
) animals that underwent DSS colitis induction. (a) MPO content in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the EC-M17-treated, DSS-treated control group (P < 0·05). (b) Histology scores in DSS-treated and water-treated animals. Mean value was significantly different from that of the vehicle-treated, DSS-treated control group: *P < 0·05, ***P < 0·001. † Mean value was significantly different from that of the metronidazole-treated, DSS-treated control group (P < 0·05). Values are means with their standard errors represented by vertical bars.
The vehicle/DSS-treated mice had evidence of more histological damage than their vehicle-treated counterparts that received water throughout the study. The mean colonic histology scores were significantly (P < 0·05) reduced in EC-M17-treated mice, in metronidazole-treated mice, and in EC-M17 plus metronidazole-treated mice (Fig. 4 (b)). The combination of EC-M17 plus metronidazole reduced mean histology scores to a greater extent than metronidazole alone (25·0 %; P < 0·05).
A normal colonic architecture is evident in a representative photograph of a histology specimen obtained from an animal that did not receive DSS (Fig. 5 (a)). By comparison, the administration of DSS resulted in crypt damage (black arrows), significant numbers of leucocytes in the lamina propria (white arrow), as well as evidence of leucocytes in the submucosa (grey arrow; Fig. 5 (b)). In this vehicle/DSS-treated mouse there was also evidence of leucocytes (i.e. apparently macrophages) at the luminal surface. In the histology specimen of an EC-M17-treated mouse shown in Fig. 5 (c), there is evidence of some surface epithelial cell damage (black arrow), as well as mild inflammatory cell influx in the lamina propria (white arrow) and submucosa (grey arrow). In the animal treated with EC-M17 plus metronidazole (Fig. 5 (d)) the crypt architecture was preserved. There was only mild leucocyte infiltration in the lamina propria (white arrow). The pattern of colonic histology in this animal generally resembled that found in the vehicle/water-treated mouse.

Fig. 5 Representative histology photographs of colonic specimens obtained on study day 13 following the oral administration of vehicle (0·6 % saline), Escherichia coli strain M-17 (EC-M17) (5 ml/kg per d of a 5 × 109 concentration), metronidazole (40 mg/kg per d) and EC-M17 plus metronidazole (5 ml/kg per d of a 5 × 109 concentration and 40 mg/kg per d, respectively) in C57 BL/6 mice that did (EC-M17, metronidazole, and EC-M17 plus metronidazole) or did not (vehicle) undergo dextran sulfate sodium (DSS) colitis induction. (a) Colonic histology of a vehicle-treated animal that did not receive DSS. (b) Colonic histology of a vehicle-treated animal that underwent DSS colitis induction. DSS caused crypt damage ( → ), significant numbers of leucocytes in the lamina propria (![]() ), as well as evidence of inflammatory cells in the submucosa (
), as well as evidence of inflammatory cells in the submucosa (![]() ). (c) Colonic histology of an EC-M17-treated animal that underwent DSS colitis induction. There was evidence of surface epithelial cell damage ( → ), as well as mild leucocyte influx in the lamina propria (
). (c) Colonic histology of an EC-M17-treated animal that underwent DSS colitis induction. There was evidence of surface epithelial cell damage ( → ), as well as mild leucocyte influx in the lamina propria (![]() ) and submucosa (
) and submucosa (![]() ). (d) Colonic histology of an EC-M17 plus metronidazole-treated animal that received DSS. Notice that the crypt architecture was well preserved and only mild inflammatory cell influx is evident in the lamina propria (
). (d) Colonic histology of an EC-M17 plus metronidazole-treated animal that received DSS. Notice that the crypt architecture was well preserved and only mild inflammatory cell influx is evident in the lamina propria (![]() ). This relatively normal colonic histology resembled that found in the vehicle-treated mouse that did not undergo DSS colitis induction. All images are at 200 × magnification.
). This relatively normal colonic histology resembled that found in the vehicle-treated mouse that did not undergo DSS colitis induction. All images are at 200 × magnification.
Effects of Escherichia coli strain M-17 on colonic nuclear factor-κB p65 in dextran sulfate sodium-induced colitis
As found in the previous DSS-induced colitis study (see above), the mean DAI was significantly reduced (P < 0·05 v. vehicle/DSS) by EC-M17 treatment. Specifically, mean DAI scores on day 13 were: 0 (sem 0) for vehicle/water, 0 (sem 0) for EC-M17/water, 2·2 (sem 0·2) for vehicle/DSS and 0·8 (sem 0·3) for EC-M17/DSS. In conjunction with the acute DSS colitis model paradigm, expression of the NF-κB p65 subunit was examined. Representative Western blots from mouse colonic nuclear extracts are shown in Fig. 6. The actin data confirmed equal protein loading in the Western blot experiments. Overall, relatively little expression of p65 was found in the colons of animals that received water over a 13 d period (vehicle/water or EC-M17/water; Fig. 6 (a)). Specifically, relative densitometry values in these colonic samples were 0·04 (lane 1), 1·96 (lane 2), 0·78 (lane 3), 1·54 (lane 4) and 0·39 (lane 5). In contrast, more prominent p65 expression was observed in animals that received 2 % DSS in drinking water (vehicle/DSS group; Fig. 6 (b)). Relative densitometry values were 6·05 (lane 1), 1·02 (lane 2) and 4·75 (lane 3). However, the expression of p65 was less evident in DSS-treated mice that received the probiotic (EC-M17/DSS; Fig. 6 (b)). These densitometry values were 1·56 (lane 4), 0·05 (lane 5) and 1·09 (lane 6). Relative densitometry analyses compared all the p65 data to vehicle/water-treated mice (normalised value = 1·0). These analyses yielded the following fold changes in mean densitometry values: 0·91 for EC-M17/water, 2·52 for vehicle/DSS and 1·37 for EC-M17/DSS. These data suggest that p65 expression was enhanced in vehicle/DSS-treated animals and reduced in mice treated with EC-M17.

Fig. 6 The effects of vehicle (0·6 % saline) in mice that did not consume dextran sulfate sodium (DSS) (n 2), Escherichia coli strain M-17 (EC-M17) (5 ml/kg per d of a 5 × 109 concentration) in mice that did not consume DSS (n 3), vehicle in mice that underwent DSS colitis induction (n 5) and EC-M17 (5 ml/kg per d of a 5 × 109 concentration) in mice that underwent DSS colitis induction (n 4) on colonic p65 expression. Nuclear extracts were obtained from colonic homogenates. The Western blot analysis of NF-κB p65 was performed using 30 μg protein per sample. The actin bands confirmed equal protein loading in these experiments. (a) Representative colonic p65 expression results in mice that did not receive DSS colitis. Lanes 1 and 2, vehicle; lanes 3 to 5, EC-M17. Relative densitometry values for these colonic extracts, as calculated in the Methods section, were: 0·04 (lane 1), 1·96 (lane 2), 0·78 (lane 3), 1·54 (lane 4) and 0·39 (lane 5). (b) Representative colonic p65 expression in mice that were given 2 % DSS in the drinking water. Lanes 1 to 3, vehicle; lanes 4 to 6, EC-M17. Relative densitometry values for these colonic samples were: 6·05 (lane 1), 1·02 (lane 2), 4·75 (lane 3), 1·56 (lane 4), 0·05 (lane 5) and 1·09 (lane 6).
Discussion
IBD is a chronic inflammatory condition involving the intestine. IBD appears to involve the complex interactions between the immune system, endogenous intestinal epithelial cells and the local luminal environment that contains high concentrations of commensal bacteria. These interactions lead to an unrestrained, exaggerated immune response resulting in intestinal inflammation(Reference Bouma and Strober30).
This immune response is characterised by activation of the NF-κB signalling system as well as secretion of relevant pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6(Reference Jobin and Sartor8, Reference Schottelius and Baldwin11). In this regard, NF-κB is clearly up-regulated in the intestines of IBD patients(Reference Neurath, Becker and Barbulescu31–Reference Thiele, Bierhaus, Autschbach, Hofmann, Stremmel, Thiele and Ziegler33). For example, the p65 component of the NF-κB heterodimer was reported to be highly activated in epithelial cells and in lamina propria macrophages obtained from patients with active Crohn's disease and ulcerative colitis(Reference Neurath, Becker and Barbulescu31). Long-standing therapies, such as corticosteroids and 5-aminosalicylic acid, may work, in part, by inhibiting NF-κB(Reference Thiele, Bierhaus, Autschbach, Hofmann, Stremmel, Thiele and Ziegler33–Reference Yan and Polk35). More specific approaches to inhibit NF-κB (for example, p65 NF-κB antisense therapy) have also demonstrated efficacy in animal models of IBD(Reference Spiik, Ridderstad, Axelsson, Midtvedt, Bkork and Pattersen18, Reference Murano, Maemura, Hirata, Toshina, Nishiwaka, Hammamoto, Sasaki, Saitoh and Katsu19, Reference Fitzpatrick, Wang and Le26, Reference Fitzpatrick, Wang and Le29). Recently, it was demonstrated that various bacteria strains (including probiotics) are also capable of inhibiting NF-κB and pro-inflammatory cytokines in various types of cells, including macrophages(Reference Petrof, Kojima, Ropeleski, Musch, Tao, De Simone and Chang4, Reference Jijon, Backer, Diaz, Yeung, Thiel, McKaigney, De Simone and Madsen12, Reference Hauf and Chakraborty36, Reference Selvaraj and Prasadarao37).
Our in vitro results showed that the novel probiotic agent, EC-M17, at a relatively low concentration (1 × 108 cfu/ml), could significantly attenuate the activation of NF-κB in two cell lines. Moreover, EC-M17 attenuated NF-κB in response to different inducing agents (TNF-α or LPS), thereby suggesting that this inhibition occurred at the post-receptor level. In concordance with the inhibition of LPS-induced p65 nuclear binding in a murine macrophage cell line, the secretion of NF-κB-dependent cytokines (TNF-α, IL-1β and IL-6) was dramatically inhibited by treatment with live EC-M17.
Interestingly, probiotic bacteria have shown varying effects on NF-κB in studies conducted in different cell lines. For example, Bifidobacterium infantis and Lactobacillus salivarius inhibited NF-κB to some degree in the HT-29 epithelial cell line(Reference O'Hara, O'Regan, Fanning, O'Mahony, Macsharry, Lyons, Bienenstock, O'Mahony and Shanahan38). In a clinically relevant study, using a tissue culture system with intestinal mucosal biopsies from IBD patients, B. longum clearly inhibited NF-κB activation in lamina propria mononuclear cells(Reference Bai, Ouyang, Xiao and Li39). By comparison, a commonly used probiotic (E. coli Nissle 1917) was found to activate the NF-κB pathway in intestinal epithelial cells, as well as in isolated murine lymphocytes(Reference Grabig, Paclik and Gurzy40, Reference Wehkamp, Harder and Wehkamp41). Similarly, B. lactis activated NF-κB in intestinal epithelial cell lines(Reference Ruiz, Hoffman, Szcesnys, Blaut and Haller42). Therefore, bacterial strain and species differences apparently exist with respect to the effects of probiotics on NF-κB. Based on our in vitro results (Figs. 1 and 2), EC-M17 and E. coli Nissle 1917 may have distinct effects on the NF-κB signal transduction system(Reference Grabig, Paclik and Gurzy40, Reference Wehkamp, Harder and Wehkamp41). It would be interesting in future studies to directly compare the effects of these two E. coli probiotics on the NF-κB pathway by conducting parallel studies in the same cell lines.
In our studies, only live EC-M17 were effective in inhibiting NF-κB signalling in murine macrophages; therefore, it is possible that EC-M17 delivers effector proteins directly into host cells, as has been suggested for other strains of E. coli (Reference Hauf and Chakraborty36, Reference Maresca, Miller, Quitaed, Dean and Kenny43). This possibility is reinforced by our data from the RAW 264·7 cell line, which showed that heat-killed EC-M17, as well as EC-M17 conditioned media, was not effective in reducing cytokine secretion by macrophages. These results suggest that the robust inhibitory effects of EC-M17 on these NF-κB-dependent cytokines required live bacteria, and not a secreted metabolite or a degradation product. Our findings are not in agreement with those of some other investigators. For example, Menard et al. (Reference Menard, Candalh, Bambou, Terpend, Cerf-Bensussan and Heyman44) found that secreted metabolites from two probiotics (B. breve and Streptococcus thermophilus) inhibited NF-κB signalling and TNF-α secretion by monocytes.
Metronidazole has been used to treat IBD patients for decades(Reference Blichfeldt, Blomhoff, Myhre and Gjone45, Reference Ursing and Kamme46). This antibiotic was selected as one of the pharmacological interventions in the present study because of its historical use as an IBD therapy, as well as to test for possible additive effects when given together with EC-M17. While metronidazole may have some intrinsic anti-inflammatory properties, its efficacy, as well as the efficacy of other antibiotics similarly employed, is probably related to a reduced burden of pro-inflammatory mucosal bacteria(Reference Lahat, Halperin, Barazovsky, Shalit, Rabau, Klausner and Fabian47, Reference Rutgeerts, Hiele, Geboes, Peeters, Penninckx, Aerts and Kerremans48). In this regard, we found no evidence that metronidazole inhibited LPS-induced cytokine secretion by murine macrophages.
The aetiology of DSS-induced colitis is thought to be multi-factorial in nature, involving both direct toxic effects on the mucosa, as well as the influx of inflammatory cells(Reference Egger, Bajaj-Elliott, MacDonald, Inglin, Eyesselein and Buchler15–Reference Kitajima, Takuma and Morimoto17). As with IBD, bacteria probably play a role in the pathogenesis of DSS-induced colitis, because it has been reported that antibiotics improve this colitis(Reference Strober, Fuss and Blumberg16, Reference Rath, Schulz, Freitag, Dieleman, Li, Linde, Scholmerich and Sartor24, Reference Hans, Scholmerich, Gross and Falk49). Moreover, various probiotics also improve DSS-induced colitis in rodents(Reference Osman, Adawi, Ahrne, Jeppsson and Molin13, Reference Arakai, Andoh, Takizawa, Takizawa and Fujiyama14).
An increased DAI, a reduction in colon length, enhanced colonic levels of pro-inflammatory cytokines, increased colonic MPO activity, and more severe colonic histological damage are consistent findings in DSS-treated animals. These parameters are often used as markers of colonic injury(Reference Jijon, Backer, Diaz, Yeung, Thiel, McKaigney, De Simone and Madsen12, Reference Fitzpatrick, Wang and Le26, Reference Gaudio, Taddei, Vetuschi, Sferrea, Frieri, Ricciardi and Caprilli50, Reference Schultz, Strauch, Linde, Watzl, Obermeir, Gottl, Dunger, Grunwald, Scholmerich and Rath51). Our findings indicate that both symptomatic (DAI) and gross morphological (colon length) parameters of colitis were significantly improved in the antibiotic and probiotic treatment groups, individually and also when administered in combination. In this regard, there was only a 40 % incidence of colonic symptoms (loose and bloody stools) in mice treated with the probiotic plus antibiotic, while this incidence was 100 % in vehicle-treated controls. Our data are also consistent with the findings of other investigators who found that probiotics can inhibit the production of pro-inflammatory cytokines(Reference Jijon, Backer, Diaz, Yeung, Thiel, McKaigney, De Simone and Madsen12, Reference Schultz, Strauch, Linde, Watzl, Obermeir, Gottl, Dunger, Grunwald, Scholmerich and Rath51). Interestingly, our data suggest that more prominent reductions of some pro-inflammatory cytokines were obtained with combined EC-M17 and metronidazole treatment (Fig. 3 (b) and (c)). Consistent with this cytokine data, the overall magnitude of the inhibitory effects on MPO and colonic histology were also more profound in probiotic plus antibiotic-treated animals (Fig. 4).
Since production of these pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) is dependent on activation of NF-κB, the effects of EC-M17 on the NF-κB signalling system were also examined in the DSS-induced colitis model(Reference Jobin and Sartor8–Reference Schottelius and Baldwin11). Interestingly, the administration of EC-M17 to DSS-treated mice resulted in attenuated nuclear expression of the NF-κB p65 subunit (Fig. 6 (b)). The nuclear expression of the p65 subunit has previously been shown to be up-regulated during DSS-induced colitis, and is thought to play a critical role in promoting intestinal inflammation(Reference Spiik, Ridderstad, Axelsson, Midtvedt, Bkork and Pattersen18, Reference Murano, Maemura, Hirata, Toshina, Nishiwaka, Hammamoto, Sasaki, Saitoh and Katsu19). Taken as a whole, these results suggest that reductions in the parameters of DSS-induced colitis, including pro-inflammatory cytokine levels, may have resulted from the inhibition of NF-κB signalling by EC-M17. This statement is supported by our in vitro data showing that EC-M17 inhibited the nuclear binding of NF-κB in murine macrophages (Fig. 2).
In the present study, the colonic level of IL-10 was decreased in vehicle-treated mice given DSS. It is possible that this attenuation of IL-10 contributed to the development of colitis, since IL-10 is a well-known immunomodulatory cytokine(Reference Strober, Fuss and Blumberg16, Reference Di Giacinto, Marinaro, Sanchez, Strober and Boirvant52). Previously, other investigators reported that the administration of probiotics to mice could induce the production of IL-10 and transforming growth factor-β-bearing regulatory cells, and that these cells contributed to the attenuation of murine colitis(Reference Di Giacinto, Marinaro, Sanchez, Strober and Boirvant52). EC-M17 treatment (alone or in combination) did not induce colonic levels of the regulatory cytokine, IL-10. These differences in IL-10 induction most probably relate to species differences between EC-M17 and VSL#3 (a probiotic food supplement), which contains a combination of Bifidobacterium, Lactobacillus and Streptococcus bacteria(Reference Petrof, Kojima, Ropeleski, Musch, Tao, De Simone and Chang4, Reference Jijon, Backer, Diaz, Yeung, Thiel, McKaigney, De Simone and Madsen12).
Our data support the contention that the major anti-colitis action of EC-M17 is by modulation of immune processes(Reference Fedorak and Madsen1). Further, the additive effects of EC-M17 and metronidazole in DSS-induced colitis suggest that the predominant mechanisms for the antibiotic and probiotic are distinct. Immunomodulation, as a mechanism of action for EC-M17, is consistent with the results reported herein on the modulation of NF-κB p65 and pro-inflammatory cytokine levels in mice administered EC-M17. Our theory is reinforced by the fact that metronidazole did not directly inhibit the secretion of pro-inflammatory cytokines by RAW 264·7 macrophages, while EC-M17 clearly showed an anti-cytokine profile. Changes in the intestinal environment due to antibiotic treatment may have eliminated pro-inflammatory bacteria in the murine colonic milieu. In this regard, metronidazole is known to reduce anaerobic bacteria (for example, Bacteroides species) that appear to contribute to the aetiology of DSS-induced colitis(Reference Rath, Schulz, Freitag, Dieleman, Li, Linde, Scholmerich and Sartor24). A similar metronidazole-mediated mechanism may have been operative in the present study. However, it also remains possible that other mechanisms may have contributed to the anti-colitis activity observed in the present study. For example, metronidazole treatment could also have better enabled EC-M17 to become a more dominant bacterial species in the intestinal environment. Thereby, EC-M17 would persist in greater amounts within the host organism relative to other commensal bacteria, by a competitive exclusion type of mechanism(Reference Fedorak and Madsen1). This possibility remains to be determined in future studies with EC-M17.
In summary, in vitro treatment of two cell lines with live EC-M17 resulted in inhibition of the NF-κB signalling pathway and p65 nuclear binding. Moreover, the probiotic attenuated the secretion of pro-inflammatory cytokines by macrophages. In vivo, EC-M17 effectively improved various histological, biochemical, morphological and symptomatic parameters of DSS-induced colitis in mice. Combined treatment with EC-M17 plus metronidazole proved to be generally more effective in DSS-induced colitis in mice than either alone. The data from this study suggest that EC-M17 could prove to be clinically useful for the treatment of intestinal inflammatory diseases. Moreover, further research with the probiotic is warranted. Finally, the combination of EC-M17 plus an antibiotic may afford therapeutic advantages that should also be investigated in future studies.
Acknowledgements
The authors acknowledge the following potential conflicts of interests. The study was supported by a grant from The BioBalance Corporation to L. R. F. Also, R. A. H. and E. F. B. are employees of The BioBalance Corporation.
All authors contributed to the intellectual aspects (L. R. F., R. A. H., E. F. B., W. A. K.) and/or technical aspects (L. R. F., J. S., L. M.) of this manuscript.











