The intestinal microbiota plays a pivotal role in human health by preventing pathogen colonisation, and shaping and maintaining normal mucosal immunity(Reference Candela, Perna and Carnevali1). To preserve this beneficial relationship, the immune system should remain hyporesponsive to commensal bacteria (mucosal tolerance)(Reference Duerkop, Vaishnava and Hooper2, Reference Honda and Takeda3), but at the same time, it has to combat pathogenic bacteria(Reference Honda and Takeda3). The breakdown of the delicate balance of the intestinal immune responses causes the development of disease states with bowel inflammation(Reference Honda and Takeda3). In this context, intestinal epithelial cells (IEC) play an important role in immune homeostasis(Reference Zeuthen, Fink and Frokiaer4, Reference O'Hara, O'Regan and Fanning5). IEC are thought to contribute to immunomodulation of mucosal leucocytes by at least two different mechanisms(Reference Parlesak, Haller and Brinz6), by acting as a physical barrier between gut luminal content (including bacteria) and the underlying immune cells, and by transmitting signals coming from the intestinal content and microbiota to the resident mucosal immune system(Reference Zeuthen, Fink and Frokiaer4). IEC secrete many mediators involved in protective responses against potentially pathogenic organisms, such as defensins, mucins, chemokines and cytokines(Reference O'Hara, O'Regan and Fanning5).
Bifidobacteria, which are important components of the human intestinal microbiota particularly of breast-fed (BF) infants(Reference He, Ouwehand and Isolauri7), have shown the capacity to modulate cytokine production by IEC, monocyte-derived dendritic cells and peripheral blood mononuclear cells (PBMC) in in vitro experiments(Reference Candela, Perna and Carnevali1, Reference Latvala, Pietila and Veckman8, Reference Niers, Timmerman and Rijkers9). In addition, the differences observed in the composition of bifidobacterial species of the intestinal microbiota of BF and formula-fed (FF) infants have been suggested to influence the incidence of immune-mediated diseases(Reference Haarman and Knol10, Reference Mountzouris, McCartney and Gibson11). These findings have led to the proposal to use some Bifidobacterium strains as potential probiotics in the prevention and treatment of pathologies with underlying immune alterations, such as inflammatory bowel diseases, allergy and coeliac disease(Reference Furrie, Macfarlane and Kennedy12–Reference Sanz, Sánchez, De Palma, Overton and Ewente14).
Following all of the aforementioned facts and hypothesis, the objective of the present study was to investigate the effect of strains of different bifidobacterial species (Bifidobacterium adolescentis, B. angulatum, B. breve, B. catenulatum, B. infantis and B. longum) and their mixtures, corresponding to the typical microbiota present in the faeces from BF and FF children, on the modulation of cytokine production by IEC and PBMC in an in vitro co-culture system, simulating the intestinal environment.
Materials and methods
Bacteria
The following strains of six different Bifidobacterium species were individually evaluated: B. adolescentis ATCC 15703; B. angulatum ATCC 27535; B. breve ATCC 15700; B. catenulatum LMG 11043; B. longum biovar infantis LMG 11046T; B. longum biovar longum ATCC 15707. In addition, two combinations of these bifidobacteria were also used to simulate the percentage of each species in the microbiota from BF and FF infants(Reference Haarman and Knol10). The BF mixture included B. infantis (59·0 %), B. breve (21·6 %), B. longum (13·5 %), B. catenulatum (3·5 %), B. angulatum (1·8 %) and B. adolescentis (0·6 %); the FF mixture included B. infantis (62·1 %), B. catenulatum (14·8 %), B. longum (10·9 %), B. breve (7·2 %) and B. adolescentis (5·0 %) (no B. angulatum).
Bifidobacteria were grown routinely in de Man Rogosa and Sharpe agar (Scharlau Chemie SA, Barcelona, Spain) with 0·05 % cysteine broth and incubated at 37°C under anaerobic conditions (AnaeroGen, Oxoid, Basingstoke, UK) for 22 h. Cells were harvested by centrifugation (6000 g for 15 min) until the stationary growth phase, washed two times in PBS (130 mm-NaCl, 10 mm-sodium phosphate, pH 7·4, and resuspended in PBS containing 20 % glycerol). Aliquots of these suspensions were frozen in liquid N2 and stored at − 80°C until used. The number of live cells after storage was determined by colony-forming unit counting on de Man Rogosa and Sharpe-cysteine after 48 h incubation in optimal conditions. For all strains tested, >90 % of cells were alive upon thawing. For every new experiment, one fresh aliquot was thawed to avoid variability in cultures between the experiments.
Leucocyte isolation and bacterial stimulation of peripheral blood mononuclear cells
Human PBMC from seven healthy volunteers were isolated from heparinised blood samples using standard Ficoll gradient centrifugation (lymphocyte isolation solution; Rafer, Zaragoza, Spain). The isolated PBMC were washed twice with Roswell Park Memorial Institute 1640 medium (Bio-Whittaker, Verviers, Belgium) and suspended in the same medium, supplemented with heat-inactivated fetal bovine serum (100 ml/l; Bio-Whittaker), after decomplementation, and containing 1 % penicillin–streptomycin (5000 IU/ml (3 mg/ml), 5000 mg/ml; Bio-Whittaker). The PBMC suspension was adjusted to 2 × 106 cells/ml, and 1 × 106 cells were used per well in all experiments.
Live bacterial cell suspensions of each individual Bifidobacterium strain or the combinations representing the faecal microbiota composition of BF and FF infants were washed in the culture medium and incubated at a final concentration of 107 colony-forming units/ml with PBMC (proportion bacteria:PBMC, 10:1)(Reference Hua, Lin and Lai15, Reference Ghadimi, Fölster-Holst and de Vrese16) during 48 h (5 % CO2 and 37°C). The supernatant was collected, centrifuged and frozen in aliquots at − 80°C until cytokine analysis.
Co-culture of Caco-2/peripheral blood mononuclear cells and bacterial stimulation
The colonic adenocarcinoma cell line Caco-2 (ECACC no. 86 010 202, Salisbury, Wiltshire, UK) was cultured at 37°C and 5 % CO2 in Eagle's minimal essential medium (Bio-Whittaker) supplemented with 10 % fetal bovine serum (Bio-Whittaker), 1 % non-essential amino acid solution (Bio-Whittaker), 1 % l-glutamine (Bio-Whittaker) and 1 % penicillin–streptomycin (Bio-Whittaker). Caco-2 cells were seeded at a density of 8 × 104 cells/well in standard twenty-four-well culture plates, and at 4 × 104 cells/well on 12 mm inserts in twenty-four-well cell culture plate assemblies (Millipore, Madrid, Spain) with a semipermeable polyethylene terephthalate membrane (1 μm in pore size). During cell growth and differentiation, the medium was changed every 2 or 3 d. Once the cells were confluent and differentiated, the experiments were performed 10–11 d after seeding. Confluence was followed by microscopic visualisation and transepithelial resistance measurements (Millicell ERS Ohmmeter; Millipore).
Co-cultures of the bifidobacteria with Caco-2 cells and PBMC from healthy donors were performed in seven different experiments. To that end, a transwell cell culture system was used as described earlier. Caco-2 monolayers were challenged by apical addition of 2 × 106 colony-forming units/insert of a Bifidobacterium strain or a combination of strains corresponding to the species composition in the faecal samples from BF and FF infants. The PBMC suspension (500 μl) was added at a concentration of 2 × 106 cells/ml in the basal compartment of the culture well for a 12 h incubation. Thereafter, further 36 h incubation was allowed after disassembly of the system. In order to measure the cytokine production by the sensitised Caco-2 and PBMC separately, the basolateral compartment of Caco-2 cells was replenished with a fresh culture medium. After the incubation period, culture media, from both the separated PBMC and Caco-2 cell plates, were collected and frozen in aliquots at − 80°C. The PBMC supernatant was centrifuged before freezing to avoid the presence of cells in aliquots.
In two different wells, two more conditions, which served as a control of the Caco-2 cell conditioning by the underlying PBMC, were carried out: the BF and FF mixtures were added to the Caco-2 monolayers in transwells with no PBMC in the basal compartment.
Cytokine quantification in culture supernatants
TNF-α, IL-1β, IL-10, IL-8 and IL-6 cytokines were measured in the basolateral medium with Caco-2 cells, and TNF-α, interferon-γ (IFN-γ), IL-6, IL-10, IL-2 and IL-4 were measured in the PBMC supernatant. All cytokine measurements were performed using a cytometric bead array system (Inflammation Kit and either a Th1/Th2 kit or a Flex set; BD Biosciences, San Agustín de Guadalix, Madrid, Spain), according to the manufacturer's protocols, and analysed by flow cytometry (FACScalibur; BD Biosciences). Data were analysed using Cellquest software (BD Biosciences). The cytometric bead array limit of detection for each cytokine was as follows: IFN-γ, 7·1 pg/ml; TNF-α, 2·8 pg/ml; IL-10, 0·13 pg/ml; IL-6, 1·6 pg/ml; IL-8, 1·2 pg/ml; IL-4, 2·6 pg/ml; IL-2, 2·6 pg/ml; IL-1β, 7·2 pg/ml. IFN-γ was also measured with high-sensitivity Immunoassay xMAP Technology (Millipore) in a Luminex 100 equipment, with a sensitivity of 0·29 pg/ml.
Statistical analyses
Statistical analyses were performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). To establish the homogeneity of variances and the distribution of the data, the Levene test was run. As a result of the non-normal distribution of the data and the non-homogeneity of the variances, the Mann–Whitney U test was used to assess the effect of every experimental condition compared with the other conditions. Data are expressed as medians and quartiles. Significant differences were established at P < 0·05. Correlations between different bacterial stimulatory conditions were analysed by Spearman's correlation test and considered significant at a P level < 0·05.
Ethical approval
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethics Committees of the Hospital Puerta de Hierro (Madrid, Spain) and CSIC organisation. Written informed consent was obtained from all subjects/patients.
Results
Cytokine production by peripheral blood mononuclear cells cultured with bifidobacteria
In order to determine the immunological effect of bifidobacteria on PBMC, the production of IFN-γ, TNF-α, IL-10, IL-6, IL-4 and IL-2 was measured in the supernatants of PBMC cultured in direct contact with the different Bifidobacterium strains (individually or mixed). Among all cytokines analysed, only IL-2 was not stimulated (Fig. 1(A)), with levels below 20 pg/ml (except for the positive control with phytohaemaglutinin; data not shown). All the other cytokines were significantly stimulated by all bifidobacterium species and their mixtures (compared with the control with only medium).
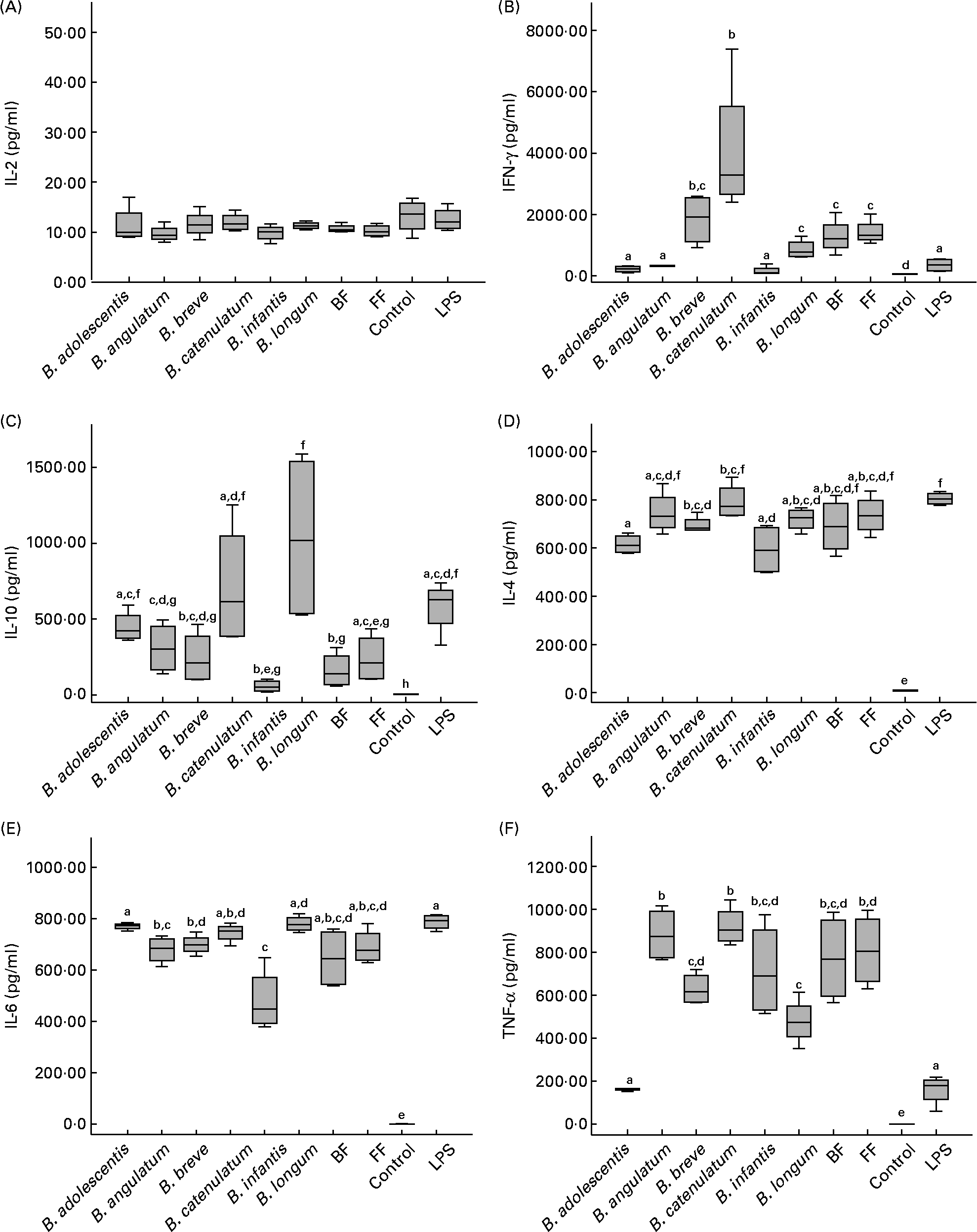
Fig. 1 Cytokine production by peripheral blood mononuclear cells after 48 h incubation with individual bifidobacterium strains and their mixtures (breast-fed (BF) and formula-fed (FF)) in a 10:1 (bacteria:cell) ratio. Each box represents median (50th percentile) and interquartile range (25th and 75th percentiles). a,b,c,d,e,f,g Mean values with unlike letters were significantly different (P < 0·05; Mann–Whitney U test). No differences were observed in IL-2 production between the conditions. (A) IL-2; (B) interferon-γ (IFN-γ); (C) IL-10; (D) IL-4; (E) IL-6; (F) TNF-α. LPS, lipopolysaccharide.
Regarding IFN-γ production (Fig. 1(B)), B. catenulatum and B. breve were the strongest enhancers, followed by the FF and BF mixtures (no significant differences were found between the two mixtures). B. catenulatum induced a higher IFN-γ production than all the other stimuli (except for B. breve). B. breve induced a higher IFN-γ production than B. adolescentis, B. angulatum and B. infantis, but similar to that induced by B. catenulatum, B. longum and the mixtures. The total percentage of B. catenulatum and B. breve was similar in the FF and BF mixture (22·05 and 25·12 %, respectively). This might explain why the levels of IFN-γ produced by PBMC stimulated with the FF and BF mixtures were not statistically different.
B. longum and B. catenulatum induced the highest IL-10 production by PBMC, showing significant differences in IL-10 production in the presence of B. infantis and the BF mixture (Fig. 1(C)). IL-10 production induced by B. longum was also significantly higher than that induced by B. angulatum, B. breve and the FF mixture. The percentages of B. longum in the FF and BF mixtures were very similar (10·87 v. 13·52 %), but B. catenulatum was approximately four times higher in the FF mixture than in the BF mixture (14·84 v. 3·50 %). The low proportion of B. catenulatum and B. adolescentis, together with the high proportion of B. infantis and B. breve in the BF mixture, might explain the significantly lower production of IL-10 induced by the BF mixture compared with that induced by B. adolescentis, B. catenulatum and B. longum individually (Fig. 1(C)). Regarding IL-4, B. catenulatum also induced a significantly higher production than B. adolescentis and B. infantis (Fig. 1(D)).
All Bifidobacterium strains stimulated PBMC to produce very high levels of IL-6, over 4000 pg/ml (Fig. 1(E)). B. adolescentis induced the highest IL-6 production, significantly higher than B. angulatum, B. breve and B. infantis (P = 0·029 in every case). B. infantis induced the lowest effect among the assayed strains on cytokine production, not only for IL-6, but also for IFN-γ, IL-10 and IL-4.
With the exception of B. adolescentis, all Bifidobacterium strains also stimulated PBMC to produce very high levels of TNF-α (Fig. 1(F)). A significantly higher TNF-α production was induced by B. angulatum and B. catenulatum compared with B. adolescentis, B. breve and B. longum. While B. longum and B. adolescentis induced a high production of IL-10, they both mildly induced TNF-α production (Fig. 1(C) and (F)). On the other hand, while B. infantis and B. angulatum induced a mild production of IL-10, they both highly induced TNF-α production (Fig. 1(C) and (F)).
Cytokine production by peripheral blood mononuclear cells in co-culture with Caco-2 cells and bifidobacteria
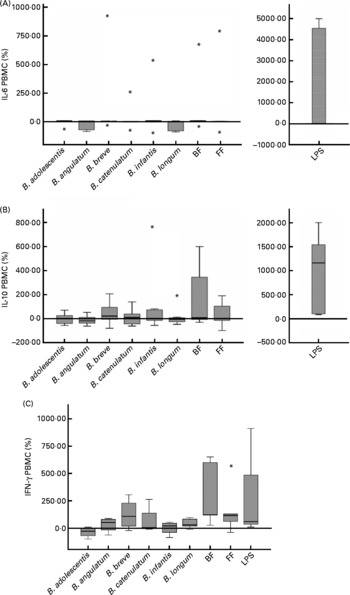
To analyse cytokine production by PBMC conditioned by previous co-culture with Caco-2 cells stimulated with bifidobacteria, IFN-γ, TNF-α, IL-10, IL-6, IL-4 and IL-2 were measured in PBMC supernatants. IL-2 and IL-4 were not detectable, and TNF-α was also below the limit or approaching the limit of detection (data not shown). No significant differences in IL-10 and IL-6 production were found, either between different bifidobacteria alone or in mixtures (Fig. 2(A) and (B)).

Fig. 2 Cytokine production in a 48 h culture of peripheral blood mononuclear cells (PBMC) sensitised by a 12 h incubation in a transwell co-culture system with Caco-2 cells apically stimulated with bifidobacteria. Values are given as percentage of the control (spontaneous production with no added bacteria). Each box represents median (50th percentile) and interquartile range (25th and 75th percentiles). Asterisks represent outliers. No differences were observed in IL-10 and IL-6 production between the different bifidobacterium conditions employed; however, lipopolysaccharide (LPS)-stimulated production was always significantly higher than the rest of the conditions. (A) IL-6 PBMC; (B) IL-10 PBMC; (C) interferon-γ PBMC. BF, breast fed; FF, formula fed.
The production of IFN-γ by PBMC was low in this system (range 1–93 pg/ml and under the detection limit in two of the seven PBMC donors). Using the available data from the other five donors, we found induction of IFN-γ production by the BF mixture in four of them (>100 % v. control) and in three of them also with B. breve (>50 % v. control), which is singularly high in the BF combination. Moreover, three donors showed stimulation with the FF mixture (>100 % v. control). The BF mixture was the stimulus that induced the highest IFN-γ production (Fig. 2(C)), significantly higher than B. adolescentis (P = 0·014), B. infantis (P = 0·050) and B. longum (P = 0·047) individually. Although B. breve also induced the production of IFN-γ, this effect was not significantly different from the other bifidobacteria (Fig. 2(C)). The effect of B. adolescentis on IFN-γ induction was inhibitory relative to the control condition and was significantly different from the stimulatory effect observed with the bifidobacteria mixtures and B. longum (Fig. 2(C)).
Cytokine production by Caco-2 cells in co-culture with peripheral blood mononuclear cells and bifidobacteria
To assess the effects of bifidobacteria and bifidobacteria mixture stimulation on Caco-2 cells in co-culture with PBMC, TNF-α, IL-1β, IL-10, IL-8 and IL-6 cytokines were measured in both apical and basolateral media. All cytokines were not detectable in the apical medium, while in the basolateral medium only IL-8 and IL-6 were in a measurable concentration range (IL-8, 120–14 000 pg/ml; IL-6, 30–600 pg/ml). When Caco-2 cells were stimulated with the bifidobacteria alone, with no PBMC in the underlying compartment, the stimulation of both cytokines was three to four times lower than in the co-culture system (data not shown).
When in co-culture with PBMC, B. breve highly stimulated the production of IL-6 and IL-8 on Caco-2 cells (66·8 and 45·5 %, respectively; Fig. 3(A) and (B)). For IL-8, this production was significantly higher, compared with B. adolescentis (P = 0·035), B. infantis (P = 0·025) and the FF mixture (P = 0·013) (Fig. 3(B)). Although the BF mixture also induced IL-6 and IL-8 production (36·0 and 20·7 %, respectively), these values were not significantly higher than those induced by the FF mixture (Fig. 3(A) and (B)). No significant differences were observed for IL-6 production between the different stimuli assayed (Fig. 3(A)).
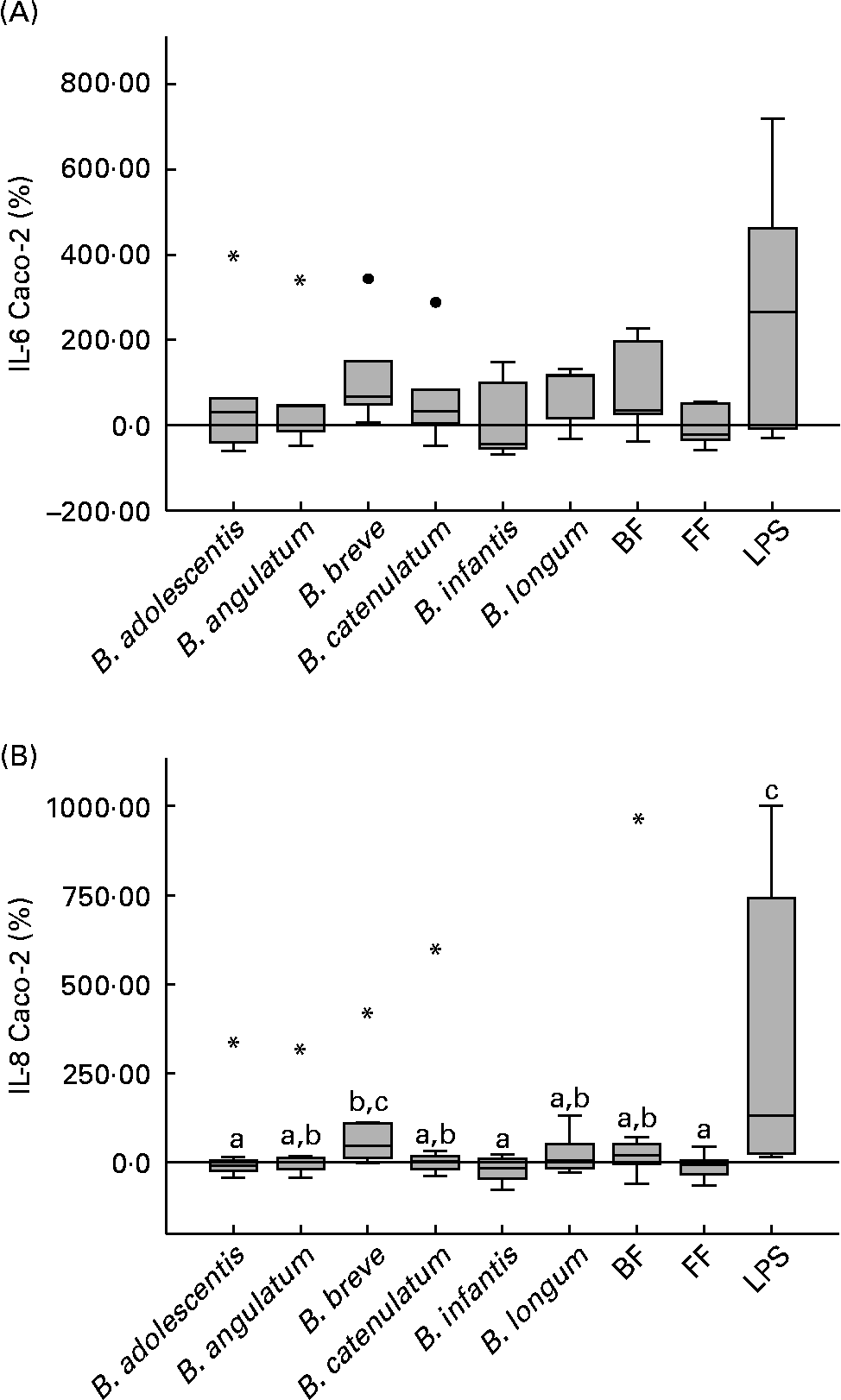
Fig. 3 Bifidobacteria-stimulated cytokine production by Caco-2 cells in a 36 h culture (basolateral medium) following prior 12 h sensitisation with peripheral blood mononuclear cells in a transwell co-culture system. Values are given as percentage of the control (spontaneous production with no added bacteria). Each box represents median (50th percentile) and interquartile range (25th and 75th percentiles). Asterisks and dots represent outliers and extreme values, respectively. a,b,c Mean values with unlike letters were significantly different (P < 0·05; Mann–Whitney U test). No differences were observed in IL-6 production between the conditions. (A) IL-6 Caco-2; (B) IL-8 Caco-2. BF, breast fed; FF, formula fed; LPS, lipopolysaccharide.
Considering the PBMC donors individually, IL-8 and IL-6 production stimulated by the FF mixture was positively and significantly correlated with IL-8 and IL-6 production stimulated by B. infantis (P < 0·001 for both cytokines). On the other hand, IL-8 production stimulated by the BF mixture was correlated with B. angulatum, B. breve and B. catenulatum (P < 0·05), and IL-6 stimulated by the BF mixture correlated with B. adolescentis and B. catenulatum (P < 0·05).
Discussion
Bifidobacterium strains have shown the capacity to modulate cytokine production by IEC, monocyte-derived dendritic cells and PBMC in in vitro experiments(Reference Candela, Perna and Carnevali1, Reference Latvala, Pietila and Veckman8, Reference Niers, Timmerman and Rijkers9). Trying to define this immunomodulatory capacity seems relevant in order to understand their contribution to the establishment of mucosal tolerance and balanced intestinal immune responses in the early stages of life. Both these processes have been linked to the prevention of immune-mediated disorders later in life, such as allergies or inflammatory bowel disease(Reference Kelly, King and Aminov17, Reference Conroy, Shi and Walker18). Several studies have evaluated the effect of different bifidobacteria in the production of cytokines by Caco-2 cells and PBMC(Reference Parlesak, Haller and Brinz6, Reference Niers, Timmerman and Rijkers9, Reference Medina, Izquierdo and Ennahar19–Reference Haller, Bode and Hammes21), but according to our knowledge, this is the first time that the Bifidobacterium strains used in the present study have been employed in co-culture experiments, and that the mixtures in the proportions of a FF and BF infant's typical microbiota have been used to stimulate these cell types.
In the present study, the levels of several cytokines were measured in two different systems: (1) a direct stimulation of PBMC with bifidobacteria and (2) a PBMC/Caco-2 cell co-culture with bifidobacteria stimulating the top layer of Caco-2 cells, which, in turn, can interact with underlying PBMC through soluble mediators. Reciprocally, PBMC were able to influence Caco-2 cell activity as well. The profile of cytokine production by PBMC exposed directly to the Bifidobacterium strains shows relevant differences compared with the profile of cytokine production by PBMC in the co-culture system, where Caco-2 cells constitute a physical barrier preventing the access of PBMC to the bifidobacteria. The first differential finding was that the level of cytokine production was much lower in the co-culture system. For instance, while three out of six cytokines measured were above 1000 pg/ml when both bifidobacterial mixtures were used, and two out of the remaining three gave results higher than 100 pg/ml in direct contact, only IL-6 by PBMC in the co-culture system gave results higher than 1000 pg/ml. It is worth noting that while in direct contact, IL-6 and TNF-α were the cytokines most highly induced, in the co-culture system, not only IL-6 but also IL-10 was the cytokine most highly produced by PBMC. In this sense, Niers et al. showed in a single culture system that the production of IL-10 by PBMC is boosted by several Bifidobacterium strains, and this down-regulates the production of TNF-α and IL-12p70 by these cells. When they used a monoclonal antibody against IL-10, they found a huge increase in the production of these inflammatory cytokines.
Different cytokines (IL-8 and IL-6) were also stimulated on Caco-2 cells, but only when they were previously co-cultured with PBMC; no cytokine production was measured if the Caco-2 cells were cultured alone with the Bifidobacterium strains. Therefore, the presence of PBMC is an essential factor for the sensitisation of Caco-2 cells to respond to bifidobacteria, which is presumably exerted by the communication between the two cell types through soluble mediators. In the present study and other studies, Caco-2 cells alone have been found to be hyporesponsive to bifidobacteria stimulation(Reference Morita, He and Fuse22) and also to other probiotic bacteria(Reference Haller, Bode and Hammes21, Reference Morita, He and Fuse22). Moreover, since cytokine production by Caco-2 cells in the co-culture system was only detectable in the basolateral medium and not in the apical medium, it demonstrates a polarised secretion by Caco-2 cells, as have been found earlier by other authors(Reference Haller, Bode and Hammes21). In a similar co-culture system, in which Caco-2 cells were stimulated with non-pathogenic Escherichia coli and Lactobacillus sakei, an induction of TNF-α secretion into the subepithelial compartment was observed, and this cytokine was signalled as the fundamental candidate for cellular crosstalk(Reference Haller, Bode and Hammes21). In contrast, we found no detectable production of TNF-α, which might be explained by a differential effect from different bacterial species and strains.
Regarding the immunomodulatory effects of specific strains used in these experiments, the most relevant findings have been found regarding the immunostimulatory effects of B. breve. This strain stimulated most of the production of IL-8 and IL-6 on both Caco-2 cells and PBMC. In the microbiota of BF infants, B. breve is the most representative Bifidobacterium species (after B. infantis, common in all milk-fed babies), and this could explain the high IL-8 and IL-6 levels produced by Caco-2 and PBMC stimulated with the BF mixture. This link between B. breve and the BF mixture was supported by the correlation found between IL-8 levels produced by Caco-2 cells stimulated by B. breve and the BF mixture. Moreover, B. breve and the BF mixture also stimulated the production of IL-10 and INF-γ by PBMC (in co-culture with Caco-2 cells). All these observations might indicate that the proportion of different Bifidobacterium species is an important determinant of the overall contribution to the stimulation of cytokines on the intestinal mucosa. In this sense, it is interesting to note that there was a correlation between the relative inhibition of IL-8 production by Caco-2 cells induced by the FF mixture and B. infantis. It seems that the differences in the proportions of the different strains between the mixtures and the stimulatory/inhibitory capacities shown by the individual strains might explain the results found with their combinations in the BF and FF mixtures.
According to the results, B. breve induced a slight pro-inflammatory response, which could turn the mucosal immune system on stand-by and prevent the release of a severe inflammation. It has already been reported that infants from 4 to 6 months old, who daily consumed infant formula fermented with B. breve and Streptococcus thermophilus, presented less severe episodes of acute diarrhoea than the standard formula group(Reference Thibault, Aubert-Jacquin and Goulet23). Furthermore, Li et al.(Reference Li, Shimizu and Hosaka24) showed that the administration of B. breve to low-birth-weight infants was useful in promoting the colonisation by other bifidobacteria, which might contribute to the establishment of a healthier microbiota. More recently, it has been found that the administration of B. breve to pre-term infants can up-regulate transforming growth factor-β1 signalling and may possibly be beneficial in attenuating inflammatory and allergic reactions in these infants(Reference Fujii, Ohtsuka and Lee25).
In allergic models, some probiotic bifidobacteria have the capacity to suppress IL-4 production, in vitro (Reference Ghadimi, Fölster-Holst and de Vrese16) and in vivo (Reference Zhang, Chen and Zheng26). We have observed that not all bifidobacterial species induce the same IL-4 production (Fig. 1(D)), indicating different effects of the interaction between bifidobacteria and PBMC related to the species.
Regarding the stimulation of the regulatory cytokine IL-10 by PBMC after direct stimulation with B. longum, a similar finding has been previously described by Medina et al., who found that several strains of B. longum are strong inducers of IL-10 secretion on PBMC. On the other hand, the finding that B. infantis is a weak inducer of cytokine secretion after direct stimulation of both PBMC and Caco-2 cells is in agreement with prior published results that have described that B. infantis attenuates baseline IL-8 secretion in HT-29 epithelial cells(Reference O'Hara, O'Regan and Fanning5) as well as pro-inflammatory IL-17 production by murine splenocytes and dextran sodium sulphate-induced intestinal inflammation(Reference Osman, Adawi and Molin27, Reference Tanabe, Kinuta and Saito28).
In conclusion, among the Bifidobacterium species tested, B. breve seems to be the most immunostimulatory strain in a co-culture system resembling the physiological layout of different cell types in the intestinal mucosa. The presence and relative proportions of different Bifidobacterium species in the microbiota of BF and FF infants could be key factors defining the immunomodulatory effect of the gut microbiota in early life.
Acknowledgements
The present study was supported by grants AGL2007-66126-C03-01/ALI and AGL2007-66126-C03-03/ALI, from the Spanish Ministry of Science and Innovation, and grants 200570F0091, 200570F0093 and 200870I183 from the CSIC. T. P.-R. was the recipient of a personal grant from the JAE/I3P Program of CSIC (Spain). The authors have no conflicts of interest in the present study. E. N., A. M. and Y. S. designed the study; T. P.-R., E. P. and E. N. conducted most of the study; I. N. performed all the microbiological experiments; T. P.-R., J. R. M. and E. N. contributed equally to the discussion and to the writing of the manuscript; Y. S. and A. M. reviewed and contributed to the writing of the final version of the manuscript.