Nutritional support is based on the assumption that critically ill patients are prone to develop malnutrition, especially protein-energy deficit, this condition being associated with morbidity and mortality(Reference Wray, Mammen and Hasselgren1–Reference Reid5). Indeed, protein-energy deficit seems common in intensive care units (ICU), occurring in 43–88 % of critically ill patients(Reference Giner, Laviano, Meguid and Gleason6, Reference Barr, Hecht, Flavin, Khorana and Gould7). Underfeeding has been reported as associated with an increased rate of infection, poor wound healing, reduced respiratory muscle mass, delayed weaning from mechanical ventilation, increased length of ICU stay and increased health care costs(Reference Wray, Mammen and Hasselgren1, Reference Rubinson, Diette, Song, Brower and Krishnan8–Reference Rodriguez13).
Perturbations of the normal metabolic response to starvation with hyperglycaemia, high lactate level, hypertriglyceridaemia and high NEFA concentrations due to insulin resistance characterize the hypermetabolic state of the critically injured patients(Reference Plank and Hill2, Reference Chiolero, Revelly and Tappy14, Reference Trager, DeBacker and Radermacher15). Energy deficit results from a combination of hypermetabolism and reduced intake due to frequent interruptions in feedings because of gastrointestinal intolerance, diagnostic and therapeutic procedures(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16–Reference O'Leary-Kelley, Puntillo, Barr, Stotts and Douglas18). In intubated and mechanically ventilated patients, the great variability of resting energy expenditure (REE) and nutrient delivery compared to prescription, partly due to frequent use of sedatives, analgesics or vasoconstrictors, increases the risk of mismatch between energy requirements and intakes(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Uehara, Plank and Hill19–Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21).
Enteral nutrition (EN) became widespread in ICU during the last two decades in order to reduce parenteral catheter-related sepsis, nutrition cost and translocation-induced multiple organ failure(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Heyland, MacDonald, Keefe and Drover22). Even if technical problems have limited its application, early EN is the recommended method of artificial feeding in ICU patients and some recent data suggest that EN is safe in patients with severe haemodynamic failure and that early EN could be an appropriate tool to reduce energy deficit and to improve ICU outcome(Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Jolliet, Pichard and Biolo23–Reference Taylor, Fettes, Jewkes and Nelson25). However, the individual fluctuations in REE of ICU patients and the inaccuracy of predictive formulas used to evaluate REE complicate the assessment of the energy requirements and do not facilitate feeding and its early administration by ICU practitioners(Reference Reid3, Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference McClave, Lowen, Kleber, Nicholson, Jimmerson, McConnell and Jung10, Reference Ibrahim, Mehringer, Prentice, Sherman, Schaiff, Fraser and Kollef26). Consequently, there is a deficit of ‘energy input — REE’ resulting in negative energy balance in surgical ICU patients, particularly those treated with mechanical ventilation and nourished by the enteral route(Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Berger, Revelly, Cayeux and Chiolero24, Reference Dock-Nascimento, Tavares and de Aguilar-Nascimento27). In non-surgical critically ill mechanically ventilated patients, hypoenergetic feeding has been also frequently associated with inappropriate prescription rate and/or with inadequate delivery of the EN(Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference Adam and Batson28, Reference Petros and Engelmann29). The causes and consequences of energy deficit have been investigated in medical mechanically ventilated patients who received, at least in part, both EN and parenteral nutrition(Reference Petros and Engelmann29, Reference Dvir, Cohen and Singer30)
The estimation of daily energy deficit requires indirect calorimetry because the usual predictive equations for estimating REE are based on individual anthropometric characteristics(Reference Petros and Engelmann29, Reference Dvir, Cohen and Singer30) However, indirect calorimetry, the gold standard for REE assessment, is not always applicable in severe ICU patients, needs costly equipment and technical expertise, is time consuming and is not available everywhere(Reference Dvir, Cohen and Singer30, Reference Faisy, Guerot, Diehl, Labrousse and Fagon31). In a previous prospective study, we found that indirect calorimetry was not applicable in 43 % of severe ICU patients because they had conditions invalidating calorimetric measurements(Reference Faisy, Guerot, Diehl, Labrousse and Fagon31). We developed and validated a simple and practicable REE equation incorporating static (weight, height) and dynamic biometric parameters (temperature, minute ventilation) that measure the very variable and rapidly changing REE in severe medical ICU patients(Reference Faisy, Guerot, Diehl, Labrousse and Fagon31, Reference Savard, Faisy, Lerolle, Guérot, Diehl and Fagon32). The present study identifies the determinants of the energy balance, estimated from our equation, and its relationship to ICU outcome in severely ill patients requiring prolonged acute mechanical ventilation and receiving exclusive EN.
Subjects and methods
Patients and study design
We conducted an observational, retrospective study in the twenty-bed medical ICU of a tertiary teaching hospital. The retrospective design protected from the Hawthorne effect (a change in behaviour when clinicians or nurses are being observed)(Reference Campbell, Maxey and Watson33). Because of the observational design of the study, no local Institutional Review Board authorization was needed according to French bioethics laws. Adult patients admitted in the ICU over a 1-year period requiring endotracheal intubation and mechanical ventilation < 6 h after ICU admission and intubated for at least 7 d were eligible for analysis. The least duration of 7 d for intubation allowed selection of patients requiring prolonged acute mechanical ventilation (≧96 h)(Reference Zilberberg, Luippold, Sulsky and Shorr34) and allowed a realistic nutritional survey requiring ventilator measurements for REE calculation to be performed. Patients who were not receiving EN because of the following contraindications were not selected for analysis: complete intestinal obstruction or ileus, intractable vomiting or severe diarrhoea, intestinal ischaemia, gastrointestinal haemorrhage, high-output enterocutaneous fistulas(Reference Barr, Hecht, Flavin, Khorana and Gould7, 35). We performed a systematic nutritional survey (estimation of REE, energy prescribed and delivered) from ICU admission to day 14 in order to focus our investigations on the level of early energy balance and its impact on outcome. Nutritional survey ceased when invasive mechanical ventilation was stopped before day 14 (death or extubation) at which stage EN intakes became difficult to estimate accurately and the calculation of REE (requiring ventilator measurements) was not possible.
Definitions
Total energy prescribed was calculated from glucose infusions and exclusive EN prescriptions based on local protocol described later. Energy delivered by nurses included energy actually administered per day by enteral feeds and glucose infusions. Propofol was not used for continuous sedation in our ICU. Recommended energy corresponded to target feeding based on nutritional protocol described later. The daily REE (kcal per d) was retrospectively calculated with the following predictive equation(Reference Faisy, Guerot, Diehl, Labrousse and Fagon31, Reference Savard, Faisy, Lerolle, Guérot, Diehl and Fagon32): (8 × body weight (kg))+(14 × height (cm))+(32 × minute ventilation (litres/min))+(94 × body temperature (°C)) − 4834. No stress factor modifications were required(Reference Faisy, Guerot, Diehl, Labrousse and Fagon31). Energy values were then given in kJ using the conversion factor 1 kcal = 4·184 kJ. Body weight, minute ventilation and body temperature were assessed from daily observational charts as described later. Height, assessed with measuring tape and the patient lying in a supine position, was collected from the medical file. Energy balance (kJ per d of mechanical ventilation) was calculated as follows: energy delivered — calculated REE. The energy stored (adipose tissues, intramuscular TAG, and blood fatty acids or TAG) was ignored for the calculation of energy balance(Reference Plank and Hill2, Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference Berger, Revelly, Cayeux and Chiolero24). Energy deficit was assumed to correspond to a negative energy balance.
Exclusive enteral nutrition
EN was started within 24 h of ICU admission when gastrointestinal tract was functional(Reference Thuong and Leteurtre36) and patients were haemodynamically stable. Patients received polymeric isoenergetic solution (Sondalis Iso®; Nestlé Clinical Nutrition, France; 4·184 kJ/ml, 50 % carbohydrates, 35 % lipids, 15 % proteins). EN was delivered by continuous, pump-driven infusion via a nasogastric tube. Post-pyloric delivery was not performed. The target feeding recommended by the Société de Réanimation de Langue Française was 125·5 kJ/kg (30 kcal/kg) per d (or per kg ideal body weight per d if BMI was >25 kg/m2)(Reference Thuong and Leteurtre36). Parenteral nutrition was not used to compensate for insufficient amount of energy in our ICU. The gastric position of the tube was checked before the onset of feeding, and later by daily chest radiography. EN was to be started within 24 h of ICU admission at 20 ml/h, and increased as previously reported(Reference Genton, Dupertuis, Romand, Simonet, Jolliet, Huber, Kudsk and Picharg37), in the absence of gastrointestinal intolerance, until the recommended target energy was reached. Occurrence of gastrointestinal intolerance (diarrhoea, vomiting, abdominal distension or ileus) necessitated reducing the feeding rate by 50 %(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21, Reference Taylor, Fettes, Jewkes and Nelson25). EN was stopped in the event of procedures out of ICU or, if required, in ICU, and in case of persistence of severe gastrointestinal intolerance and then restarted after recovery. Gastric residual volumes were not routinely measured and gastric prokinetic drugs and stress ulcer prophylaxis were not systematically used in our ICU(Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21, Reference Faisy, Guerot, Diehl, Iftimovici and Fagon38). The gastric lumen tube was systematically washed with 20 ml sterile water after administration of drugs by this route. In our unit, intubated patients were maintained with head-of-bed >30° to reduce the risk of aspiration(Reference Chastre and Fagon39). EN was not discontinued when the prone position was used in patients with acute respiratory distress syndrome or when ventilator weaning test procedures were achieved.
Patient data
Baseline demographic data at the time of ICU admission included age, height, weight, main diagnosis and Simplified Acute Physiology Score II (SAPS II). The diagnosis of septic shock was retained in the presence of documented infection including bacteraemia, urinary tract infection and pneumonia(Reference Chastre and Fagon39). On each day of mechanical ventilation, the following data were retrospectively collected from charts prospectively completed by nurses: weight measured with a mobile electronic scale, temperature (mean of four values measured electronically in the ear at 6 h intervals during the day) and minute ventilation (mean of four values determined with the respiratory device at 6 h intervals during the day). We assessed from charts the total duration of EN interruptions (expressed as percentage of survey time) due to gastrointestinal intolerance (vomiting, severe diarrhoea, ileus) or diagnostic and therapeutic procedures. We also estimated the total length (percentage of survey time) of conditions that could decrease nutrient absorption or limit the volume of feeding rate prescription(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Uehara, Plank and Hill19–Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21Reference Ravasco and Camilo40): use of sedatives, opioids, neuromuscular blocking agents or vasopressors, and presence of renal failure (defined as creatinine clearance < 50 ml/min) with fluid overload.
Statistical analysis
Results were expressed as means with their standard errors for quantitative variables and numbers (n) or percentages (%) for categorical variables. Paired t tests, Mann–Whitney or Fisher's tests were applied for comparison of relevant variables. Logistic regression was used for multivariate analysis. Relationships between ICU mortality and quartiles of mean energy balance were assessed with the Mantel–Haenszel test. A receiver operating characteristics curve was generated with a non-parametric method (JLABROC4 Eng Software®; Johns Hopkins University, Baltimore, MD, USA) to determine the best operating point as mean energy deficit threshold for predicting ICU mortality. ICU survival was analysed by Kaplan–Meier curves, and comparison between groups was performed by the log-rank test. Statistics were calculated with the StatView® 4.5 (Abacus Concept Inc., Berkeley, CA, USA) and EPI INFO 3.4 (Centers for Disease Control and Prevention, Atlanta, GA, USA) software. We considered a difference to be significant when the α risk was < 5 % (P < 0·05).
Results
Patients
Forty-two patients out of 684 consecutive patients (6 %) admitted to our ICU were eligible for analysis over a 1-year period. Among non-eligible patients, 198 were not intubated during their ICU stay, 444 were intubated >6 h after ICU admission or intubated < 7 d or had contraindication for EN during acute mechanical ventilation. Four eligible patients were excluded because of incomplete data or missing files. Finally, thirty-eight patients were included in the nutrition survey. Main diagnoses at ICU admission were: septic shock (n 19), acute respiratory failure due to congestive heart failure (n 11), acute respiratory distress syndrome (n 4) and non-traumatic coma (n 4); characteristics and demographic data are summarized in Table 1.
Table 1 Description of the patients (n 38) requiring prolonged acute mechanical ventilation
(Mean values with their standard errors)
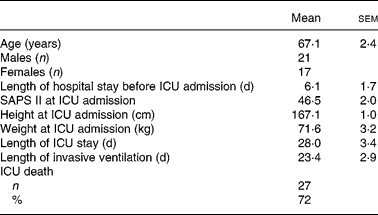
ICU, intensive care unit; SAPS II, Simplified Acute Physiology Score II.
Energy balance
Mean prescribed, delivered and recommended energies, mean calculated REE and mean energy balance are summarized in Table 2. Mean energy deficit was stable from day 3 to day 14 of mechanical ventilation (Fig. 1 (A)). The ratio of delivered/prescribed energy was between 60 and 70 % during the first 14 d of mechanical ventilation whereas prescribed energy/estimated REE ratio remained close to 40 % from day 3 (Fig. 1 (B)). The total duration of EN interruptions and the length of conditions limiting feeding absorption or feeding rate prescription for the first 14 d of mechanical ventilation are summarized in Table 2. The main causes of EN interruptions were diagnostic procedures in or out of ICU and vomiting.
Table 2 Mean energy/d of mechanical ventilation, and presence of conditions limiting feeding administration or feeding prescription in the first 14 d of respiratory support in patients (n 38) requiring prolonged acute mechanical ventilation‡
(Mean values with their standard errors)
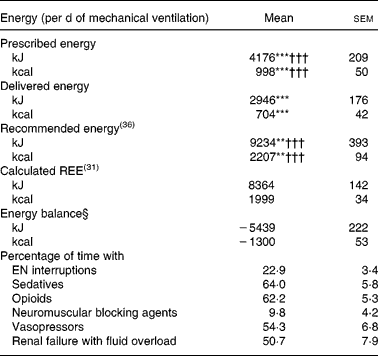
EN, enteral nutrition; REE, resting energy expenditure.
Mean values were significantly different from those of the calculated REE: **P < 0·01, ***P < 0·0001.
Mean values were significantly different from those of the delivered energy (paired t test): †††P < 0·0001.
‡ For details of subjects and procedures, see the Subjects and methods section and Table 1.
§ Energy balance = delivered energy − calculated REE.
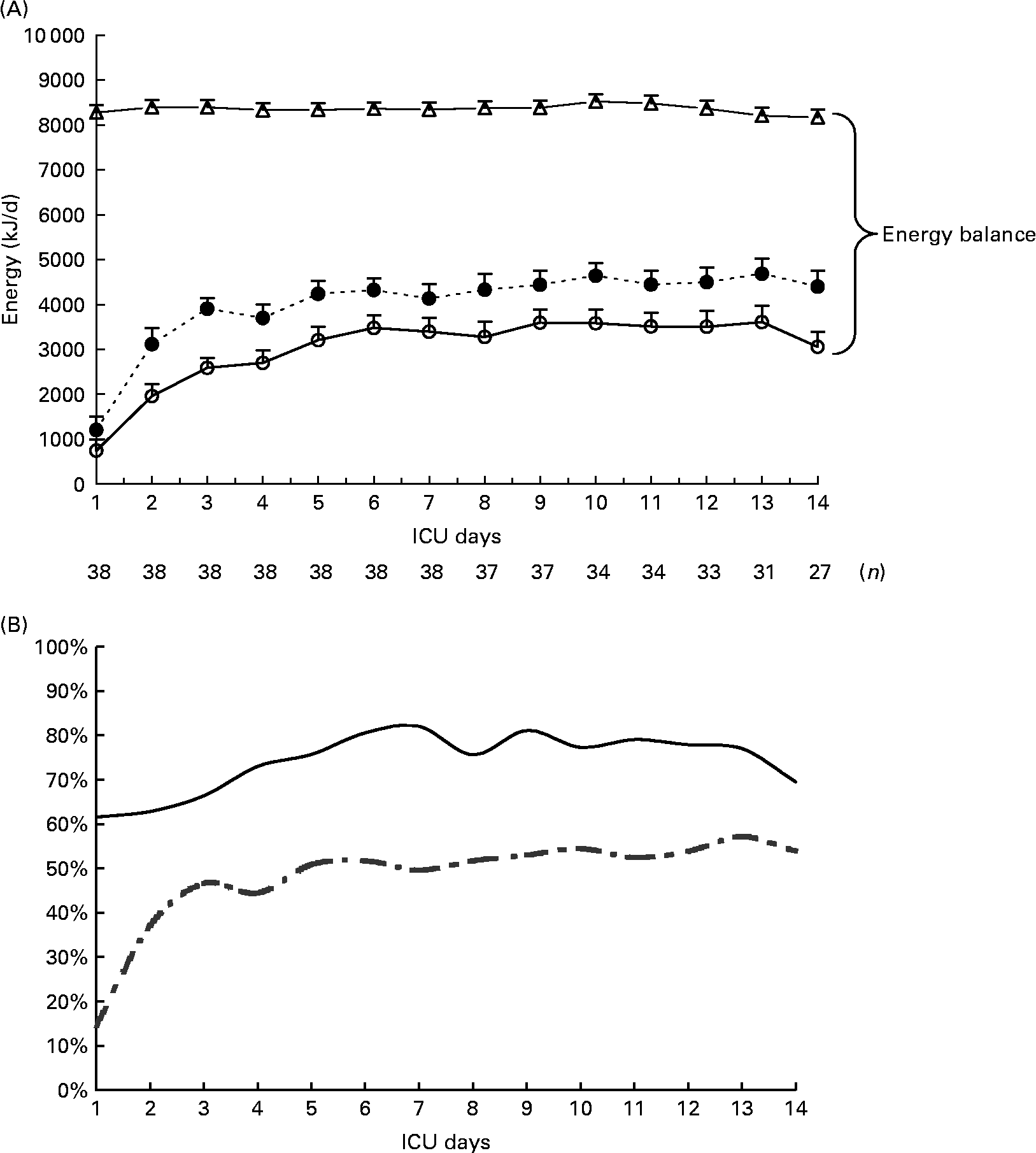
Fig. 1 (A), Evolution of prescribed energy (●), delivered energy (○) and resting energy expenditure (REE) calculated with the Faisy 2003 equation(Reference Faisy, Guerot, Diehl, Labrousse and Fagon31) (△) in patients requiring prolonged acute mechanical ventilation (n 38). Energy balance = delivered energy – calculated REE. Values are means with their standard errors depicted by vertical bars. (B), Delivered/prescribed (—) and delivered/calculated REE energy ratios (- -).
Impact of energy deficit on intensive care unit outcome
Non-survivors had higher mean energy deficit than ICU survivors (Table 3). Furthermore, non-survivors had a daily energy deficit higher than ICU survivors and that difference was statistically significant from day 10 of mechanical ventilation (Fig. 2). Non-survivors were similar to survivors for demographic characteristics, causes of ICU admission, factors influencing feeding administration or feeding prescription but spent more time sedated (Table 3). Logistic regression analysis, performed with factors influencing ICU outcome, identified mean energy deficit as independently associated with ICU death (Table 4). By the Mantel–Haenszel test, there was an increasing trend in ICU mortality across the quartiles of mean energy deficit estimated in the first 14 d of mechanical ventilation (Fig. 3 (A)). We used the best operating point of the receiver operating characteristics curve (Fig. 3 (B)) of mean energy deficit to determine a threshold of 5021 kJ (1200 kcal) per d of mechanical ventilation for predicting ICU death after the fourteenth ICU day (sensitivity 80 %, specificity 65 %, OR 6·12 (95 % CI 1·33, 28·2), positive likelihood ratio 2·28). Twenty-five patients had a mean energy deficit ≧5021 kJ (1200 kcal) per d of mechanical ventilation and they had a higher ICU mortality rate than patients with lower mean energy deficit (n 13) after the fourteenth ICU day (Fig. 4).
Table 3 Characteristics and conditions limiting feeding administration or feeding prescription in the first 14 d of respiratory support in intensive care unit (ICU) deaths and in ICU survivors who experienced prolonged acute mechanical ventilation‡
(Mean values with their standard errors)
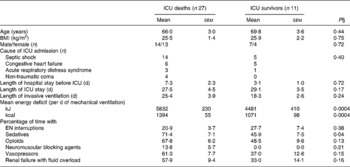
EN, enteral nutrition; SAPS II, Simplified Acute Physiology Score II.
‡ For details of subjects and procedures, see the Subjects and methods section and Table 1.
§ Statistical analysis was by Mann–Whitney test for quantitative variables and by Fisher's test for categorical variables (Male/female and Cause of ICU admission).
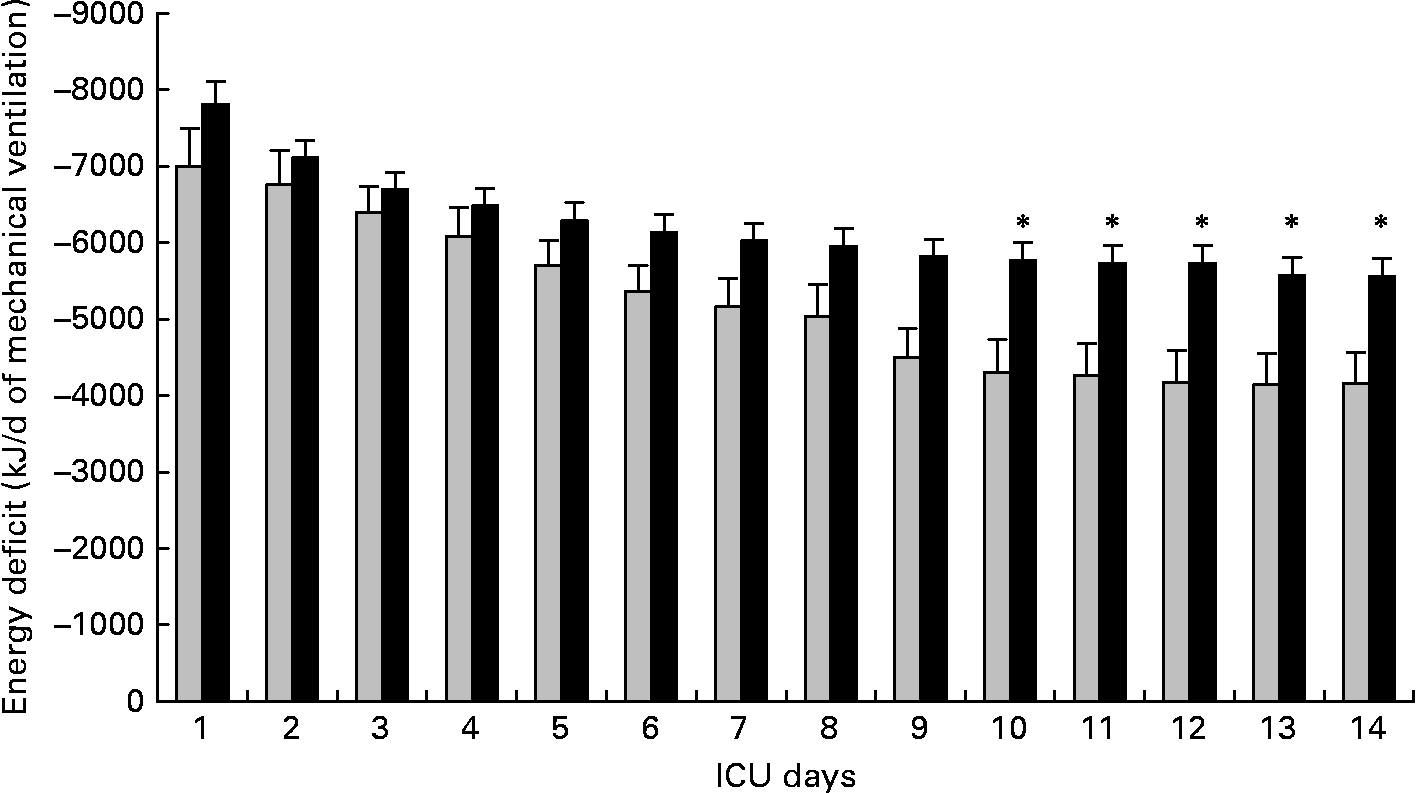
Fig. 2 Evolution of daily energy deficit of intensive care unit (ICU) survivors (░; n 11) and of ICU deaths (■; n 27) in patients requiring prolonged acute mechanical ventilation. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of the ICU survivors: *P < 0·05.
Table 4 Logistic model coefficients table for intensive care unit (ICU) death in patients requiring prolonged acute mechanical ventilation (multivariate analysis)‡
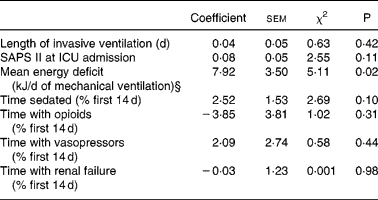
SAPS II, Simplified Acute Physiology Score II.
‡ For details of subjects and procedures, see the Subjects and methods section and Table 1.
§ Mean energy deficit was independently associated with ICU death in patients requiring prolonged acute mechanical ventilation.

Fig. 3 (A), Relationship between intensive care unit (ICU) mortality and quartiles of mean energy deficit in the first 14 d in patients requiring prolonged acute mechanical ventilation. Values are percentages with 95 % CI depicted by vertical bars. OR, comparison v. the first quartile. Values were significantly different from that of the first quartile (Mantel–Haenszel test): P = 0·003. (B), Receiver operating characteristics (ROC) curve of mean energy deficit for ICU mortality. The area under the ROC curve is 0·80 (sem 0·08). ← , 5021 kJ (1200 kcal) per d.
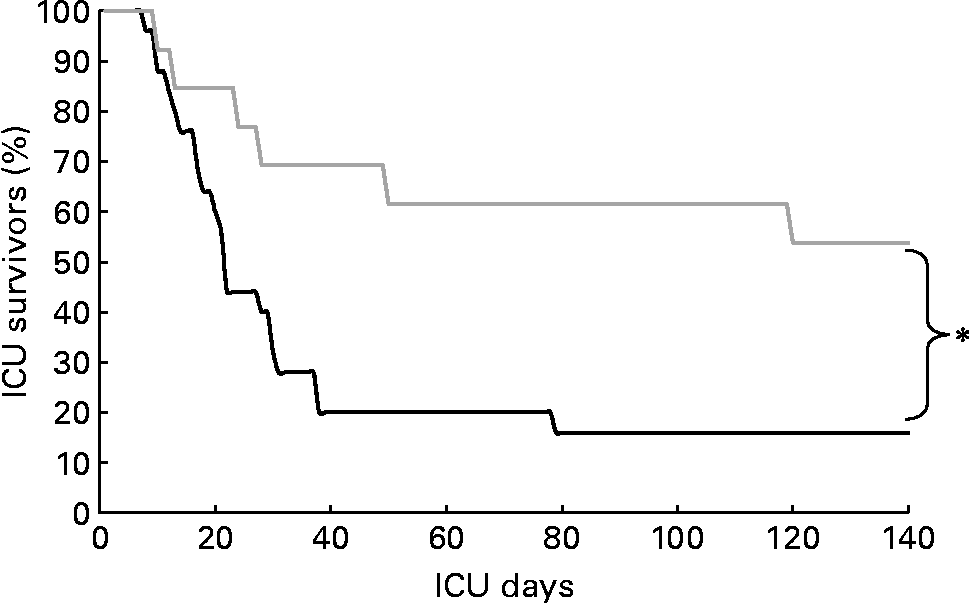
Fig. 4 Kaplan–Meier analysis of intensive care unit (ICU) survival rate in patients with mean energy deficit ≧5021 kJ (1200 kcal)/d of mechanical ventilation (—; n 25) and with mean energy deficit < 5021 kJ (1200 kcal)/d of mechanical ventilation (![]() ; n 13). *Values were significantly different (P = 0·01; log-rank test).
; n 13). *Values were significantly different (P = 0·01; log-rank test).
Discussion
The present study shows that negative energy balance, calculated with a predictive estimation of REE based on static and dynamic biometric parameters, is associated with ICU mortality in the most severely ill medical patients requiring acute prolonged mechanical ventilation and receiving exclusive EN. Our predictive method was applicable to the ICU patients measured in the current study and the present results are in agreement with recent works performed with calorimetric devices confirming the usefulness of our predictive equation when indirect calorimetry is not available or inapplicable(Reference Petros and Engelmann29, Reference Dvir, Cohen and Singer32). However, the magnitude of the deficit between estimated energy requirements and measured energy intakes was unexpected considering the procedures used to enable adequate feeding. The present result may be explained, at least in part, by the inclusion of older and very seriously ill patients with a high incidence of clinical situations pre-disposing to gastroparesis or interruption of feeding. Moreover, the retrospective observational design of the present study, by limiting the Hawthorne effect, underlines the gap between evidence and practice (‘the true life’) for EN support in critically ill patients(Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21, Reference Campbell, Maxey and Watson33, Reference Heyland, Dhaliwal, Day, Jain and Drover41).
Also, unexpectedly, we found that estimated REE remained stable during the first 14 d of mechanical ventilation instead of a hypometabolic ‘ebb’ phase during the first 24–48 h, followed by ‘flow’ phase(Reference Cuthbertson42). Other investigators described similar classic evolution in critically ill subjects by using indirect calorimetry to estimate REE but the limitations of this method in unstable patients were not considered(Reference Uehara, Plank and Hill19, Reference Faisy, Guerot, Diehl, Labrousse and Fagon31). Nevertheless, methodological biases in previous studies do not appear sufficient to explain the absence of significant change in REE in the present study. It is likely that many patients were already in the ‘flow’ phase by the time they were admitted to our ICU and the modern fluid resuscitation would shorten the period of hypotension resulting in a short ‘ebb’ phase. In addition, our patients were frequently infected and/or shocked at ICU admission and metabolic alterations in sepsis (elevated REE) associated with metabolic overstimulation caused by inotropic-vasopressor agents could also explain the absence of early hypometabolism, increasing energy deficit in the present study(Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Trager, DeBacker and Radermacher15, Reference Faisy, Guerot, Diehl, Labrousse and Fagon31).
Recent studies of energy deficit in severely ill patients estimated the prescribed/REE energy ratio close to 70 % with relatively great variability(Reference Plank and Hill2, Reference Reid5, Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21, Reference Berger, Revelly, Cayeux and Chiolero24, Reference Petros and Engelmann29, Reference Kyle, Genton, Heidegger, Maisonneuve, Karsegard, Huber, Mensi, Romand, Jolliet and Pichard43) compared to 40 % after the third day in the current study. The difference between the intakes recommended by French guidelines and our REE estimation using the Faisy 2003 equation possibly suggests that the former are not adapted for the patients studied. Moreover, the energy deficit compared to the Faisy equation is large, suggesting that protocols for ensuring adequate EN are not successful. The causes of delayed or underdelivered EN are well known: gastro-intestinal intolerance enhanced by a high rate of splanchnic O2 extraction in patients with shock or cardiac failure(Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Berger, Revelly, Cayeux and Chiolero24); drugs such as sedatives and vasopressors resulting in gastroparesis(Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Ravasco and Camilo40); various diagnostic or therapeutic procedures, especially in mechanically ventilated patients(Reference Rice, Swope, Bozema and Wheeler17, Reference Berger, Revelly, Cayeux and Chiolero24, Reference Petros and Engelmann29, Reference Kyle, Genton, Heidegger, Maisonneuve, Karsegard, Huber, Mensi, Romand, Jolliet and Pichard43); lack of medical and paramedical management of feeding, particularly the delays in restarting EN after procedures(Reference Reid5, Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference O'Leary-Kelley, Puntillo, Barr, Stotts and Douglas18, Reference Adam and Batson28, Reference Kyle, Genton, Heidegger, Maisonneuve, Karsegard, Huber, Mensi, Romand, Jolliet and Pichard43, Reference Marshall and West44). Our acute mechanically ventilated patients frequently had compromised haemodynamic status at ICU admission and most of them received prolonged infusion of vasopressors and sedatives. These conditions were also present in other studies but without the large energy deficit found in the current study(Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference De Jonghe, Appere-De-Vechi, Fournier, Tran, Merrer, Melchior and Houtin21, Reference Berger, Revelly, Cayeux and Chiolero24). One explanation may be the high incidence of renal failure in our patients necessitating limitation of feed volume to limit fluid overload. Moreover, similar to previous studies, 71 % of the energy prescribed was delivered suggesting that energy deficit was not mostly caused by nursing practices(Reference Reid5, Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Berger, Chiolero, Pannatier, Cayeux and Tappy16, Reference Dock-Nascimento, Tavares and de Aguilar-Nascimento27, Reference Petros and Engelmann29, Reference Marshall and West44). Additionally, the duration of EN interruptions for procedures or severe gastrointestinal intolerance was close to 23 % of the total time of survey and explained most of the differences between delivered and prescribed energy. We did not routinely use post-pyloric feeding, prokinetic drugs administration or measurement of gastric residual volumes to manage poor gastric emptying as their use is controversial and can involve premature cessation or delayed EN(Reference Reid5Reference Barr, Hecht, Flavin, Khorana and Gould7Reference Heyland, Dhaliwal, Day, Jain and Drover41, Reference Davies, Froomes, French, Bellomo, Gutteridge, Nyulasi, Walker and Sewel45–Reference Montejo, Grau and Acosta47). We merely raised the patient's head and shoulders(Reference Marshall and West44, Reference Adam48), questioning this practice. We conclude that underestimation of energy requirements, delay in starting or increasing EN, and overprecautionary interruptions in EN resulted in underfeeding in our ICU patients(Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Kyle, Genton, Heidegger, Maisonneuve, Karsegard, Huber, Mensi, Romand, Jolliet and Pichard43, Reference Marshall and West44). In addition, herein, we found that the percentage of time with situations limiting feed administration was higher than periods with EN interruptions, suggesting the inappropriate medical practices were the major cause of the large negative energy balance reported in the present study. Contrary to recent prospective studies, our survey suggests that implementation of a standardized nutritional management protocol in ICU does not prevent such conservative prescribing patterns(Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Heyland, Dhaliwal, Day, Jain and Drover41). The lack of consensus concerning the energy requirement for critically ill patients and the uncertainty about the safety of early EN in mechanically ventilated patients may explain this conservative approach(Reference Reid5, Reference Barr, Hecht, Flavin, Khorana and Gould7, Reference Ibrahim, Mehringer, Prentice, Sherman, Schaiff, Fraser and Kollef26, Reference Dock-Nascimento, Tavares and de Aguilar-Nascimento27, Reference Kyle, Genton, Heidegger, Maisonneuve, Karsegard, Huber, Mensi, Romand, Jolliet and Pichard43). However, the present study indicates that the gap between recommended nutrition care and practice regarding enteral feeding still exists and it results from lack of knowledge and interest of the importance of nutritional assessment by nurses and doctors(Reference Lennard-Jones, Arrowsmith, Davison, Denham and Micklewright49–Reference Lindorff-Larsen, Hojgaard Rasmussen, Kondrup, Staun and Ladefoged51). Confusion between medical and nursing staff over responsibility for EN probably plays a part in underestimation of energy requirements in ICU(Reference Adam and Webb52). Importantly, no qualified dietitian was regularly present in our ICU while recent surveys emphasized that doctors and nurses supported by clinical dietitians had better focus on clinical nutrition, especially in daily ICU practice(Reference Fulbrook, Bongers and Albarran53, Reference Thorensen, Rothenberg, Beck and Irtun54). Taken together, the present findings highlight the causes of the discrepancy between recommended and prescribed or delivered energy in our severe ICU patients. Moreover, they also underline the limitations of written standardized nutritional protocols and the relevance of a nutritional support team for improving energy balance of the most severely injured patients receiving EN.
We showed that negative energy balance during the first 14 d in the ICU is an independent factor of ICU mortality in patients requiring acute prolonged mechanical ventilation. Moreover, the present results confirm recent findings indicating that length of acute mechanical ventilation does not influence ICU discharge(Reference Zilberberg, Luippold, Sulsky and Shorr34). It has been shown that early energy deficit is strongly correlated with infectious complications and organ failures in surgical and medical ICU patients(Reference Villet, Chiolero, Bollmann, Revelly, Cayeux, Delarue and Berger4, Reference Berger, Revelly, Cayeux and Chiolero24, Reference Dvir, Cohen and Singer30). However, correlations do not mean causality and the relationships between ICU mortality and energy deficit were not clearly evidenced in these studies. Interestingly, in the present study the patients who died, unlike survivors, continued to have a large negative energy balance ≥ 5021 kJ (1200 kcal) per d after the first week of mechanical ventilation. Taken together, the present findings suggest that energy deficit contributes to ICU mortality and that this deficit results from persistent hypermetabolism combined with inadequate nutrient intake for >1 week of mechanical ventilation. This highlights the possibility that ICU mortality could be reduced by ensuring the energy deficit is less than 5021 kJ (1200 kcal) per day for the first 2 weeks of mechanical ventilation. Alternative routes of feeding (post-pyloric or parenteral) should be considered if EN via a nasogastric tube failed to achieve this goal. Furthermore, assessment of body composition could be a useful means to detect and prevent the effects of underfeeding in ICU patients(Reference Faisy, Rabbat, Kouchakji and Laaban55).
Further investigation to confirm the present findings is required before extrapolating the present results beyond our patient population and procedures of enteral feeding. However, it is important to keep in mind that energy deficit may impact on outcome mainly in the most severely ill ICU patients and progress in nutritional assessment of this ‘targeted’ population could improve significantly their ICU survival. The present results support this hypothesis confirming the significant impact of negative energy balance on outcome and highlighting the causes of underfeeding in the very sick medical ICU population. We expect the present results could contribute to develop better focus on nutrition assessment practices in the most severely injured patients for whom the impact of negative energy balance is clinically relevant. Our nutrition practices were modified on the basis of the present study, especially special attention for the most severely ill patients with prolonged acute mechanical ventilation.
In summary, the present study shows that large, negative energy balance occurs during acute prolonged mechanical ventilation even in the presence of standardized nutritional management protocol. Energy deficit seems to be an independent determinant of ICU mortality and its causes, under prescription and interruption of feeding, must be addressed through an educational policy. Specifically, feed prescription based on accurate REE estimation appears to be a prerequisite for improving nutritional management of ICU patients. In this way, the use of our simple predictive estimation of REE based on static and dynamic biometric parameters could be an alternative approach when indirect calorimetry is not available or inapplicable.
Acknowledgements
The authors appreciate the diligent revision of this manuscript by Dr Stephen J. Taylor, from the Department of Nutrition and Dietetics, Frenchay Hospital, Bristol, UK. No author has any financial or personal interest in any company or organization relevant to the field of research in connection with this work. The funding source was Service de Réanimation Médicale, Hôpital Européen Georges Pompidou, Paris, France. This study was not sponsored by gifts or fellowships. C. F. and F. D. were responsible for the conception and design of the study, data collection and analysis, and drafting the report. N. L. and J.-F. S. were responsible for interpretation of the data and drafting the report. I. A., J.-M. T. and J.-Y. F. were responsible for revising the report.










