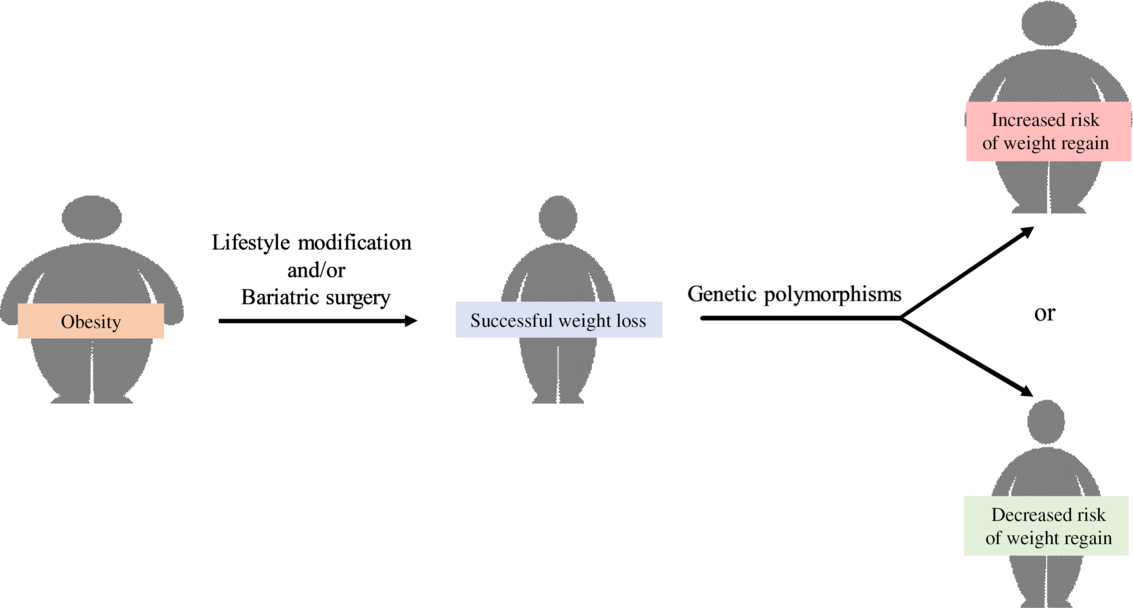
Obesity is associated with an increased risk of numerous chronic diseases. These include the metabolic syndrome, CHD, obstructive sleep apnoea, cancer, depression and infertility(Reference Hruby and Hu1). Although nearly 50 % of adults worldwide were reported to be trying to lose weight(Reference Santos, Sniehotta and Marques2), obesity prevalence has doubled in seventy-three countries since 1980(Reference Friedrich3) and adult obesity exceeded 50 % in several countries(Reference Hruby and Hu1). Moreover, the increase in obesity prevalence resulted in a 28·3 % increase in the rate of obesity-related mortality and a 35·8 % increase in the rate of disability-adjusted life years from 1990 to 2015(Reference Friedrich3), which led to a substantial economic burden conferred by obesity in both developed and developing countries(Reference Friedrich3,Reference Tremmel, Gerdtham and Nilsson4) . One factor that hinders weight loss-induced decrease in obesity prevalence is weight regain. In fact, about half of the weight lost is regained in the first year following weight loss and the weight regain continues thereafter(Reference Byrne, Cooper and Fairburn5,Reference Wadden, Sternberg and Letizia6) . In the 3- to 5-year period following weight reduction, many subjects (about 85 %) have returned to, or exceeded, their initial weight(Reference Byrne, Cooper and Fairburn5,Reference Wadden, Sternberg and Letizia6) . Factors that are associated with weight regain have been reviewed in many previous articles. These include large initial weight loss(Reference Marinilli Pinto, Gorin and Raynor7,Reference Garcia Ulen, Huizinga and Beech8) , high levels of orexigenic peptides after weight loss(Reference Garcia Ulen, Huizinga and Beech8,Reference Wing, Papandonatos and Fava9) , increased consumption of high glycaemic index food and beverages(Reference Bosy-Westphal and Muller10,Reference Phelan, Wing and Loria11) , low levels of exercise(Reference Garcia Ulen, Huizinga and Beech8,Reference Wing, Papandonatos and Fava9) , sedentary activities including excessive television watching(Reference Garcia Ulen, Huizinga and Beech8,Reference Raynor, Phelan and Hill12) , infrequent self-weighing(Reference Wing, Papandonatos and Fava9), internal disinhibition of eating(Reference Garcia Ulen, Huizinga and Beech8,Reference Wing, Papandonatos and Fava9) and dichotomous thinking(Reference Byrne, Cooper and Fairburn5,Reference Garcia Ulen, Huizinga and Beech8) . Indeed, it is widely accepted that weight regain is multifactorial involving physiological, psychological and behavioural factors. However, the information regarding the association between weight regain and genetics, which play an important role in obesity(Reference Elks, den Hoed and Zhao13,Reference Herrera and Lindgren14) , has not been previously summarised.
Heritability estimates of BMI and/or the development of obesity are between 40 and 80 %(Reference Elks, den Hoed and Zhao13,Reference Herrera and Lindgren14) , and therefore, genetic polymorphisms have been believed to be one of the key determinants of weight regain in individuals with obesity following successful weight loss. Genetic polymorphism is defined as the inheritance of a trait controlled by a single genetic locus with two alleles, in which the least common allele has a frequency of about 1 % or greater(Reference Ismail and Essawi15). In this article, we have reviewed genetic polymorphisms associated with weight regain in adults and children with obesity after successful weight loss.
Search strategy and selection criteria
A literature search was performed using PubMed from database inception to April 2020. Only articles written in English are included. The search terms included weight regain, gene and obese. Only relevant articles pertaining to the topic of interest are included. Finally, we identified thirty-one polymorphisms from twenty genes that are associated with weight regain and categorised them depending on their functions, including: (1) nutrient metabolism, (2) food intake and energy expenditure, (3) adipocyte differentiation and (4) inflammation, extracellular matrix and bone metabolism.
Nutrient metabolism-related genes and their association with weight regain
It has been shown that nutrient-related metabolite levels in plasma differ between lean subjects and individuals with obesity. These include branched-chain amino acids, aromatic amino acids, NEFA and acylcarnitine intermediates of fatty acid oxidation and branched-chain amino acid catabolism(Reference Newgard, An and Bain16). Additionally, a study into skeletal muscle revealed lower levels of citric acid cycle metabolites, end products of nutrient metabolism, in rats with obesity compared with their lean counterparts(Reference Koves, Ussher and Noland17). Moreover, they found impaired switching from fat to carbohydrate utilisation during the fasted-to-fed transition in the skeletal muscle of rats with obesity(Reference Koves, Ussher and Noland17). Those results suggest that obesity is associated with alteration in nutrient metabolism; therefore, it is not surprising that the polymorphisms of several nutrient metabolism-related genes are also associated with weight regain. These include perilipin (PLIN), brain-derived neurotrophic factor (BDNF), leptin, PPARγ2, protein phosphatase, Mg2+/Mn2+ dependent 1K (PPM1K), β-2 adrenergic receptor (ADRB2), IL-6 and glucocorticoid receptor (GRL). A comprehensive summary of the reports describing these genes is shown in Table 1.
Table 1. SNP or the restriction fragment length polymorphisms (RFLP) of nutrient metabolism-related genes that are associated with weight regain
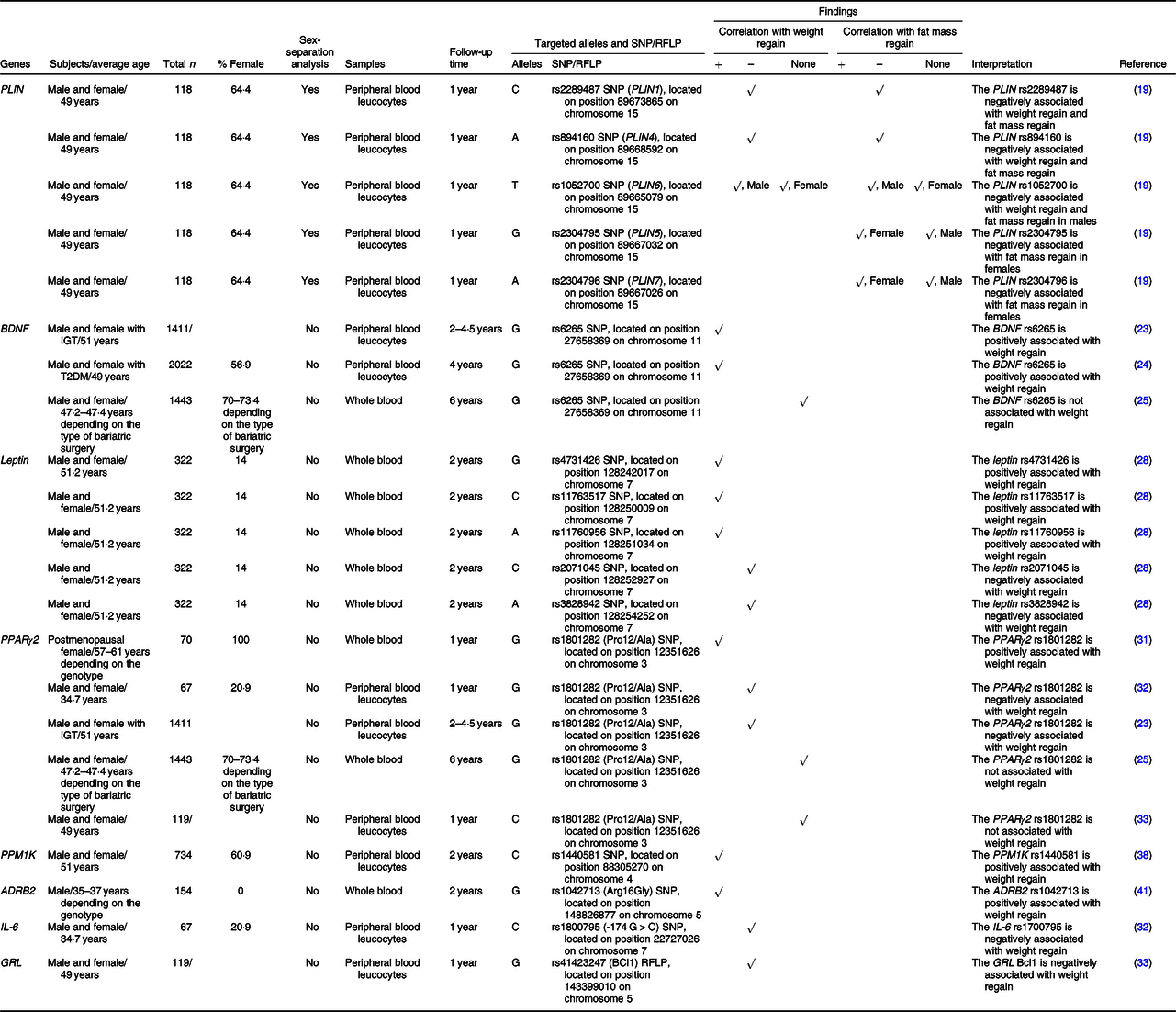
+, Positively associated with; –, negatively associated with; ADRB2, β-2 adrenergic receptor; BDNF, brain-derived neurotrophic factor; GRL, glucocorticoid receptor; IGT, impaired glucose tolerance; PLIN, perilipin; PPM1K, protein phosphatase, Mg2+/Mn2+ dependent 1K; T2DM, type 2 diabetes.
PLIN coats intracellular lipid droplets and regulates adipocyte lipolysis(Reference Qi, Corella and Sorli18). Soenen and colleagues identified the linkage equilibria between PLIN1 and PLIN4 and between PLIN5 and PLIN7 (Reference Soenen, Mariman and Vogels19). They also revealed that males carrying the C-allele of PLIN1 plus the A-allele of PLIN4 had a lower BMI and body fat at baseline, weight loss phase and weight maintenance phase, whereas females carrying these alleles had larger weight loss and fat loss at both weight loss and weight maintenance periods(Reference Soenen, Mariman and Vogels19). In the case of PLIN6, males with the T/T genotype had a lower BMI and body fat at all time points(Reference Soenen, Mariman and Vogels19). In females, those who carried the G allele of PLIN5 plus the A/A genotype of PLIN7 showed greater reduction on fat mass after weight loss and during weight maintenance(Reference Soenen, Mariman and Vogels19). These findings indicate an association between PLIN gene polymorphisms and risks of weight and fat mass regain in a sex-specific manner. These sex-specific effects of PLIN on obesity risk suggest a particular characteristic of PLIN in obesity-related conditions. A further in-depth study regarding the association between PLIN and sex hormones on obesity and weight regain could be useful in the identification of molecular mechanisms responsible for this association.
BDNF influences various cell types involved in glucose metabolism. These include increased insulin production from pancreatic β-cells, decreased glucose production in hepatocytes and increased insulin sensitivity in skeletal muscle(Reference Marosi and Mattson20). Deletions or mutations of the BDNF gene result in a phenotype of obesity(Reference Xu and Xie21,Reference An Juan, Liao and Kinney Clint22) . Considering the effect on weight regain, it was observed that the G allele of the rs6265 SNP of the BDNF gene was positively associated with weight regain after successful weight loss using lifestyle intervention at two to four and a half years of follow-up(Reference Delahanty, Pan and Jablonski23,Reference McCaffery, Papandonatos and Huggins24) . However, there was no effect of that SNP on weight regain in subjects who underwent bariatric surgery at 6 years of follow-up(Reference Sarzynski, Jacobson and Rankinen25). These findings suggest that: (1) bariatric surgery overwhelms the effect of this polymorphism on weight regain and (2) the effects of this polymorphism on weight regain are not persistent. A shorter-term study in the bariatric surgery model and a longer-term study in the lifestyle intervention model are required to confirm these hypotheses.
Leptin also plays a critical role in glucose and lipid metabolism. Leptin has been shown to decrease glucose production and fat accumulation in the liver and to increase glucose uptake and fatty acid oxidation in skeletal muscle, in addition to increasing glucose uptake into brown adipocytes(Reference Fernandez-Formoso, Perez-Sieira and Gonzalez-Touceda26,Reference Li and Li27) . The G allele of rs4731426 SNP was found to be positively associated with weight regain, while the C allele of rs2071045 SNP was negatively associated with weight regain(Reference Erez, Tirosh and Rudich28). Additionally, a multivariate parsimonious model including rs4731426, rs2071045 and rs3828942 SNP showed a significant association with weight regain, and a model that included rs4731426, rs2071045, rs3828942, rs11763517 and 11760956 SNP also showed a significant association(Reference Erez, Tirosh and Rudich28).
PPARγ2 regulates glucose metabolism by increasing transcription of GLUT2 and glucokinase genes, leading to increased glycolysis in the liver and increased insulin secretion by pancreatic β-cells, that is, PPARγ2 improves insulin sensitivity(Reference Kim and Ahn29). In addition, PPARγ2 is responsible for lipid metabolism by increasing fatty acid oxidation in skeletal muscle(Reference Marion-Letellier, Savoye and Ghosh30). Previous studies investigated the effect of PPARγ2 polymorphism on weight regain by identifying a correlation between rs1801282 SNP of the PPARγ2 gene and weight regain. However, their results were inconsistent. In fact, a study in postmenopausal females demonstrated that the G (Ala) allele of rs1801282 showed a positive correlation with weight regain after diet-induced weight loss(Reference Nicklas, van Rossum and Berman31), whereas the other two studies in both males and females discovered a negative correlation(Reference Delahanty, Pan and Jablonski23,Reference Goyenechea, Dolores Parra and Alfredo Martinez32) . Another study found that the weight regain group had more homozygous Pro alleles (C/C genotype) than the weight maintenance group did, but the relationship of the gene with weight regain was not significant after multiple corrections (body weight, BMI and waist circumference), and also there was only one subject who carried the G/G genotype in that study(Reference Vogels, Mariman and Bouwman33). Six years after bariatric surgery, there was no effect of that SNP on weight regain as well(Reference Sarzynski, Jacobson and Rankinen25). Because there was no relationship between PPARγ2 rs1801282 and weight regain in adults who underwent bariatric surgery at the 6-year follow-up but the relationship was established in other studies using lifestyle intervention-induced weight reduction at earlier stages of follow-up, these findings suggest that: (1) bariatric surgery overcomes the effect of PPARγ2 rs1801282 on weight regain, and (2) the effect of PPARγ2 rs1801282 on weight regain is inconsistent. As in the case of BDNF rs6265, both a shorter-duration study in the bariatric surgery model and a longer-duration study in lifestyle intervention model are necessary. Also of note, the association between weight regain and PPARγ2 in postmenopausal females was different from other populations. PPARγ and oestrogen receptors have been shown to interact with each other in terms of vascular function and the treatment of cancer(Reference Tiyerili, Muller and Fung34–Reference Chu, van Hasselt and Vlantis36). Therefore, oestrogen receptors may also mediate the effect of PPARγ2 on weight regain, which may be responsible for differences in effect between pre- and postmenopausal females. Further investigation is required to clarify the relationships between PPARγ2, oestrogen receptor and weight regain.
The PPM1K gene encodes for the mitochondrial branched-chain α-ketoacid dehydrogenase phosphatase, which catalyses oxidative decarboxylation of branched-chain α-ketoacids from branched-chain amino acids(Reference Wynn, Li and Brautigam37). At 24 months after the intervention, the C allele of the rs1440581 SNP of the PPM1K gene was related to smaller weight reduction in subjects with obesity who received a high-fat diet (40 % of fat), whereas no significant genetic effect was observed in a low-fat diet group (20 % of fat)(Reference Xu, Qi and Liang38). These findings suggested that there was a gene-diet interaction on weight maintenance, and the C allele of the rs1440581 was positively associated with the risk of weight regain following high-fat diet intervention.
ADRB2 plays important roles in lipid and glucose metabolism by modulating lipolysis(Reference Schiffelers, Saris and Boomsma39) and insulin secretion(Reference Lacey, Cable and James40). Masuo and colleagues investigated the effects of the rs1042713 SNP of the ADRB2 gene and demonstrated that males with rebound weight gain had a significantly higher frequency of the Gly16 (G) allele compared with those who could maintain their weight loss(Reference Masuo, Katsuya and Kawaguchi41).
IL-6 affects glucose and lipid metabolism through various mechanisms, including decreased glucose uptake into adipocytes, decreased glycogen synthesis in the liver and increased lipolysis, resulting in obesity and insulin resistance(Reference Eder, Baffy and Falus42,Reference El-Kadre and Tinoco43) . A previous study revealed that the C allele of the rs1800795 (–174 G > C) SNP of the IL-6 gene protected against weight regain(Reference Goyenechea, Dolores Parra and Alfredo Martinez32). Moreover, the conjoined presence of the C allele of the rs1800795 and the G (Ala) allele of the rs1801282 SNP of the PPARγ2 gene further improved the ability for weight maintenance(Reference Goyenechea, Dolores Parra and Alfredo Martinez32).
GRL is a transcription factor that binds glucocorticoids and regulates the transcription of specific genes(Reference Wang, Gray and Kuo44). These GRL primary target genes further initiate the physiological and pathological responses of glucocorticoids, which include the homoeostasis involved in lipid metabolism(Reference Wang, Gray and Kuo44). A previous study showed that the G/G genotype of the Bcl1 (rs41423247) RFLP of the GRL gene was independently associated with successful weight maintenance(Reference Vogels, Mariman and Bouwman33).
In summary, evidence indicates that the polymorphisms of several genes related to lipid, glucose and branched-chain amino acid metabolism (PLIN, BDNF, leptin, PPARγ2, PPM1K, ADRB2, IL-6 and GRL) are associated with weight regain.
Food intake and energy expenditure-related genes and their association with weight regain
Obesity is the result of an imbalance between food intake and energy expenditure(Reference Reed, Chaput and Tremblay45). For weight maintenance following weight loss, energy intake must match energy expenditure as well(Reference Galgani and Ravussin46). Unfortunately, weight reduction leads to decreased energy needs, but to an augmented drive to eat, which favours weight regain(Reference Reed, Chaput and Tremblay45). Previous studies have demonstrated that there were relationships between weight regain and polymorphisms of various genes that are related to food intake and energy expenditure, including BDNF, leptin, fat mass and obesity-associated protein (FTO), dopamine receptor D2 (DRD2), K channel tetramerisation domain containing 15 (KCTD15), transmembrane protein 18 (TMEM18), ADRB2, IL-6, neuronal growth regulator 1 (NEGR1) and mitochondrial translational initiation factor 3 (MTIF3). A comprehensive summary of those reports is shown in Table 2.
Table 2. SNP or the restriction fragment length polymorphisms (RFLP) of food intake and energy expenditure-related genes that are associated with weight regain
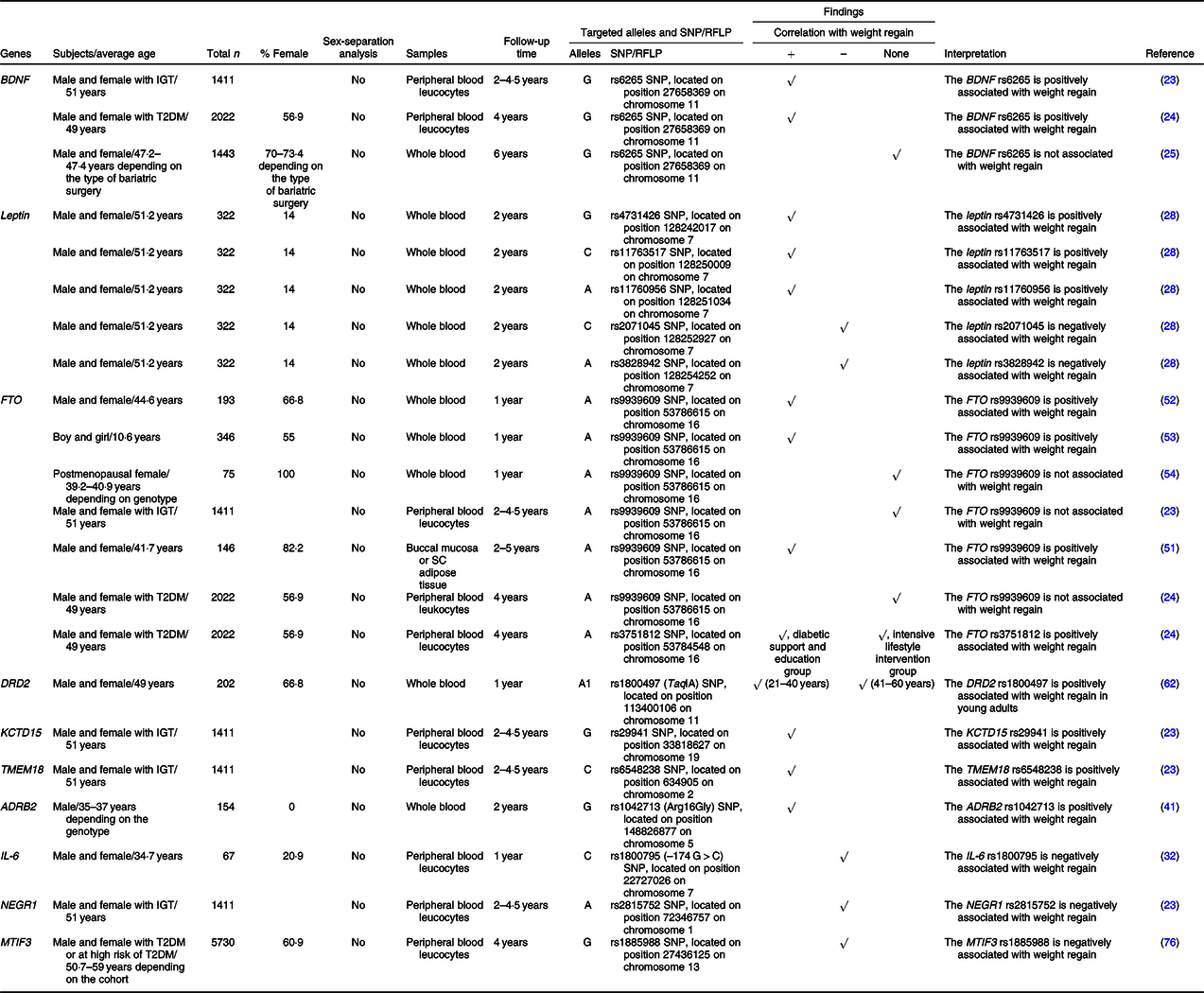
+, Positively associated with; –, negatively associated with; ADRB2, β-2 adrenergic receptor; BDNF, brain-derived neurotrophic factor; DRD2, dopamine receptor D2; FTO, fat mass and obesity-associated protein; IGT, impaired glucose tolerance; KCTD15, K channel tetramerisation domain containing 15; MTIF3, mitochondrial translational initiation factor 3; NEGR1, neuronal growth regulator 1; SC, subcutaneous; T2DM, type 2 diabetes mellitus; TMEM18, transmembrane protein 18.
BDNF suppresses food intake by acting on neurons in the hypothalamus(Reference Urabe, Kojima and Chan47) and increases energy expenditure by increasing adaptive thermogenesis via the activation of a hypothalamic-adipocyte axis(Reference Cao, Choi and Liu48). Deletions or mutations of the BDNF gene lead to impaired thermogenesis and clinical manifestations of hyperphagia and obesity(Reference Xu and Xie21,Reference An Juan, Liao and Kinney Clint22) . An association between the G allele of the rs6265 SNP of the BDNF gene and weight regain was previously described in ‘Nutrient metabolism-related genes and their association with weight regain’.
Similarly to BDNF, leptin acts on neurons in the hypothalamus and promotes satiety(Reference Fernandez-Formoso, Perez-Sieira and Gonzalez-Touceda26,Reference Li and Li27) . Several SNP of the leptin gene were previously found to be correlated with weight regain, including rs4731426, rs2071045, rs3828942, rs11763517 and rs11760956 SNP, as previously mentioned in ‘Nutrient metabolism-related genes and their association with weight regain’.
Previous studies showed that polymorphisms of the FTO gene were associated with increased food intake(Reference Speakman49,Reference Dang, Samanez-Larkin and Smith50) and obesity(Reference Speakman49). The SNP of the FTO gene that was commonly investigated regarding weight regain was rs9939609, but the results were inconsistent. Specifically, three previous studies, which include a study in children, showed that the A allele of that SNP was positively associated with weight regain(Reference Rodrigues, Resende and Durso51–Reference Reinehr, Wolters and Roth53). On the other hand, a study in postmenopausal females and another two studies in subjects with impaired glucose tolerance and type 2 diabetes found there was no relationship of that SNP with weight regain(Reference Delahanty, Pan and Jablonski23,Reference McCaffery, Papandonatos and Huggins24,Reference Rauhio, Uusi-Rasi and Nikkari54) . The A allele of another SNP of the FTO gene, rs3751812, was also positively associated with weight regain in individuals who received diabetic support and education, but not in those who received the intensive lifestyle intervention(Reference McCaffery, Papandonatos and Huggins24). All of these findings suggest that the impact of FTO rs9939609 on weight regain seems to have disappeared in postmenopausal females. There is evidence that oestrogen regulates FTO via the PI3K/AKT, MAPK and mTOR (mammalian target of rapamycin) signalling pathways, resulting in endometrial cancer cell proliferation(Reference Zhu, Shen and Gao55,Reference Zhang, Zhou and Lai56) . Thus, it is possible that oestrogen regulates the effect of FTO on weight regain also and therefore oestrogen deficiency hinders the association between FTO and weight regain in postmenopausal females. A future study is needed to prove this possibility. Interestingly, studies in individuals with impaired glucose tolerance and type 2 diabetes demonstrated no correlation between FTO rs9939609 and weight regain following weight loss. However, a correlation was exhibited in studies in general populations and even in children, indicating the overwhelming effect of impaired glucose tolerance and type 2 diabetes on FTO-associated weight regain. Furthermore, FTO rs9939609 was reportedly associated with insulin resistance and the risk of type 2 diabetes(Reference Takeuchi, Yamamoto and Katsuya57,Reference De Luis, Aller and Izaola58) . Nevertheless, further studies are required to identify the molecular mechanisms responsible for the relationships between FTO, insulin resistance and weight regain.
DRD2 is known to be responsible for the circuit of food reward(Reference Baik59). Genetic variations of the DRD2 gene were previously found to be related to emotional eating(Reference Yeh, Trang and Henning60,Reference Obregon, Valladares and Goldfield61) . It was shown that young adults (21–40 years old) carrying the A1 allele of the rs1800497 (TaqIA) SNP of the DRD2 gene had greater weight regain during the weight maintenance phase, while there was no effect of that SNP on weight regain among older adults (41–60 years old)(Reference Winkler, Woehning and Schultz62). Particularly, age modulates the effect of the DRD2 rs1800497 on weight regain. This might be due to age-induced improvement of response-inhibition capacities, alterations in several hormones and involvement of other acquired factors which are a result of the ageing process(Reference Winkler, Woehning and Schultz62). However, the reduction in D2 receptor binding and endogenous dopamine with age(Reference Backman, Ginovart and Dixon63,Reference Reeves, Bench and Howard64) may be another possible explanation.
KCTD15 plays an important role in the regulation of eating behaviour and reward(Reference Gamero-Villarroel, González and Rodríguez-López65). A previous study observed treatment-specific effects of the KCTD15 rs29941 SNP on weight regain(Reference Delahanty, Pan and Jablonski23). Specifically, the G allele of that SNP was positively correlated with weight regain in subjects who underwent an intensive lifestyle modification programme(Reference Delahanty, Pan and Jablonski23).
A study in TMEM18-deficient mice found that the loss of TMEM18 expression resulted in increased appetite(Reference Larder, Sim and Gulati66). Similarly to KCTD15, a treatment-specific effect of the TMEM18 rs6548238 SNP on weight regain was exhibited(Reference Delahanty, Pan and Jablonski23). Indeed, the C allele of that SNP was positively associated with weight regain in subjects who underwent an intensive lifestyle modification programme(Reference Delahanty, Pan and Jablonski23).
Not only does ADRB2 modulate lipolysis, but it also regulates energy expenditure via the thermogenic effects of catecholamines(Reference Leite, Silva and Jesus67,Reference Szendrei, Gonzalez-Lamuno and Amigo68) . A study in Japanese males revealed that the Gly16 (G) allele of the rs1042713 SNP of the ADRB2 gene was positively related to weight regain(Reference Masuo, Katsuya and Kawaguchi41).
IL-6 impacts regulation of energy expenditure through several mechanisms, including actions in the hypothalamic-pituitary-adrenal axis, and activation of the sympathetic nervous system(Reference Papanicolaou, Petrides and Tsigos69,Reference Torpy, Papanicolaou and Lotsikas70) . A previous study demonstrated an association between the C allele of the rs1800795 (–174 G > C) SNP of the IL-6 gene and weight regain(Reference Goyenechea, Dolores Parra and Alfredo Martinez32), as previously described in ‘Nutrient metabolism-related genes and their association with weight regain’.
Previous studies suggested that the expression of NEGR1 in the hypothalamus was related to food intake(Reference Speakman71,Reference Boender, van Gestel and Garner72) . Delahanty and colleagues demonstrated that the A allele of the rs2815752 SNP of the NEGR1 gene was negatively associated with weight regain irrespective of treatment arms(Reference Delahanty, Pan and Jablonski23).
MTIF3 promotes the formation of the initiation complex on the mitochondrial 55S ribosome, which is essential for energy balance in the mitochondria(Reference Behrouz, Vilarino-Guell and Heckman73). This gene has also been identified as an obesity susceptibility gene due to its relationship with BMI(Reference Speliotes, Willer and Berndt74,Reference Conn, Vaughan and Garver75) . Papandonatos and colleagues showed that the G allele of the MTIF3 rs1885988 was associated with greater weight loss following lifestyle intervention over 4 years of follow-up(Reference Papandonatos, Pan and Pajewski76). In other words, their result implies that G allele of the MTIF3 rs1885988 is related to lower-risk of weight regain.
In summary, evidence indicates that the polymorphisms of the genes that regulate satiety, food reward, eating behaviour, as well as energy expenditure (BDNF, leptin, FTO, DRD2, KCTD15, TMEM18, ADRB2, IL-6, NEGR1 and MTIF3) are associated with the ability of long-term weight maintenance.
Adipocyte differentiation-related genes and their association with weight regain
Adipocytes are a key regulator of body weight, and obesity is a condition characterised by excess adipose tissue(Reference Stephens77). The generation of new adipocytes consists of two steps: the proliferation of preadipocytes and the differentiation from preadipocytes to adipocytes (adipocyte differentiation)(Reference MacLean, Higgins and Giles78). Taken together, adipocyte differentiation is a process that determines body weight and obesity(Reference Auwerx, Martin and Guerre-Millo79). The polymorphisms of adipocyte differentiation-related genes that are associated with weight regain were previously evidenced, including BDNF, PPARγ2, TMEM18 and NEGR1. A comprehensive summary of these relevant reports is shown in Table 3.
Table 3. SNP or the restriction fragment length polymorphisms (RFLP) of adipocyte differentiation-related genes that are associated with weight regain
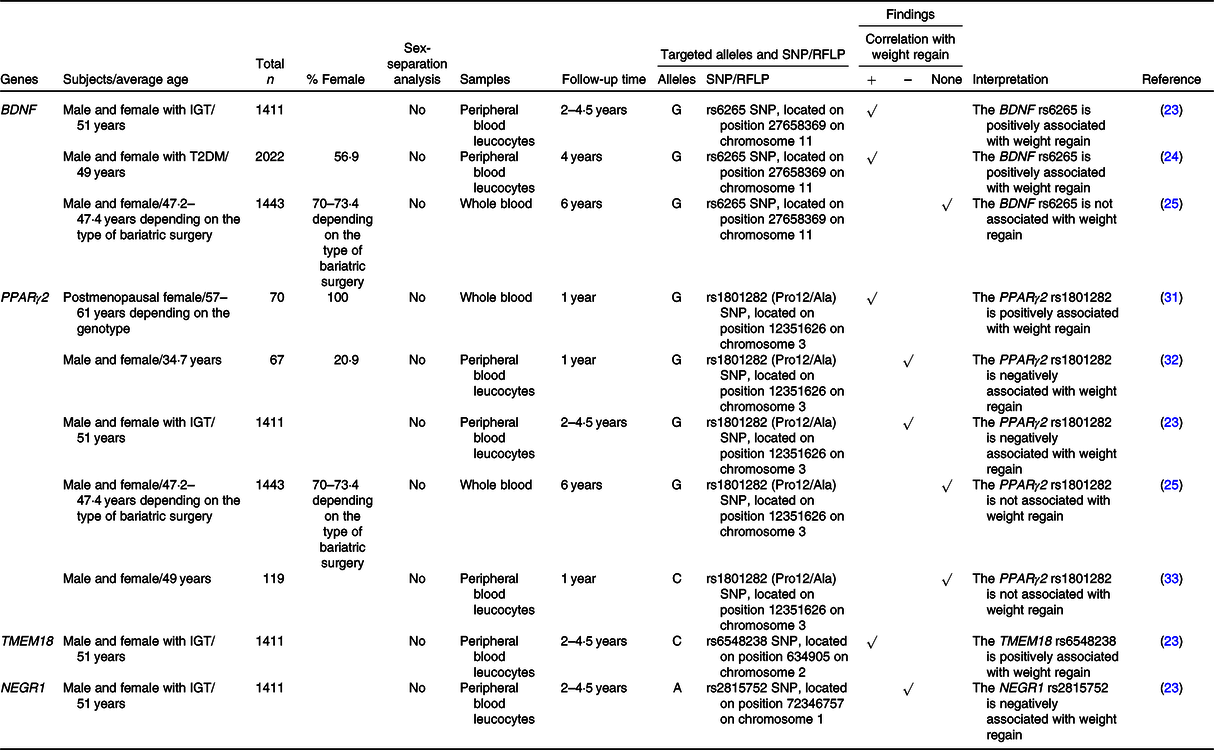
+, Positively associated with; –, negatively associated with; BDNF, brain-derived neurotrophic factor; IGT, impaired glucose tolerance; NEGR1, neuronal growth regulator 1; T2DM, type 2 diabetes mellitus; TMEM18, transmembrane protein 18.
A previous study demonstrated that BDNF gene expression was down-regulated during adipocyte differentiation and knockdown of BDNF inhibited adipocyte differentiation(Reference Bernhard, Landgraf and Kloting80). There was an association between the G allele of the rs6265 SNP of the BDNF gene and weight regain as previously described in ‘Nutrient metabolism-related genes and their association with weight regain’.
It is widely known that PPARγ2 is a master regulator of adipocyte differentiation(Reference Marion-Letellier, Savoye and Ghosh30,Reference Poulos, Dodson and Culver81) . The effect of PPARγ2 polymorphism on weight regain was focused on the rs1801282 SNP, but the results were inconsistent, as previously mentioned in ‘Nutrient metabolism-related genes and their association with weight regain’.
Knockdown of TMEM18 inhibited adipocyte differentiation(Reference Bernhard, Landgraf and Kloting80). Delahanty and colleagues demonstrated that there was a treatment-specific effect of the TMEM18 rs6548238 SNP on weight regain. In other words, the C allele of that SNP showed a positive correlation with weight regain in individuals who underwent an intensive programme of lifestyle modification(Reference Delahanty, Pan and Jablonski23).
Unlike BDNF, NEGR1 gene expression was up-regulated during adipocyte differentiation, yet knockdown of NEGR1 did also inhibit adipocyte differentiation(Reference Bernhard, Landgraf and Kloting80). As previously mentioned, a negative correlation between the A allele of the rs2815752 SNP of the NEGR1 gene and weight regain was observed regardless of treatment arms(Reference Delahanty, Pan and Jablonski23).
In summary, evidence indicates that there is a relationship between adipocyte differentiation and weight regain, which is mediated by the polymorphisms of adipocyte differentiation-related genes (BDNF, PPARγ2, TMEM18 and NEGR1).
Inflammation, extracellular matrix and bone metabolism-related genes and their association with weight regain
Obesity is associated with chronic inflammation owing to NEFA-induced activation of inflammatory pathways(Reference Ellulu, Patimah and Khaza’ai82,Reference Stepien, Stepien and Wlazel83) . This inflammatory response leads to increased extracellular matrix synthesis but reduced extracellular matrix degradation, resulting in increased deposition and remodelling of extracellular matrix, especially in adipocytes(Reference Lin, Chun and Kang84,Reference Williams, Kang and Wasserman85) . Bone metabolism is also correlated with body fat and lean mass(Reference Gomez-Ambrosi, Rodriguez and Catalan86). In fact, bone has recently been determined as an endocrine organ that affects body weight regulation via the actions of bone-derived factors such as osteocalcin and osteopontin(Reference Gomez-Ambrosi, Rodriguez and Catalan86). The associations between weight regain and polymorphisms of inflammation, extracellular matrix and bone metabolism-related genes include IL-6, periostin (POSTN), collagen type XXIII α1 chain (COL23A1), fibulin 5 (FBLN5), laminin subunit β1 (LAMB1), fibronectin 1 (FN1) and TNF receptor superfamily member 11a (TNFRSF11A). These reports are comprehensively summarised in Table 4.
Table 4. SNP or the restriction fragment length polymorphisms (RFLP) of inflammation, extracellular matrix and bone metabolism-related genes that are associated with weight regain
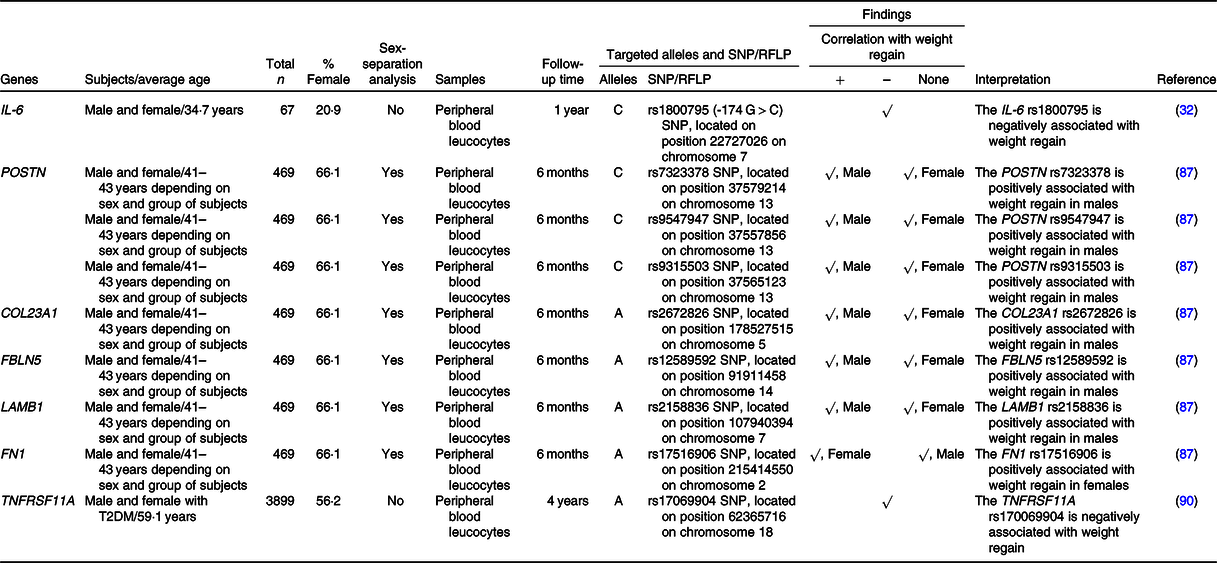
+, Positively associated with; –, negatively associated with; COL23A1, collagen type XXIII α1 chain; FBLN5, fibulin 5; FN1, fibronectin 1; LAMB1, laminin subunit β1; POSTN, periostin; T2DM, type 2 diabetes mellitus; TNFRSF11A, TNF receptor superfamily member 11a.
IL-6 is an inflammatory mediator that is oversecreted in response to excessive macronutrients in adipose tissue(Reference Ellulu, Patimah and Khaza’ai82). A prior study demonstrated an association between the C allele of the rs1800795 (–174 G > C) SNP of the IL-6 gene and weight regain(Reference Goyenechea, Dolores Parra and Alfredo Martinez32), as previously described in ‘Nutrient metabolism-related genes and their association with weight regain’.
Roumans and colleagues investigated the effects of one hundred and twenty-four extracellular matrix-related genes on weight regain, revealing six SNP (males) and one SNP (females) that were significantly associated with the weight maintenance score(Reference Roumans, Vink and Gielen87). Indeed, they found that the risks of weight regain in males were increased in C-allele carriers of three SNP (rs7323378, rs9547947 and rs9315503) of the POSTN gene, A-allele carriers of the COL23A1 rs2672826, A-allele carriers of the FBLN1 rs12589592 and A-allele carriers of the LAMB1 rs2158836(Reference Roumans, Vink and Gielen87). In females, however, the risk of weight regain was higher in A-allele carriers of the FN1 rs17516906(Reference Roumans, Vink and Gielen87). As previously mentioned, obesity is associated with extracellular matrix remodelling in adipocytes(Reference Lin, Chun and Kang84,Reference Williams, Kang and Wasserman85) and adipocyte characteristics differ based on sex(Reference Karastergiou and Fried88). Altogether, these facts support the sex-specific effects of extracellular matrix-related genes on weight regain.
TNFRSF11A, or more commonly known as RANK, involves in a signalling pathway that regulates bone metabolism(Reference Boyce and Xing89). A previous study revealed that the A allele of TNFRSF11A rs17069904 was associated with greater weight loss in subjects who received intensive lifestyle intervention-induced weight reduction at 4 years of follow-up(Reference McCaffery, Papandonatos and Huggins90). Indeed, the result from that study implies that A allele of TNFRSF11A rs17069904 is related to lower risk of weight regain.
In summary, not only inflammation, extracellular matrix and bone metabolism-related genes are associated with obesity but also the polymorphisms of these genes (IL-6, POSTN, COL23A1, FBLN5, LAMB1, FN1 and TNFRSF11A) are also associated with weight regain in individuals with obesity after successful weight loss.
Conclusion
There is a growing body of evidence which suggests that genetic polymorphism is associated with weight regain in both adults and children with obesity. Specifically, these genes are responsible for the pathophysiology of obesity, including nutrient metabolism, regulation of food intake and energy expenditure, adipocyte differentiation, inflammation, structure of the extracellular matrix and bone metabolism as summarised in Fig. 1. Additionally, some of these genes affect weight regain in a distinct manner, such as intervention-specific, age-specific, sex-specific, oestrogen status-specific and insulin sensitivity status-specific.
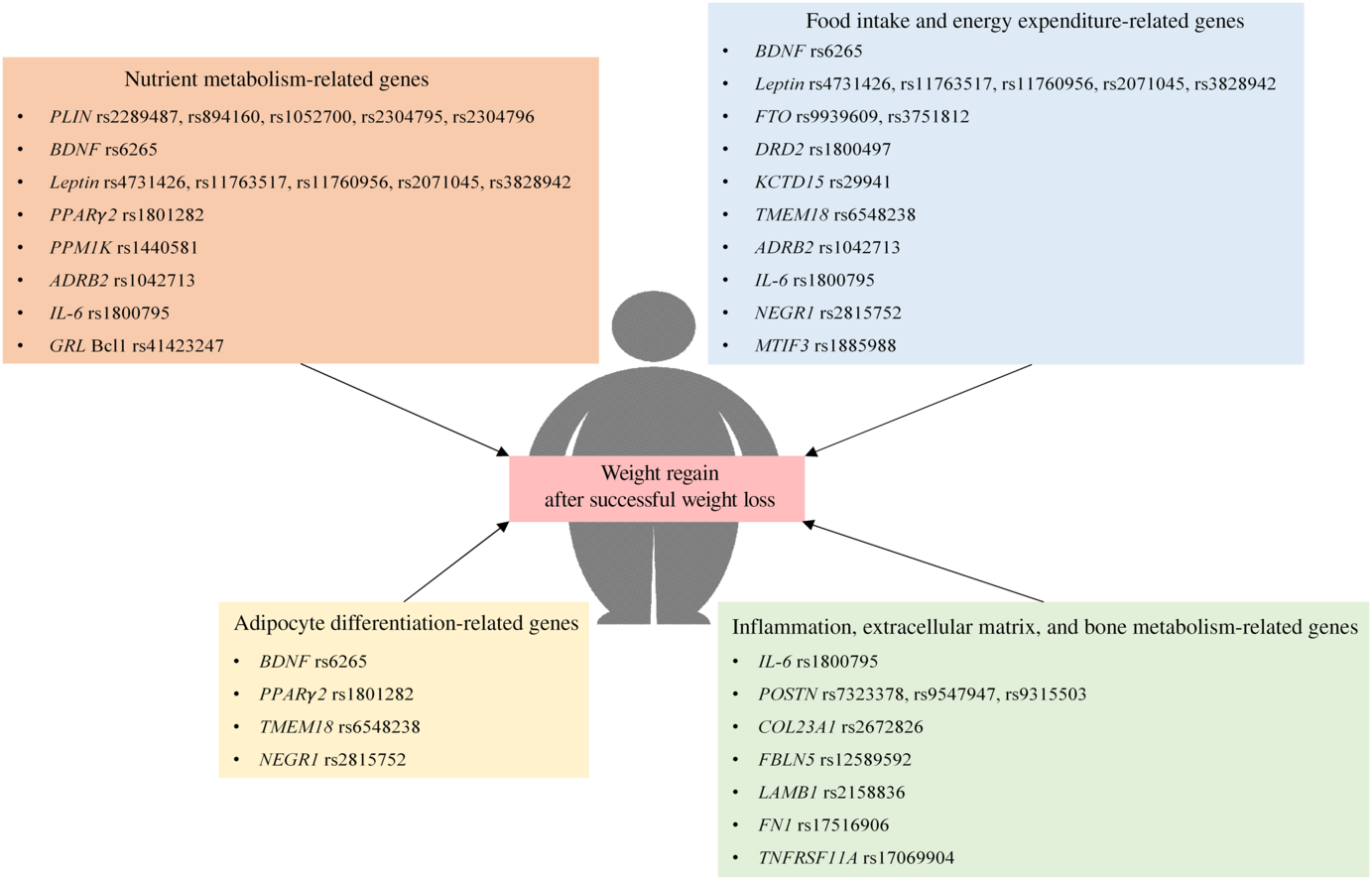
Fig. 1. Polymorphism of genes that are associated with weight regain after successful weight loss. ADRB2, β-2 adrenergic receptor; BDNF, brain-derived neurotrophic factor; COL23A1, collagen type XXIII α1 chain; DRD2, dopamine receptor D2; FBLN5, fibulin 5; FN1, fibronectin 1; FTO, fat mass and obesity-associated protein; GRL, glucocorticoid receptor; KCTD15, K channel tetramerisation domain containing 15; LAMB1, laminin subunit β1; MTIF3, mitochondrial translational initiation factor 3; NEGR1, neuronal growth regulator 1; PLIN, perilipin; POSTN, periostin; PPM1K, protein phosphatase, Mg2+/Mn2+ dependent 1K; TMEM18, transmembrane protein 18; TNFRSF11A, TNF receptor superfamily member 11a.
Future directions and clinical applications
Since there is evidence that suggested relationships between genetic polymorphisms and weight regain, identification of genetic polymorphisms in patients with obesity who undergo weight loss intervention could provide useful information to estimate their risks of weight regain after successful weight reduction. However, the impact that any one of these genetic polymorphisms have on weight regain is still unclear due to short duration of follow-up in those reports. Because long-term follow-up is required for continued long-term weight loss success, it is essential that further studies with the longer duration of follow-up are needed to confirm the persistence of the impact of those genetic polymorphisms on weight maintenance. Furthermore, future studies in twins and siblings clarifying the contribution of each genetic polymorphism on weight regain are required to confirm a clinical valuable of genetic testing. Moreover, it is still controversial which genetic polymorphisms exhibit the maximum effect size on weight regain due to the difference of the design among studies. Therefore, further studies that directly compare the effect size of those polymorphisms are needed. Although most of the previous studies identified the correlations between genetic polymorphisms and weight regain after adjusting for several confounding factors (age, sex, ethnicity, study site, method of weight loss and baseline anthropometry), behavioural and psychological factors were not included in the multiple regression analyses. Adjusting for the degree of weight reduction, behavioural factors and psychological factors are necessary in future studies since these factors have been shown to be associated with weight regain(Reference Marinilli Pinto, Gorin and Raynor7–Reference Raynor, Phelan and Hill12). In addition to the genetic polymorphisms, epigenetics could also play important roles on weight regain. Although the association between weight regain and DNA methylation of some genes, including proopiomelanocortin (POMC), neuropeptide Y (NPY), mal, T cell differentiation protein 2 (MAL2), family with sequence similarity 129, member A (FAM129A), periplakin (PPL), PDZ domain containing ring finger 4 (PDZRN4) and major facilitator superfamily domain containing 3 (MFSD3), has been reported(Reference Nicoletti, Pinhel and Noronha91–Reference Bollepalli, Kaye and Heinonen93), future epigenetic studies regarding the relationship between post-transcriptional modification and the impacts of genetic polymorphisms on long-term weight maintenance in different populations are also required.
After further investigation mentioned above, the genetic-based risk estimation might be used as a guide for physicians and dietitians to provide each of their own patients with the most appropriate strategies for weight loss and for long-term weight maintenance, which might result in a decreased prevalence of obesity, decreased risk of obesity-associated diseases and mortality, as well as decreased obesity-related economic burden.
Acknowledgements
This work was supported by Thailand Research Fund grants MRG6280014 (C. T.) and RSA5880015 (K. S.), a Senior Research Scholar grant from the National Research Council of Thailand (S. C. C.), a NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (N. C.), and the Chiang Mai University Center of Excellence Award (N. C.).
C. T. designed the work, drafted the work and revised the work. K. S. revised the work. S. C. C. designed the work. N. C. designed the work, revised the work, and gave final approval of the version to be published.
The authors declare that there are no conflicts of interest.