The fetal and neonatal periods critically guide the development, growth and functional maturation of the organ systems. There are data to suggest that small birth weight with rapid postnatal catch-up growth on the one hand or large birth weight on the other hand is associated with major disorders in adult life, including CVD, hypertension, non-insulin-dependent diabetes and obesity(Reference Lucas1–Reference Parsons, Power and Manor3). Maternal nutrition constitutes a decisive factor in the in utero environment. Infants of small birth weight reflecting poor intra-uterine nutrition have a heightened risk of such chronic diseases, a phenomenon called programming(Reference Palinski, Yamashita and Freigang4). In Western societies, in contrast, the fetus is often overnourished due to the mother's excessive dietary intake of saturated fat and carbohydrates of high glycaemic index. Maternal nutrition also influences the composition of breast milk, with a marked growth-promoting and regulatory potential against infections and allergies(Reference Innis5, Reference Howie, Forsyth and Ogston6). Additionally, maternal prepregnancy weight correlates with the birth weight of the child, and maternal obesity is linked to fetal macrosomia(Reference Surkan, Hsieh and Johansson7), these being associated per se with later obesity(Reference Sorensen, Sabroe and Rothman2, Reference Parsons, Power and Manor3). Maternal obesity is also a well-known contributor of gestational diabetes mellitus (GDM), which elevates itself the risk of non-insulin-dependent diabetes and the metabolic syndrome later in life(Reference Damm8). We therefore hypothesised here that the nutritional environment during the perinatal period creates a window of opportunity to reverse the increasing trend in Western lifestyle diseases.
Recent data also point to the gut microbiota as being instrumental in the microbial, metabolic and immunological programming of the child(Reference Bäckhed, Ding and Wang9, Reference Cani and Delzenne10). Indeed, aberrant compositional development of the gut microbiota has been linked to obesity(Reference Ley, Turnbaugh and Klein11, Reference Kalliomäki, Collado and Salminen12) and an allergy risk(Reference Kalliomäki, Kirjavainen and Eerola13), and modification of the gut microbiota by probiotics early in life has thus attracted scientific interest. However, the long-term safety of probiotic intervention during the period when the bases of child's immunological and metabolic phenotypes are created remains to be established.
The objective of our Nutrition, Allergy, Mucosal immunology and Intestinal microbiota programme is to accomplish clinical intervention studies with perinatal probiotic intervention combined with balanced maternal nutrition during pregnancy and lactation and to provide a new direction in the search for scientifically validated means of reducing the risk of Western lifestyle disease. In our recent publications, we have provided evidence of perinatal dietary counselling to improve the dietary intake of the pregnant women(Reference Piirainen, Isolauri and Lagström14) and perinatal probiotic intervention to contribute to improved glucose regulation during and after pregnancy(Reference Laitinen, Poussa and Isolauri15). The present study specifically evaluates the safety of this approach. To this end, a mother–infant cohort (n 256) was followed with careful evaluation of pregnancy outcome and health and growth of the children.
Subjects and methods
Study design
The original study population comprised 256 mother–baby pairs participating in an ongoing prospective, randomised mother–infant nutrition and probiotic study (http://www.clinicaltrials.gov/ct/gui/show/NCT00167700,section3), described in detail elsewhere(Reference Piirainen, Isolauri and Lagström14–Reference Aaltonen, Ojala and Laitinen16). In brief, women were recruited in early pregnancy during their first visit to maternal welfare clinics in Turku and neighbouring areas in South-West Finland. The inclusion criterion was that the subjects had no chronic metabolic diseases; however, the allergic diseases (atopic eczema, allergic rhinitis or asthma) were allowed. At entry, the women were randomised into a dietary intervention group or a control group. All women received dietary counselling provided by welfare clinics according to a national programme. The intervention group received additionally intensive dietary counselling at every study visit provided by a nutritionist, the aim being a dietary intake complying with current recommendations(Reference Becker, Lyhne and Pedersen17, 18), combined with conventional food products with favourable fat and fibre contents for use at home. This group was further randomised at baseline in a double-blind manner to receive either probiotics (diet/probiotics group), Lactobacillus rhamnosus GG (ATCC 53 103, Valio Ltd, Helsinki, Finland) and Bifidobacterium lactis Bb12 (Chr. Hansen, Hørsholm, Denmark) at a dose of 1010 colony-forming units/d each, or placebo (diet/placebo group), microcrystalline cellulose and dextrose anhydrate (Chr. Hansen) capsules. The control group received placebo capsules in a single-blinded manner (Fig. 1). The capsules were taken once daily and the intervention period extended from the first trimester of pregnancy to the end of exclusive breastfeeding. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Ethical Committee of the Hospital District of Southwest Finland. Written informed consent was obtained from all participants.

Fig. 1 The flow of subjects.
As depicted in Fig. 1, altogether, 256 pregnant women were recruited and 238 of them continued the study throughout pregnancy. Three twin pairs were excluded from the growth follow-up. Of the 241 children delivered, 191 completed the 24 months' follow-up.
Clinical evaluation
Pregnant women
The women in all study groups visited the study clinic three times during pregnancy, in addition to their regular visits to well-women clinics. Their clinical data were collected by interview at the first visit (Table 1). The duration of pregnancy was calculated from the date of the last menstruation. The results of 75 g oral glucose tolerance tests were recorded; these were performed in welfare clinics in all risk pregnancies. The diagnosis of GDM was based on modified criteria of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus(Reference Metzger and Coustan19), according to recommendations implemented in Finland during the clinical study years 2002–2005. Specifically, an oral glucose tolerance test was considered pathological when one value exceeded ≥ 4·8 mmol/l at baseline, ≥ 10·0 mmol/l at 1 h or ≥ 8·7 mmol/l at 2 h. The estimate of fetal weight in the 20th pregnancy week ultrasound examination was recorded in cases where the women were willing to have the test performed. These examinations, provided by the mother's municipality, were performed by sonographers or doctors experienced in obstetric ultrasound. The duration of exclusive and total breastfeeding was recorded from the mother.
Table 1 Clinical characteristics of the women and impact of the intervention on pregnancy outcome

Results are given as mean (sd), mean (95 % CI) or as numbers (%) of subjects, if not stated otherwise. Total number of subjects is given if all are not included.
* None of the differences among the groups was significant except P = 0·003.
† Median (range).
Infant
After delivery, study visits were scheduled at the ages of 1, 6, 12 and 24 months. Infant weight was measured with a SECA model 727 scale (Hamburg, Germany) and length on a length board. All weights and lengths were recorded to the nearest 0·01 kg and 0·1 cm, respectively. Physical examinations were always carried out by the same research nurse at the age of 1 month and by a physician in the research group at 6, 12 and 24 months of age, apart from the regular visits to the well-baby clinics. The clinical evaluation of the infants included inspections of skin, ears, eyes, nose, genitalia and anus, auscultation of heart and lungs, palpation of abdomen and femoral pulsations, evaluation of growth and nutritional status, muscle tone and neurological development.
Statistical methods
Pregnancy outcome and infant growth were the primary outcome variables. The baseline variables and other variables in Table 1 were analysed using the χ2 test, ANOVA and Kruskal–Wallis test (5-min Apgar score). Perinatal death was defined as stillbirth at 23 weeks or more of gestation or death of an infant less than 1 week of age. Very premature delivery was defined as spontaneous live birth at weeks 23–31, premature delivery as spontaneous live birth at weeks 32–36, term delivery as spontaneous live birth at weeks 37–41 and post-term delivery as spontaneous live birth ≥ 42 weeks of gestation.
All growth measurements were estimated to precise daily ages (1, 6, 12 and 24 months) using linear interpolating by reason of variable measuring ages (1, 0·6 and 2·16 months; 6, 5·0 and 7·7 months; 12, 10·8 and 16·7 months; 24, 22·3 and 33·6 months). Missing data for length (n 12) and weight (n 8) were linearly interpolated using the exact daily ages; no extrapolation was made. Growth rate was defined as grams and centimetres gained per month between the two measurements. Comparison of growth rates during the periods 0–6 months, 6–12 months and 12–24 months among the study groups was made by ANOVA for repeated measurements.
As confounders, we considered mothers' BMI before pregnancy (kg/m2), maternal smoking before pregnancy (yes or no), highest maternal education in terms of college or university (yes or no), GDM, sex and breastfeeding at infant's age of 6 months (yes or no). Associations between confounders and infant growth rates at separate time periods were studied by including those confounders as continuous or dichotomous covariates in multivariate models. Logistic regression analysis was used to compare the incidence of GDM among the study groups. The results are given as OR with 95 % CI. Two-way ANOVA with two explaining factors, study group and GDM, was used to analyse the birth size variables. Due to significant interactions between dietary intervention and GDM, subgroup analyses were conducted to estimate the effect of GDM in study groups.
Data were analysed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). A P value of < 0·05 was considered statistically significant.
Results
As shown in Table 1, the mean age of the women was 30·0 (range 17·6–44·2) years. All participants were Caucasian and healthy; 201 (79 %) had previously been diagnosed with an allergic disease. The mean BMI of women was 23·6 (sd 3·8) kg/m2 (Reference Aaltonen, Ojala and Laitinen16). The majority had college or university education and were primipara.
Pregnancy outcome
Pregnancy outcome is depicted in Table 1. The frequency of GDM was significantly different between study groups (P = 0·003, Table 1). The risk of GDM was significantly reduced in the diet/probiotics group when compared to the control group (OR = 0·27 (95 % CI 0·11, 0·62); P = 0·002), whereas in the diet/placebo group, the risk was not significantly different when compared to the control group (OR = 1·08 (95 % CI 0·55, 2·12); P = 0·823). There were no significant differences among the study groups in the duration of gestation. There were altogether 4/238 (1·7 %) preterm deliveries, only one of them, a twin pregnancy, coming very preterm (30th gestation week). Post-term deliveries were more common, in 16/238 (7 %) pregnancies, with no significant differences in frequency among the study groups.
Rates of caesarean delivery were 14–16 % in all study groups. There were no perinatal deaths or serious adverse incidences such as sepsis in mothers or newborns. The 5-min Apgar scores were inline in all study groups (Table 1). Likewise, the mean duration of exclusive breastfeeding and thus the duration of probiotic/placebo intervention did not differ among the study groups; 2·9 (sd 1·7) months in the diet/probiotics group, 3·4 (sd 1·8) months in the diet/placebo group and 3·0 (sd 1·6) months in the control group. Neither did the groups diverge significantly with regard to breastfeeding status at 6 months of age; altogether, 68 % in the diet/probiotics group, 75 % in the diet/placebo group and 76 % in the control group were breastfed to any degree at least for 6 months.
Growth
The mean birth weight of the neonates (n 241) was 3551 (range 1610–4750) g and birth length 50·9 (range 44·0–57·0) cm, showing no significant differences among the study groups (Table 1). Moreover, when the birth weights and lengths were adjusted to mothers' prepregnancy BMI (kg/m2), smoking before pregnancy, education, GDM, sex of the child and duration of breastfeeding, no statistically significant differences emerged among the study groups. GDM tended to increase birth weight and birth length in all study groups without a significant main effect (P = 0·183 and 0·278, respectively, two-way ANOVA; Table 2). In spite of non-significant main effects, the interactions (P = 0·054 for birth weight and 0·034 for birth length) and the following subgroup analyses indicated that the impact of GDM on birth size was manifested most distinctly in the control group (426 g in weight and 1·7 cm in length; P = 0·001), and the effect of GDM on birth size was lessened to non-significant effects of 190 and 73 g in weight and 0·6 and 0·1 cm in length by dietary intervention with or without probiotics (Table 2). The effect of dietary intervention and GDM on birth size was analysed further and the dietary intervention groups were combined (Table 3). Again, interactions were detected between dietary intervention and GDM (P = 0·035 for birth weight and 0·028 for birth length), indicating that the dietary intervention modified the effect of GDM. GDM increased the birth weight on the average 144 (95% CI − 7, 295) g and 0·4 (95% CI − 0·2, 1·1) cm in the combined dietary intervention group and 426 (95% CI 193, 660) g and 1·7 (95% CI 0·7, 2·7) cm in the control group.
Table 2 The birth weight and birth length of the infants (n 221) in the study groups according to mothers' gestational diabetes mellitus (GDM)
(Mean values and 95 % confidence intervals)
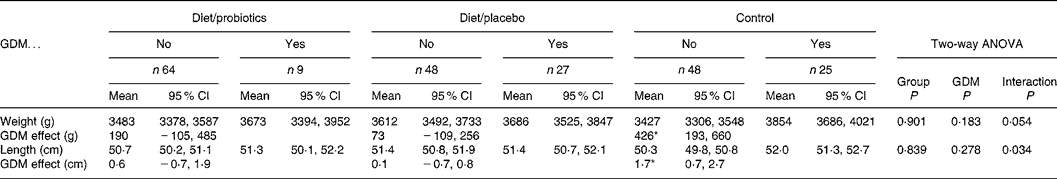
Subgroup analysis: the effect of GDM in the control group and intervention groups with and without probiotics.
* P = 0·001 in the control group and non-significant in other subgroup comparisons.
Table 3 The birth weight and birth length of the infants (n 221) in the control group and in the integrated dietary intervention groups according to mothers' gestational diabetes mellitus (GDM)
(Mean values and 95 % confidence intervals)
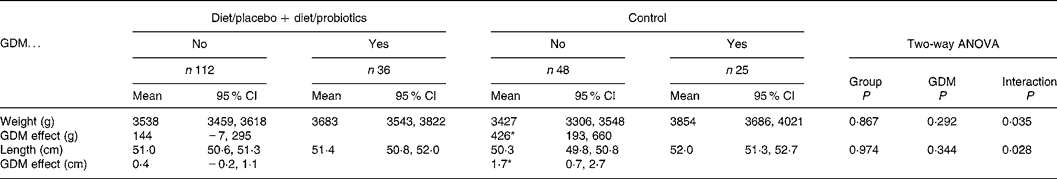
Subgroup analysis: the effect of GDM in the control group and in the integrated dietary intervention groups.
* P = 0·001 in the control group and non-significant in other subgroup comparisons.
Weight gain and growth in length during the periods 0–6 months, 6–12 months and 12–24 months showed no statistically significant differences between the diet/probiotics, diet/placebo and control groups. The respective mean weight gains (g/month) over 0–6 months were 759 (sd 160), 762 (sd 165) and 756 (sd 148); over 6–12 months were 323 (sd 80), 296 (sd 99) and 315 (sd 91); over 12–24 months were 211 (sd 76), 230 (sd 61) and 218 (sd 52) (P = 0·983 for group effect; P < 0·001 for time effect; P = 0·520 for group × time interaction; ANOVA for repeated measurements). The mean growths in length (cm/month) in the respective periods were 2·84 (sd 0·35), 2·89 (sd 0·29) and 2·93 (sd 0·35); 1·40 (sd 0·19), 1·38 (sd 0·21) and 1·36 (sd 0·24); 0·95 (sd 0·14), 0·94 (sd 0·15) and 0·93 (sd 0·12) in the diet/probiotics, diet/placebo and control groups, respectively (P = 0·872 for group effect; P < 0·001 for time effect; P = 0·325 for group × time interaction; ANOVA for repeated measurements).
In the subgroup of infants undergoing the 20th pregnancy week ultrasound examination (diet/probiotics group n 28, diet/placebo group n 25 and control group n 17), there were no statistically significant differences among the study groups in perinatal weight gain or weight gain and growth in length over the 24 months' follow-up (P = 0·809, ANOVA for repeated measurements, group effect; Fig. 2).

Fig. 2 Impact of probiotic-supplemented dietary intervention on perinatal growth from 20th pregnancy week to delivery and over the follow-up period to 24 months' age in diet/probiotics group (n 28), diet/placebo group (n 25) and control group (n 17). Values are mean weights (95 % CI). P = 0·809, ANOVA for repeated measurements, group effect.
As an additional analysis, the adjusted group comparisons for periods of 0–6, 6–12 and 12–24 months were performed separately in order to evaluate the effect of other confounders. In the 0–6 months' age period, the adjusted group comparisons to explain weight gain and growth in length were non-significant (P = 0·905 and 0·441, respectively). However, male sex increased the weight gain by 94 g/month (P < 0·001), and breastfeeding for at least 6 months decreased the weight gain by 70 g/month (P = 0·003). Likewise, male sex increased growth in length during the 0–6 months' age period by 0·23 cm/month (P < 0·001), and breastfeeding for at least 6 months decreased growth in length by 0·15 cm/month (P = 0·002). The adjusted group comparisons with respect to weight and length gain were also non-significant during the periods 6–12 months and 12–24 months of age. Breastfeeding for at least 6 months decreased statistically significantly the growth in length during the 6–12 months' age period by 0·07 cm/month (P = 0·028). Male sex decreased the growth in length 0·05 cm/month (P = 0·144) during 6–12 months' and 0·04 cm/month (P = 0·029) during 12–24 months' age period.
Discussion
Current research interest in nutrition is directed towards the invention of novel dietary compounds with specific effects in health promotion and reduction of the risk of disease(Reference Schwager, Mohajeri and Fowler20). This also holds true for specific strains of probiotics, which are associated with immune regulation, improved gut barrier function and reduction of the risk of intestinal infections and allergic diseases, thereby furnishing documentation of efficacy(Reference Douglas and Sanders21). Safety documentation, again, is mandatory in extending application to the general population. In the present study, we provide evidence of normal growth rates in children receiving probiotics, indicating the long-term safety of the approach. Specifically, this evidence of optimal pre- and postnatal growth encourages anticipating safe protection against metabolic diseases later in life.
The present paper is the first to report the safety aspect of the probiotic intervention starting as early as the first trimester of pregnancy. The merit of this approach lies in the possibility to evaluate the effect, if any, on pregnancy outcome. This is important in that specific strains of probiotics have been shown to contribute to the generation of Th1, Th3 and Tr1 regulatory cells(Reference He, Morita and Ouwehand22, Reference Christensen, Frøkiær and Pestka23), with a counter-regulatory activity on the Th2-skewed immune responder type, with this prevailing in utero (Reference Piccini, Beloni and Livi24, Reference Prescott, Macauba and Smallacombe25). The normal pregnancy outcome reported here confirms no interference with the physiological equilibrium and duration of the pregnancy, concomitantly confirming the safety aspect. In view of the fact that impaired maternal glycaemia predisposes not only the mother but also the child to perinatal and later health problems(Reference Damm8), the diminished risk of GDM reported in the present study may have been a contributor on reducing the adverse pregnancy outcomes.
The size of the mother and especially the diet during pregnancy influence fetal growth and development, these being mirrored in birth size(Reference Godfrey, Robinson and Barker26, Reference Barker27). Prepregnancy obesity, excessive weight gain during pregnancy and GDM overnourish the fetus and lead to fetal macrosomia(Reference Catalano28). Larger birth size, again, predisposes to later obesity(Reference Baird, Fisher and Lucas29, Reference Druet and Ong30) with associated comorbidities(Reference Boney, Verma and Tucker31). Previous reports from this project have already demonstrated, firstly, that dietary counselling during pregnancy improved the dietary intake, particularly the amount of fibre and the quality of fat intake, of the pregnant women(Reference Laitinen, Poussa and Isolauri15). Secondly, we have provided clinical evidence of consistently improved plasma glucose concentrations and insulin sensitivity in healthy women during pregnancy and 12 months post partum when advantageous dietary intake is combined with probiotics(Reference Piirainen, Isolauri and Lagström14). The demonstration here extends these effects to fetus and neonate. Interestingly, nutrition counselling and probiotic intervention were shown in the present study to have a distinct effect on GDM; probiotics diminished the risk of the disorder, and dietary counselling during pregnancy reduced the risk of fetal overgrowth associated with it.
Considering that the maternal microbiota is a first inoculum to the development of the child's microbiota(Reference Mackie, Sghir and Gaskins32), it is important to recognise that the gut microbiota has recently come to be seen as a key organ involved in host energy homoeostasis(Reference Bäckhed, Ding and Wang9), in affecting the harvest and storage of energy from the diet. Furthermore, it modulates plasma lipopolysaccharide concentrations and sets the inflammation tone to affect insulin sensitivity and thereby the risk of the metabolic syndrome(Reference Bäckhed, Ding and Wang9, Reference Cani and Delzenne10, Reference Turnbaugh, Ley and Mahovald33, Reference Cani, Amar and Iglesias34). Specific probiotic consumption may modify the intestinal microbiota composition and activity in such a way that fermentation of dietary polysaccharides is altered and gut barrier function is improved, not forgetting the capability to regulate the inflammatory pathways(Reference Isolauri35, Reference Cani, Possemiers and Van de Wiele36). It is conceivable that the protection against GDM provided by probiotics, as described in the present report, may have been mediated via both immunomodulatory pathways and energy harvest. The diminished risk of GDM-associated fetal overgrowth, again, provided by dietary intervention may be explained by the change achieved in dietary fat composition, which also modifies the gut microbiota composition and the development of innate immunity(Reference Calder37). Indeed, microbes and fatty acids are both capable of exploiting the same pathways in the innate immune system, which participates in the regulation of glucose metabolism and insulin resistance(Reference Shi, Kokoeva and Inouye38).
The high incidence of GDM observed in the present study may be explained by differences in the diagnostic criteria(Reference Yogev, Metzger and Hod39). This notwithstanding, elevated maternal glucose levels, even within non-diabetic ranges, are of concern, because they have been shown to be related to larger offspring birth size and increased risk of interventional deliveries(Reference Metzger and Lowe40). Hence, the findings of the present study invite acceptance of new universal guidelines for the type of threshold selection of GDM.
In view of the recent demonstration that obesity and allergic diseases share common environmental influences, i.e. a low-grade systemic inflammation(Reference Weiss41, Reference Shore42), we have for the first time targeted a metabolically healthy at -risk population, which would most likely benefit from the dietary intervention combined with probiotics.
Conclusion
Taken together, long-term health benefits for mothers and children may be conferred by balanced maternal nutrition during pregnancy and lactation and by promoting the healthy gut microbiota in the mother and the child. The results of the present study add weight to the argument that the continuing burden of Western lifestyle diseases is modifiable. Based on the present findings, perinatal dietary counselling combined with probiotics could provide a safe and cost-effective tool in addressing the obesity epidemic.
Acknowledgements
The present study was supported by grants from the Academy of Finland, the Sigrid-Jusélius Foundation, Juho Vainio Foundation and the Social Insurance Institution of Finland. Provision of food products was by Raisio plc (Raisio, Finland), B. lactis Bb12 by Chr. Hansen (Hoersholm, Denmark) and L. rhamnosus GG by Valio Ltd (Helsinki, Finland). We would like to thank Ms Ulla-Maija Eriksson for her clinical work with the study subjects, Tuija Poussa, MSc, for statistical consultation and Robert MacGilleon, MSA, for language review of the manuscript. The authors declare that there is no personal or financial conflict of interest associated with the present paper. The authors' responsibilities were as follows: K. L. and E. I. made the study concept and design, M. N. and R. L. did the collection of the data and all authors participated in preparation of the manuscript. Conflict of interest: The authors have no conflict of interest related to the present article. The trial registration: http://www.clinicaltrials.gov/ct/gui/show/NCT00167700,section3







