Excessive iodine intake caused by iodine-rich underground drinking-water was first reported in China in the early years of 1980s, which was mainly characterised by elevated urinary iodine content and high goitre prevalence( Reference Yu, Zhu and Chen 1 ). The spatial distributions of high-iodine area (HIA) in China have been identified, which are concentrated in the downstream of Yellow River, affecting a population of nearly 40 million in eleven provinces( Reference Shen, Liu and Sun 2 ).
Goitre is the most visible manifestation of iodine excess( Reference Zhang and Li 3 ). Endemic goitre prevails in areas with the median water iodine content (MWIC) above 300 μg/l in drinking-water in China( Reference Li, Liu and Qu 4 , Reference Zhao, Wang and Shang 5 ). In two of our recent studies, it has also been revealed that goitre prevalence in children aged 8–10 years in the HIA with the MWIC ranging from 150 to 300 μg/l reached 11·0 % as defined by the Chinese criteria for thyroid volume( Reference Lv, Zhao and Xu 6 ) and 24·6 % by the WHO's criteria( Reference Lv, Xu and Wang 7 ).
Universal salt iodisation was implemented in China in 1995 to eliminate iodine-deficiency disorders (IDD)( Reference Lv, Xie and Zhou 8 ). HIA were included as they had not been identified at that time. Since then, iodine excess has emerged as a noticeable public health issue in HIA. To prevent the potential health consequences caused by iodine excess, a policy of removing iodised salt from HIA was enforced by the Chinese government in 2010. Due to the impact of the Fukushima nuclear disaster in March 2011, consumption of iodised salt was not completely ended until March 2012. To date, the effectiveness of this intervention measure on goitre prevalence caused by iodine excess in the local population residing in the HIA has not been evaluated.
Hebei is one of the provinces with a widespread distribution of HIA. It has 173 towns with the MWIC above 150 μg/l, involving a population of about 8 million( Reference Ma, Zhou and Jia 9 ). In the present study, we selected three towns with the MWIC ranging from 150 to 300 μg/l in Hengshui City, Hebei Province to compare the prevalence of goitre in children aged 8–10 years using the WHO's criteria for thyroid volume before and after the removal of iodised salt from their diet. The aim of the present study was to evaluate the effect of removing iodised salt on goitre status in children aged 8–10 years.
Materials and methods
Selection of high-iodine towns
Hengshui City is located in the southern plain of Hebei Province administrating 114 towns, in which fifteen towns were identified as high-iodine towns (HIT), with the MWIC of 150–300 μg/l, in a survey conducted in 2004( Reference Ma, Zhou and Jia 9 ). The fifteen HIT are located in a small geographical area where a field survey is easier to be conducted. The local residents have a similar diet and lifestyle that result in less variation in terms of confounding to their iodine nutrition. Therefore, Hengshui City was chosen to conduct the study. By the random sampling method, the following three towns were randomly selected from the fifteen HIT to measure urinary iodine content and goitre prevalence in children: Qinghan; Fangzhuang; Miaozhen. The baseline survey was conducted in May 2010 when iodised salt was still available. The second survey was carried out in October 2013 by which time the iodised salt was withdrawn for about 1·5 years.
Selection of children aged 8–10 years
The sample size was calculated according to the equation for simple random sampling( Reference Li, Ding and Gao 10 ). Goitre prevalence in children in the HIT was assumed at 50 %, and α and δ were both set at 0·05; the minimal sample size was 384. Only those children who lived in the three towns since birth were included. Children migrating from other towns were excluded. In each of the three towns chosen, two to five village schools were randomly selected. From each of these schools, all the children aged 8–10 years old were selected. A total of 452 and 459 children aged 8–10 years who lived in twelve villages in the three HIT were included in the baseline and second surveys, respectively.
All the pupils chosen underwent thyroid volume measurement by ultrasound on the spot. Meanwhile, more than half of the classes, which the selected pupils belonged to in each school, were randomly selected. All the pupils in the selected classes were asked to collect their spot urine samples. A total of 326 and 302 spot urine samples were collected in the baseline and second surveys, respectively.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Hebei Provincial Bureau of Science and Technology. Since ultrasound examination and urine sample collection are non-invasive, oral consents for thyroid volume measurement and urinary sample collection were obtained from the headmasters of the investigated schools. Verbal consent was witnessed and formally recorded.
Measurements of thyroid volume by ultrasound
Thyroid volume was measured using an Aloka SSD-500 echocamera (Aloka) equipped with 7·5 MHz linear transducers. Measurement was performed while the child lay in a bed with the neck fully exposed. For each thyroid lobe, the maximum perpendicular anteroposterior and mediolateral dimensions were measured on a transverse image of the largest diameter, without including the isthmus. Then, the maximum craniocaudal diameter of each thyroid lobe was measured on a longitudinal image. However, the thyroid capsule was not included. Ultrasound measurements were taken by one experienced examiner who had specialised in thyroid measurement by ultrasound for 10 years.
Thyroid volume was calculated by using the equation of Brunn et al. ( Reference Brunn, Block and Ruf 11 ), in which the volume of each thyroid lobe (ml) is equal to anteroposterior diameter (cm) × mediolateral diameter (cm) × craniocaudal diameter (cm) × 0·479, and the lobe volumes are summed. According to the WHO's criteria for thyroid volume measurement( 12 ), if a child's thyroid volume exceeds the 97th percentile for boys or girls by age-specific thyroid volume, the child is judged as goitrous.
Collection of drinking-water samples and edible salt samples
Drinking-water and edible salt samples were collected to measure their iodine content from the households of the villages where those investigated children had lived since birth. Because the drinking-water supply was centralised (tap water) in all of them, two water samples were randomly collected from two households in each village. As for salt sample collection, a systematic sampling method was employed according to the east, west, north, south or centre location of the village. From each location, four households were randomly selected to collect edible salt samples, resulting in the collection of twenty salt samples in each village.
Biological and environmental sample measurement and analysis
The detection of iodine content in the biological and environmental samples was conducted in the laboratory of Hengshui Municipal Center for Disease Control and Prevention. The iodine content of urine samples was measured by the method of Sandell–Kolthoff, which is based on the reduction of ceric ion in the presence of arsenious acid( 13 ). The WHO defines a population having a median urinary iodine concentration (MUIC) of 300 μg/l and above as iodine excess( 12 ). The iodine content of salt samples was determined quantitatively with the titration method( 14 ). The Chinese national plan for IDD surveillance defines any edible salt with less than 5 mg iodine/kg as non-iodised salt. The iodine content in drinking-water was determined by the method of Ar–Ce oxidation–reduction spectrophotometry( 15 ).
The IDD laboratory of Hengshui CDC was accredited by the National IDD Reference Laboratory to detect iodine content in urine, water and salt samples, through passing the tests for measuring spiked samples and certified reference materials. To apply quality-control materials to the measurement of iodine content in these samples, the provincial IDD laboratory conducted duplicate analysis for 5 % of all the collected samples. The accordance between the provincial IDD laboratory and the Hengshui municipal IDD laboratories was above 90 %.
Data processing and statistical analysis
Data processing and statistical analyses were performed using statistical software packages Epi–InfoTM 2002 (Centers for Disease Control and Prevention) and SPSS version 13.0 (SPSS, Inc.). Since the distributions of iodine content in edible salt, drinking-water and children's urine samples were not normal, the median was employed to describe their central tendency. The differences in the MUIC of children across the three towns, sex and age group before and after removing iodised salt were determined by the Mann–Whitney test. Prevalence was employed to indicate the magnitudes of goitre in the present study. The comparisons of goitre prevalence in children across age group and sex were performed by the χ2 test.
Results
Iodine content in drinking-water
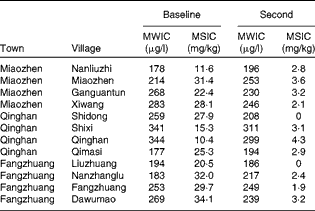
The MWIC ranged from 177 to 344 μg/l in the baseline survey and from 186 to 311 μg/l in the second survey (Table 1). No significant differences were observed.
Table 1 Median water iodine content (MWIC, n 20) and salt iodine content (MSIC, n 2) in the baseline (2010) and second (2013) surveys conducted at twelve villages in Hengshui City, Hebei Province, China
Iodine content in edible salt
All edible salt samples were iodised in the baseline survey, while none of them was iodised in the second survey. The iodine content in the samples decreased from 10·4 and 34·1 to < 5 mg/kg (Table 1).
Urinary iodine content in children aged 8–10 years
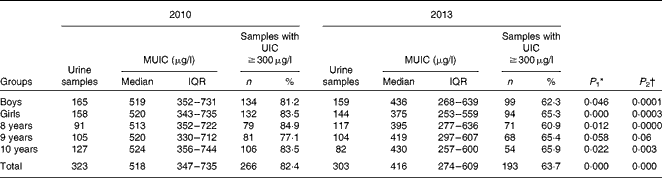
The overall MUIC of children decreased from 518 (interquartile range (IQR) 347–735) μg/l in the baseline survey to 416 (IQR 274–609) μg/l in the second survey. The results obtained from the Mann–Whitney test revealed that the MUIC of children in the three HIT in the second survey was significantly lower than that in the baseline survey (Table 2) .
Table 2 Median urinary iodine content (MUIC) and samples with UIC ≥300 μg/l in children aged 8–10 years across age group and sex in Hengshui City, Hebei Province, China in 2010 and 2013 (Median values and interquartile ranges (IQR); number of children and percentages)
* Results were obtained by Mann–Whitney test.
† Results were obtained by χ2 test.
The percentage of urine samples with iodine content >300μg/l in the three HIT was 82·4 % (n 266/323). In the second survey, the percentage decreased significantly to 63·7 % (n 193/303; Table 2).
Children's urine iodine content across sex and age group
In the baseline survey, the MUIC of children aged 8–10 years was 513 (IQR 352–722), 520 (IQR 330–712) and 524 (IQR 356–744) μg/l, respectively. In the second survey, their MUIC decreased to 395 (IQR 277–636), 419 (IQR 297–607) and 430 (IQR 257–600) μg/l, respectively. The differences in the MUIC of children across age group between the two surveys were all significant except for the age group of 9 years. The percentage of urine samples with iodine content >300 μg/l in each age group varied from 77·1 % (n 81/105) to 84·9 % (n 79/91). In the second survey, the percentage varied from 60·9 % (n 71/117) to 65·9 % (n 54/82) (Table 2).
The MUIC of boys and girls in the baseline survey was 519 (IQR 352–731) and 520 (IQR 343–735) μg/l, respectively, and decreased to 436 (IQR 268–639) and 375 (IQR 253–559) μg/l, respectively, in the second survey (boys: P= 0·046; girls: P< 0·001) (Table 2).
Children's goitre prevalence by age-specific thyroid volume
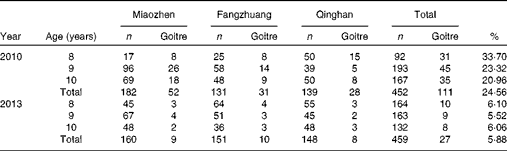
In the baseline survey, 111 children aged 8–10 years were identified as having goitre, with goitre prevalence being 24·56 % (n 111/452). Goitre prevalence in the town of Miaozhen, Fangzhuang, Qinghan was 28·57 % (52/182), 23·67 % (31/131), 20·14 % (28/139) respectively; and the differences were not sifnificant (P= 0·078).
In the second round survey, twenty-seven children were judged as goitrous and goitre prevalence was 5·88 % (27/459), with significant difference (P< 0·001). Goitre prevalence in the town of Miaozhen, Fangzhuang and Qinghan was 5·63 % (n 9/160), 6·62 % (n 10/151) and 5·40 % (n 8/148), respectively, but the differences were not significant (P= 0·43) (Table 3).
Table 3 Goitre status in children aged 8–10 years before and after removing iodised salt in the three high-iodine towns in Hengshui City, Hebei Province, China in 2010 and 2013*
*
![]() $$\chi _{8yr}^{2} = 33.24, P \ textless 0.001 $$
,
$$\chi _{8yr}^{2} = 33.24, P \ textless 0.001 $$
,
![]() $$\chi _{9yr}^{2} = 21.68, P \ textless 0.001 $$
,
$$\chi _{9yr}^{2} = 21.68, P \ textless 0.001 $$
,
![]() $$\chi _{10yr}^{2} = 13.24, P \ textless 0.001 $$
,
$$\chi _{10yr}^{2} = 13.24, P \ textless 0.001 $$
,
![]() $$\chi _{total}^{2} = 61.73, P \ textless 0.001 $$
.
$$\chi _{total}^{2} = 61.73, P \ textless 0.001 $$
.
Children's goitre prevalence across age group and sex
In the baseline survey, goitre prevalence in children aged 8–10 years was 33·70 % (n 31/92), 23·32 % (n 45/193) and 20·96 % (n 35/167), respectively. In the second survey, the prevalence decreased to 6·10 % (n 10/164), 5·52 % (n 9/163) and 6·06 % (n 8/132), respectively. The differences in the prevalence of goitre in children across age group between the two surveys were all significant (Table 3).
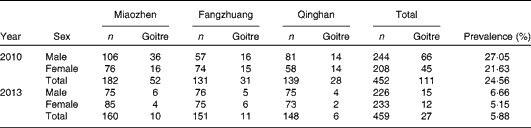
Goitre prevalence in boys and girls decreased significantly from 27·05 % (n 66/244) and 21·63 % (n 45/208) in the baseline survey to 6·66 % (n 15/226) and 5·15 % (n 12/233) in the second survey, respectively, (boys: P< 0·001; girls: P< 0·001) (Table 4).
Table 4 Goitre status of children across sex before and after removing iodised salt in the three high-iodine towns in Hengshui City, Hebei Province, China in 2010 and 2013*
*
![]() $$\chi _{male}^{2} = 34.20 $$
, P< 0.001,
$$\chi _{male}^{2} = 34.20 $$
, P< 0.001,
![]() $$\chi _{female}^{2} = 26.47 $$
, P< 0.001,
$$\chi _{female}^{2} = 26.47 $$
, P< 0.001,
![]() $$\chi _{total}^{2} = 61.73, P \ textless 0.001. $$
$$\chi _{total}^{2} = 61.73, P \ textless 0.001. $$
Discussion
Goitre is one of the common consequences of excessive iodine intake. In recent years, a number of studies conducted in China have demonstrated that endemic goitre prevails in the HIA with the MWIC >300 μg/l in drinking-water( Reference Wang, Zheng and Wang 16 – Reference Zhang, Fan and Guo 18 ). Moreover, two of our recent studies have also revealed that goitre was prevalent in children aged 8–10 years in the HIA with the MWIC ranging from 150 to 300 μg/l( Reference Lv, Zhao and Xu 6 , Reference Lv, Xu and Wang 7 ). In the HIA supplied with iodised salt, though excessive iodine intake was mainly attributed to drinking-water with high iodine content, iodised salt also played its part( Reference Lv, Wang and Xu 19 ). Also, the prevalence of goitre in children living in the HIA with iodised salt supply was higher than that in the HIA without iodised salt supply( Reference Lv, Zhao and Xu 6 ), indicating that iodised salt also fuelled the occurrence of goitre.
In the present study, after iodised salt was removed from the HIA about 1·5 years, goitre prevalence in children significantly decreased from 24·56 % (n 111/452) to 5·88 % (n 27/459). The further breakdown analysis on goitre prevalence in children across sex and age group also demonstrated the significant decrease in goitre prevalence after the removal of iodised salt. The present study also revealed that the decrease in the prevalence of goitre in children was consistent with the decrease in their MUIC, which dropped from 518 to 416 μg/l (Table 2). A significant decrease in MUIC was all identified across sex and age group except for the age group of 9 years. These findings appeared to support Zimmermann's argument that urinary iodine content ≥ 500 μg/l was associated with increasing thyroid volume( Reference Zimmermann, Ito and Hess 20 ). Therefore, in the HIA with MWIC ranging from 150 to 300 μg/l, the removal of iodised salt could significantly decrease goitre prevalence in children by reducing their iodine intake.
Given the high MWIC in the investigated villages (>200 μg/l) and the high MUIC of children (>400 μg/l), goitre prevalence in children was still above 5 %, which is the criterion defined by the WHO for goitre prevailing in a population. To lower the prevalence of goitre below 5 % in the HIA, changing the water source, i.e. consuming drinking-water with lower iodine content, is a necessary and feasible measure. A couple of small pilot studies have proved that changing the drinking-water source can effectively lower the prevalence of goitre in children to a normal level( Reference Zhu and Ma 21 , Reference Wang, Zhang and Shang 22 ).
Iodine in underground water is usually bound in humic substances with marine origin as this has been proved in Denmark( Reference Andersen, Petersen and Laurberg 23 ) and northern China( Reference Andersen, Guan and Teng 24 ). Iodine bound in humic substances is highly bioavailable and can significantly influence the iodine intake of a population( Reference Andersen, Petersen and Iversen 25 ). Moreover, some humic substances per se could promote the occurrence of goitre in humans( Reference Huang, Lu and Tsai 26 , Reference Chang, Hong and Chen 27 ). Therefore, they could play some part in the remaining occurrence of goitre besides the high iodine content and iodised salt in the present study. However, since humic substances in high-iodine water were not detected in the present study, their role on the occurrence of goitre could not be confirmed, and future studies are required for further investigation.
Both deficient and excessive iodine intakes can cause thyroid-related problems. In adults, iodine deficiency usually induces goitre and hyperthyroidism( Reference Zimmermann 28 ) and iodine excess often results in goitre and hypothyroidism( Reference Hans 29 ). To prevent these health consequences, it is essential to maintain proper iodine intake which the WHO recommended as the MUIC of the population ranging from 100 to 199 μg/l. Meeting this target in the HIA demands consuming water with a proper iodine content. This is the fundamental measure to prevent excessive iodine intake and its health consequences, which should be implemented in the near future.
Conclusion
Goitre is one of the most common manifestations of iodine excess. Previous studies have confirmed that endemic goitre prevails in children aged 8–10 years in the HIA, and iodised salt could enhance the prevalence of goitre among school children. However, the impact of removing iodised salt on goitre status in children remains unclear. Through the comparisons of goitre prevalence in children before and after the removal of iodised salt, the present study revealed that goitre prevalence in children decreased significantly after removing iodised salt from their diet for about 1·5 years in the HIA in Hebei Province. The decreases in goitre prevalence were significant across sex and age group. The present study is the first to quantitatively measure the impact of removing iodised salt on goitre status in children living in the HIA. It expanded our knowledge on how iodised salt affected goitre status in children residing in the HIA, and also provided valuable information on making intervention measures on iodine excess.
Acknowledgements
The authors thank the staff in the endemic control department of the Centre for Disease Prevention and Control of Jingxian and Gucheng County for their assistance in the field investigation.
The present study was supported by the Hebei Provincial Bureau of Science and Technology (grant no. 11276103D-3). The funder had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: S. L. was responsible for the study design, data analysis, writing of the paper and field investigation; D. X. performed the thyroid measurements by ultrasound and data entry; Y. W. was in charge of the coordination of the field investigation and laboratory detection of iodine; Z. J. was responsible for the quality control of laboratory detection; L. J. and Y. D. participated in the field investigation.
There are no conflicts of interest.






