The digestibility of dietary protein, an indirect measure of the extent of digestion and absorption of food protein as amino acids (AA), is a key determinant of protein bioavailability(Reference Fuller and Tome1). It is thus considered as an important factor for nutritional quality assessment(2). It is important to determine protein digestibility with accuracy as it differs substantially among diets, in particular between those from developing or developed countries whereby values of 0·54–0·78 (diets from India, Guatemala or Brazil) v. 0·88–0·94 (diets from North America) have been reported(Reference Gilani, Cockell and Sepehr3). Determination of digestibility at the ileal level is now recognised as being more accurate than its determination at the faecal level(Reference Fuller and Tome1, Reference Darragh and Hodgkinson4, Reference Moughan5), due to the high metabolic activity of the hindgut microflora leading to modification of the undigested dietary AA profile. Most dietary AA are absorbed in the small intestine(Reference Krawielitzki, Zebrowska and Schadereit6, Reference Fuller and Reeds7), although it remains unclear if the colon may also absorb AA to some degree(Reference Blachier, Mariotti and Huneau8).
In humans, ileal digestibility can be determined in subjects with ileostomies(Reference Moughan, Butts and van Wijk9) or by sampling via a naso-ileal tube(Reference Bos, Airinei and Mariotti10). None of these methods, however, is suitable for routine application due to technical and economic constraints. Animal models have thus been developed to determine ileal protein digestibility for humans. While the laboratory rat has been considered as a suitable animal model(11), the pig, the digestive tract of which is more similar to that of humans both anatomically and physiologically(Reference Pond and Houpt12–Reference Moughan, Birtles and Cranwell15), may be a better model for protein digestion in humans(Reference Darragh and Hodgkinson4, Reference Moughan, Cranwell, Darragh, Souffrant and Hagemeister16, Reference Moughan17). To date, however, only a very few studies have compared protein digestibility directly between pigs and human subjects(Reference Forsum, Goranzon and Rundgren18–Reference Darragh and Moughan20), and only one of these(Reference Rowan, Moughan and Wilson19) has assessed digestibility at the ileal level.
The objective of the present study was to extend the work of Rowan et al. (Reference Rowan, Moughan and Wilson19) to further evaluate the suitability of the growing pig for predicting dietary ileal N digestibility in human subjects. Whereas the previous inter-species comparison(Reference Rowan, Moughan and Wilson19) depended upon a single mixed-protein diet, the present study aimed to expand the comparison to purified protein sources based on an expected highly digestible (casein) and an expected poorly digestible (rapeseed isolate) protein. Hydrolysed casein was also included as part of a wider study, but given the paucity of comparative species data on ileal AA and N digestibility, digestibility data for hydrolysed casein were also reported here. Ileal digesta were collected via a post-valve T-caecum (PVTC) cannula in pigs and by naso-ileal intubation in human subjects. The protein sources were 15N-labelled to allow determination of the ileal endogenous N and AA losses, thus allowing for the correction of apparent to true protein digestibility.
Materials and methods
Test meals
Similar semi-synthetic test meals (Table 1) were prepared for pigs and human subjects. The test meals contained as the sole respective source of N a uniformly 15N-labelled intact casein (meal C), a 15N-labelled casein hydrolysate (meal HC; derived from the former casein) or a 15N-labelled rapeseed protein isolate (meal R; Brassica napus L., Goéland cultivar). The N content ranged from 29·3 to 32·6 g/kg diet. 15N-labelled casein (in the form of native calcium phosphocaseinate) was extracted from 15N-labelled milk collected in cows perfused in the rumen with [15N]ammonium sulfate as detailed previously(Reference Deglaire, Moughan and Bos21). The resulting casein was freeze-dried. Its isotopic enrichment was 0·54 atom%. A sample of [15N]casein was hydrolysed with pig pancreatin as detailed previously(Reference Deglaire, Moughan and Bos21). The molecular-weight profile, determined by HPLC gel filtration(Reference Deglaire, Moughan and Bos21), indicated that 21 % of the peptides were between 1 and 5 kDa in size, and 79 % were less than 1 kDa. 15N-labelled rapeseed protein isolate was obtained from rapeseed fertilised with [15N]ammonium nitrate as described previously(Reference Bos, Airinei and Mariotti10). Its isotopic enrichment reached 1·16 atom%.
Table 1 Ingredient compositions of the test meals fed to growing pigs and adult humans
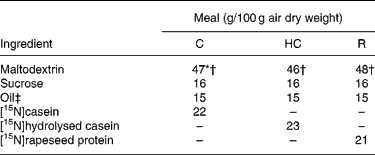
C, intact casein-based; HC, hydrolysed casein-based; R, rapeseed isolate protein-based.
* In the pig diets only, 1·8 g maltodextrin/100 g diet was replaced by sodium bicarbonate to equalise the dietary electrolyte balance between the C and HC diets. This was not done in the human diets, as the sodium bicarbonate rendered the meal unpalatable.
† In the pig diets only, 0·3 g maltodextrin/100 g diet was replaced by titanium dioxide as a dietary marker.
‡ Soyabean oil was used in the C and HC diets. Rapeseed oil was used in the R diet.
Experimental procedure: pigs
The pig experiment was conducted according to the guidelines of Massey University, New Zealand and all the experimental procedures were approved by the Massey University Animal Ethics Committee (protocol 05/29).
Eight 10-week-old Large White × Duroc entire male pigs were housed individually in steel metabolism crates in a room maintained at 24 ± 1°C.
Mean body weight (BW) at the time of surgery (day 0) was 34·4 (sd 2·0) kg. A PVTC cannula was surgically inserted into the caecum of each pig for the collection of ileal digesta(Reference van Leeuwen, van Kleef and van Kempen22). The cannulae were made of medical-grade silastic tubing (internal diameter, 24 mm; external diameter, 32 mm). The pigs were not fed for 12 h before surgery. Before the start of surgery, the pigs were given analgesics: carprofen (3 mg/kg BW; Pfizer Laboratories Ltd, Manukau, New Zealand), administered by intravenous injection, and methadone (0·2 mg/kg BW; David Bull Laboratories, Mulgrave, Victoria, Australia), administered by deep intramuscular injection. Anaesthesia was induced with an intramuscular injection of midazolam (1 mg/kg BW; Roche Products Ltd, Auckland, New Zealand) and ketamine (10 mg/kg BW; Parnell Laboratories Ltd, East Tamaki, New Zealand) followed by an intravenous injection of propofol (2 mg/kg BW; Gensia Laboratories Ltd, Irvine, CA, USA). The anaesthesia was maintained via inhalation of isoflurane (1·5 to 2 %; Merial Ltd, Auckland, New Zealand) in O2. Crystalloids were infused intravenously throughout the anaesthesia period (5–10 ml/kg BW per h) to maintain hydration. Immediately after surgery, the pigs received an intramuscular injection of antibiotic (2 ml; Duplocillin LA, Intervet international B.V., Boxmeer, The Netherlands). For the following 4 d, antibiotic powder (Mamyzim, Boehringer Ingelheim Ltd, Wiri, New Zealand) was dusted on the wound site daily. The site where the cannula was exteriorised was washed with water, and Zn ointment was applied daily throughout the experiment. The pigs regained consciousness within 1 h of surgery and were standing 7–8 h after surgery. There was a 14 d recovery period before the start of the experiment.
At day 14, the mean BW of the pigs was 39·8 (sd 2·6) kg. During the experimental period (days 14 to 37), pigs were fed at a daily level of 0·08 kg diet/kg metabolic BW (kg0·75). Except on the digesta collection day, the pigs received three meals daily (08.00, 12.00 and 16.00 hours) in equal portions. The meals were mixed with water (1:1, w/w) and water was freely available between meals. On the day of digesta collection, the pigs received (09.00 hours) the test meal (a third of the daily portion) mixed with water (2·3:1, w/w); no other food was then ingested and 200 ml water were given every 30 min until the end of the digesta collection period (19.00 hours). The pigs received the rest of their daily portion at 19.00 hours (basal meal) and water was then freely available. During the study, the pigs were weighed every sixth day and the level of food intake was adjusted accordingly.
The test meals were administered using a duplicated 4 × 4 Latin square design such that every test meal followed each other once only. A fourth test meal was included in the design but was not part of the species comparison. The pigs were randomly allocated to the Latin square and were fed their respective test meals every sixth day after having been fed a basal meal (Table 2) in the intervening 5 d periods. This was so that the feeding of the test meal was acute (one meal) to afford a similar comparison with human subjects. On the sixth day of each test period, ileal digesta were collected continuously for 10 h after the ingestion of the test meal using polythene bags attached to the cannula. The bung of the cannula was removed 2 h before the collection commenced(Reference van Leeuwen, van Kleef and van Kempen22) to allow the ileo-caecal valve to protrude into the lumen of the cannula. Digesta collection commenced 30 min before the ingestion of the test meal in order to determine the basal 15N-enrichment in digesta. The plastic bags, which contained sodium benzoate (2·3 mol/l) as a bactericide, were removed every 30 min. Digesta were immediately frozen ( − 20°C) after addition of phenylmethylsulfonyl fluoride (70 mmol/l) as an antiprotease(Reference Salgado, Freire and Mourato23). Pig digesta were freeze-dried and finely ground.
Table 2 Ingredient composition of the basal meal fed to growing pigs between the test meals
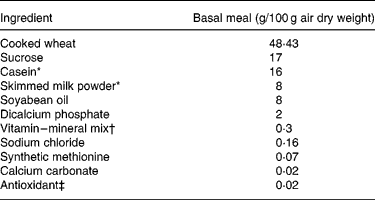
* Acid casein (NZMP, Palmerston North, New Zealand).
† Vitalean (Vitec Nutrition Ltd, Auckland, New Zealand). The mix provided (per 100 g meal): cholecalciferol, 50·0 mg; choline, 8·33 mg; niacin, 1·25 mg; pantothenic acid, 0·83 mg; vitamin A, 0·3 g; riboflavin, 0·21 mg; vitamin B6, 0·17 mg; vitamin E, 4·27 mg; vitamin K, 0·17 mg; biotin, 0·83 μg; folic acid, 41·7 μg; thiamin, 83·3 μg; vitamin B12, 0·83 μg; Cu, 10·4 mg; Fe, 8·3 mg; Mn, 3·8 mg; Zn, 10 mg; I, 83·3 μg; Co, 41·7 μg; Se, 25 μg.
‡ Ethoxyquin (Unitech Industries, Auckland, New Zealand).
Experimental procedure: human subjects
The human study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board for St-Germain-en-Laye Hospital, France. Written informed consent was obtained from all subjects.
Eighteen subjects (nine female, nine male; aged 30 (sd 8) years; mean BW 67 (sd 12) kg; mean BMI 22·5 (sd 3·5) kg/m2) were included in the study after a thorough medical examination and the conduct of routine blood tests. The volunteers were admitted to hospital for 2 d. In the morning of day 1, a 3 m polyvinyl chloride (PVC) triple-lumen tube was inserted via the nose under local anaesthesia and then swallowed by the subject so as to progress down the gastrointestinal tract. The progression of the tube was facilitated by inflating through one of the tube's lumens a balloon located at the terminal tip of the tube, after it had passed the pyloric valve. The subjects were then given a standard easily digested hospital meal and received a second meal at 19.00 hours before being food-deprived overnight. The tube was restrained from further movement once it had reached the terminal ileum, as verified under X-ray. On day 2, the protocol commenced at 09.30 hours, when a saline solution(Reference Gausserès, Mahé and Benamouzig24), containing polyethylene glycol (PEG)-4000 (20 g/l) as a liquid-phase marker for calculation of intestinal fluid flow rate(Reference Modigliani, Rambaud and Bernier25), was infused continuously through one of the tube's lumens at a constant rate of 1 ml/min. The test meal was given at 10.00 hours as a liquid drink containing 150 g test diet and 400 g deionised water. The study was performed while the subjects were resting in a semi-recumbent position and no food other than the test meal was ingested until the end of the study period. Water was given hourly. Digesta collection commenced 30 min before the ingestion of the test meal in order to determine the basal 15N-enrichment and lasted for the 8 h following the ingestion of the meal. Digesta were collected continuously on ice and pooled over 30 min periods. Digesta were immediately frozen after addition of the antiprotease di-isopropylfluorophosphate (1 mmol/l). For each pooled sample, a 4 ml sample was kept frozen ( − 20°C) for PEG-4000 determination and the remainder was freeze-dried. Subjects were given meal R in a separate part of the study which was previously undertaken using exactly the same design as described above(Reference Bos, Airinei and Mariotti10).
Chemical analysis
Pig digesta were pooled for each pig and meal type between 4 and 10 h after meal ingestion(Reference Leterme, Thewis and Francois26). Pooled digesta were analysed for TiO2, total N, AA and for 15N-enrichment of total N and single AA. Diets were analysed for TiO2.
For human subjects, the 30 min digesta samples were freeze-dried and analysed for N content and 15N-enrichment. The 30 min samples were pooled over 8 h for each meal type and subjected to the analysis of AA and their individual 15N-enrichments.
Protein sources were analysed for AA, total N and for 15N-enrichment of total N and single AA.
Total N and 15N-enrichment were measured on an isotopic ratio MS (Optima; Fisons Instruments, Manchester, UK) coupled to an elemental N analyser (NA 1500 series 2; Fisons Instruments)(Reference Gausserès, Mahé and Benamouzig24). AA were determined after acid hydrolysis for 24 h with HCl (6 mol/l containing 0·1 % phenol) using a Waters ion exchange HPLC(27). AA were not determined in digesta from animals or human subjects receiving the rapeseed protein as there was insufficient digesta from the human subjects. Cysteine, methionine and tryptophan, being destroyed during acid hydrolysis, were not determined. The 15N-enrichments of individual AA were determined by GC combustion isotope ratio MS (GC-C-IRMS, Finnigan Delta S; Thermo Fisher Scientific Inc., Bremen, Germany) as described previously(Reference Metges, Petzke and Hennig28, Reference Petzke, Feist and Fleig29). The derivative used here did not allow the determination of 15N-enrichment of arginine. TiO2 was determined using a colorimetric assay after ashing the sample and solubilisation of the minerals(Reference Short, Gorton and Wiseman30). PEG-4000 was measured using a turbidimetric method(Reference Hyden31).
Data analysis: pig data
Ileal total N flows (TNFL; g/100 g DM intake) were determined for the pooled digesta samples with reference to the dietary marker as follows:
Ileal dietary N flows (DNFL; g/100 g DM intake) were determined according to the isotope dilution as follows(Reference Hess, Ganier and Thibault32):
where Es is the 15N-enrichment in the digesta sample, Emeal is the 15N-enrichment in the meal and E0 is the baseline 15N-enrichment in digesta.
Similar equations were used to determine total and dietary AA flows.
Ileal N digestibility (%) was calculated as follows:
Similar equations were used to determine ileal AA digestibility coefficients.
Data analysis: human data
Ileal TNFL (mg per 8 h) was determined from the cumulative recovery of total N for the 8 h postprandial period using the following equation:
where Ntot-digesta-(T) is the N content of the digesta sample for the T 30 min period (mg/g), DMS-(T) is the DM of the sample (g/ml) and Ftot-(T) (ml/30 min) is the total ileal liquid flow rate over the T 30 min period. Ftot-(T) was determined as follows:
where PEGi and PEGS-(T) are the PEG concentrations in the infusion solution and in the digesta sample for the T 30 min period, respectively, Fi is the PEG infusion rate (1 ml/min) and t is the duration of the collection period (30 min).
DNFL (mg/8 h) was calculated from the cumulative recovery of undigested dietary N over the 8 h postprandial period using the following equation:

where ES-(T) is the 15N-enrichment in the digesta sample for the 30 min period.
Total AA flow (TAAFL; mg/8 h) and dietary AA flow DAAFL; mg/8 h) were determined in pooled digesta samples, reconstituted so as to be representative of the total collection, as follows:
where AAs is the AA content in the pooled digesta sample (mg/g), DM is the DM of the pooled digesta sample (g/ml) and F is the total estimated flow rate over 8 h (ml/8 h).
where Es is the 15N-enrichment of individual AA in the pooled digesta sample.
Ileal apparent and true digestibilities of N and AA were determined as described above for the growing pig.
Statistical analysis
Statistical analyses were performed using SAS (version 9.1; SAS Institute Inc., Cary, NC, USA). The dataset was first subjected to an outlier test(Reference Dixon33, Reference Dixon34) with P < 0·05 and was then analysed using the following general linear model:
where Yij is the dependent variable, μ is the general mean, αi is the fixed effect of the meal, βj is the fixed effect of the species and ɛij is the random residual error. Pearson correlation and simple linear regression analyses were performed on mean pig and human digestibility values for the meals C, HC, and where applicable for meal R. For AA, mean values for each AA were included in the correlation and regression analyses. Significance was considered to be reached at P < 0·05.
Results are mean values and standard deviations.
Results
The pigs remained healthy and grew normally throughout the study, except for one pig that was removed from the study because of internalisation of the cannula. This animal was replaced by a spare cannulated pig of a similar age and BW. Minimal leakage from the PVTC cannula occurred during digesta collections. At post-mortem dissection, no signs of adverse effects due to the cannulation were observed. Mean pig BW at the completion of the trial was 61·3 (sd 4·3) kg.
All human subjects completed the trial without complication, and complied with the experimental protocol.
For meals C and HC fed to the pigs, one observation each for apparent and true AA and N digestibility was removed from the dataset, as detected by application of the outlier test. Similarly, for meal R fed to the human subjects, one observation for apparent and true N digestibility was removed from the dataset.
The apparent and true ileal digestibilities of N for meals C, HC and R for pigs and human subjects are given in Table 3. There was a significant (P < 0·05) effect of species, with both apparent and true digestibilities being lower in human subjects compared with pigs. The species differences were marked for the apparent digestibility of N (14–16 % lower in human subjects) but were relatively small for true N digestibility (3–5 % lower in human subjects).
Table 3 Apparent and true ileal nitrogen digestibility for mixed meals based on intact casein (C), hydrolysed casein (HC) and rapeseed protein isolate (R) fed to adult humans and growing pigs
(Mean values and pooled standard deviations)
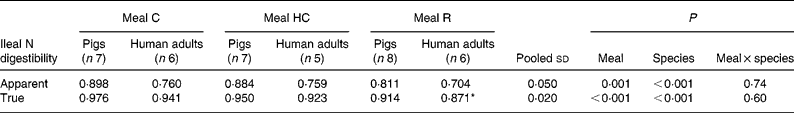
* Data originally published by Bos et al. (Reference Bos, Airinei and Mariotti10).
The apparent ileal AA digestibilities for meals C and HC for pigs and human subjects are given in Table 4. For each AA, apparent digestibility was significantly lower (P < 0·05) in human subjects compared with pigs, in both meals C and HC, except for threonine for which the difference did not reach statistical significance.
Table 4 Apparent ileal amino acid digestibility for mixed meals based on intact casein (C) or on hydrolysed casein (HC) fed to adult humans and growing pigs
(Mean values and pooled standard deviations)

The true ileal AA digestibility values for meals C and HC are given in Table 5. Pig and human true ileal digestibilities were not significantly different (P>0·05) over both meals (C and HC) for most AA except for phenylalanine, tyrosine, lysine, histidine and aspartic acid for which digestibilities were significantly lower (P < 0·05) for human subjects compared with pigs.
Table 5 True ileal amino acid digestibility for meals based on intact casein (C) or on hydrolysed casein (HC) fed to growing pigs and adult humans
(Mean values and pooled standard deviations)

The correlation coefficient between pig and human apparent ileal digestibility was high (r 0·99) but not significant (P = 0·09) for N from meals C, HC and R and was high and statistically significant (r 0·90; P < 0·0001) for AA from meals C and HC. The linear regression equation for the AA was y = 1·20x − 0·25, where y is human digestibility and x is pig digestibility.
Similarly, the correlation coefficient between pig and human true ileal digestibility was high (r 0·98) but not significant (P = 0·11) for N from meals C, HC and R, and was high and statistically significant (r 0·87; P < 0·0001) for AA from meals C and HC. Linear regression equations were derived to allow predictions of human true ileal digestibility values from determined pig true ileal digestibility values for N (Fig. 1(a)) and AA (Fig. 1(b)).

Fig. 1 Linear regression relationships between mean values for human and pig true ileal digestibilities (TID) of (a) nitrogen (y = 1·14x − 0·17; R 2 0·97; P slope = 0·11) and (b) individual amino acids (y = 0·83x+0·14; R 2 0·76; P slope < 0·0001) for meals based on casein (○) or on hydrolysed casein (●) and, when applicable, for rapeseed protein isolate (□). A, alanine; D, aspartic acid; E, glutamic acid; F, phenylalanine; H, histidine; I, isoleucine; K, lysine; L, leucine; P, proline; S, serine; T, threonine; V, valine; Y, tyrosine.
Discussion
The objective of the study was to assess the suitability of the growing pig as an animal model for determining the ileal N digestibility of dietary protein in adult human subjects. Overall, good agreement was found between species for ranking the protein sources, especially regarding true ileal N digestibility.
The study used methods of digesta collection optimised for each species and for this reason the method of collection was not common across pigs and human subjects, although it was maintained as close as possible and based in both cases on a direct access to ileal fluid as well as the use of 15N-labelled protein sources. In human subjects, ileal digesta were sampled through a naso-ileal triple-lumen tube, allowing digesta collection from subjects with an entire digestive tract, unlike that from ileostomised subjects. The intubation method has been previously used for the determination of ileal N digestibility in human subjects(Reference Gaudichon, Mahé and Benamouzig35–Reference Bos, Juillet and Fouillet38) and has been shown to allow more than 50 % of the total digesta to be sampled through the tube and total recovery (about 103 %) of an unabsorbable marker mixed within the meal (A Deglaire, unpublished results). Although it remains unknown whether the oro-ileal transit time is altered by the presence of the tube, previous studies have reported that digesta transit time, and especially gastric emptying rate, do not influence overall ileal protein digestibility(Reference Gaudichon, Mahé and Benamouzig35, Reference Weber and Ehrlein39, Reference Mariotti, Mahé and Luengo40). In the present study, ileal digesta were collected from pigs through a PVTC cannula, which, above other cannulation techniques, offers the advantage of leaving the small intestine intact, thus minimising effects on ileal muscle function. The indigestible marker ingested with the test meal was recovered at a rate of 42 (sd 15) % (data not shown) across the diets C, HC and R in the present pig digesta samples. The less than complete recovery may result from the prescribed period of digesta collection and/or from digesta bypassing the cannula and entering the large intestine. The latter may have differed among pigs resulting in some variability for marker recovery. In the present study, there were no statistically significant correlations found between marker recovery and apparent ileal N digestibility within diets, which indicates that the pig digesta samples were representative of total ileal digesta(Reference van Leeuwen, Veldman and Boisen41). Human digesta flow was detected immediately after meal ingestion and was minimal after 8 h, ensuring that the 8 h collection period was adequate to sample most of the non-digested dietary N. However, relatively high amounts of pig digesta flow were found only 4 h after the meal as observed previously(Reference Leterme, Thewis and Francois26, Reference Hodgkinson, Moughan and Morel42) and flow was still substantial 10 h after meal ingestion. The latter time period (4–10 h) is likely to correspond to maximal ileal passage of dietary N, previously observed to occur between 3–4 and 9–11 h from ingestion for pea- or wheat-based diets(Reference Leterme, Thewis and Francois26). Thus, although digesta collection times differed between the species, digesta were collected for each species when digesta flows were broadly close to maximal. A species effect inducing different rates of the 15N tracer recycling within endogenous secretions cannot be ruled out. However, overall, tracer recycling had little impact on the relative differences between the species for the true ileal N digestibility in HC (a diet allowing calculations of tracer recycling), with values after correction for recycling being 0·965 (sd 0·020) and 0·934 (sd 0·010) in pigs and in human subjects, respectively (data not shown).
Of interest in the present study was that the apparent ileal digestibility of N was markedly lower (14 to 16 %) for human subjects compared with pigs across the test meals containing different protein sources. The species difference was smaller for apparent AA digestibility, but still substantial, with values being on average 8 % lower for human subjects. The closer agreement observed for apparent digestibilities of AA as compared with those of total N might arise from differences in non-protein N transiting in the small intestine between species, such as urea. The acute (no adaptation to the test meal) feeding procedure used in the present work did not appear to influence the results of the study as the present pig apparent AA and N digestibility coefficients for C and HC were similar to those for the same diets but given to adapted (5 d) pigs, being on average only 2·3 % different (A Deglaire and PJ Moughan, unpublished results), such as has been observed previously(Reference Moughan, Butts and van Wijk9). The presently reported values for the apparent ileal digestibility of N and AA in casein and hydrolysed casein agree closely with other published values(Reference Chung and Baker43–Reference Yin, Huang and Libao-Mercado45). Roos et al. (Reference Roos, Pfeuffer and Hagemeister46) reported apparent ileal digestibility values of casein for pigs (0·76–0·80) which were lower than those found in the present study but closer to the values observed here for human subjects. Apparent ileal digestibility values for casein and hydrolysed casein in human subjects have not been reported in the literature. The mean apparent ileal N digestibility value for rapeseed isolate was in line with that determined in pigs fed dehulled and untoasted rapeseed (0·76)(Reference Grala, Verstegen and Jansman47). The present differences observed for apparent ileal digestibility between species are likely to be due to a higher proportion of endogenous protein to total ileal protein in human subjects compared with pigs, as true ileal digestibility was much closer between pigs and human subjects (less than 5 % difference for true ileal digestibility of N). Whether the different amount of endogenous protein in digesta is an actual effect of the species or is due to methodological differences is unknown, and should be the topic of further investigation.
It is more accurate to compare true ileal protein digestibility between species, as this measure represents the specific fate of dietary N and AA in the digestive tract(Reference Fuller and Tome1). True ileal digestibilities of N and of some AA were significantly lower for diet HC than for diet C. This was observed in both species. It may suggest a lower extent of digestion and/or absorption for HC and/or a higher recycling rate of the 15N tracer for HC. The exact reason remains unknown but should be investigated further. However, the present study shows a similar ranking among the protein sources (C, HC and R) between human subjects and pigs and that for most AA true ileal digestibility (C and HC) was the same between the species. Although the true ileal digestibility of N and of five AA was significantly different (P < 0·05) between the species, the coefficients were on average only 4 % lower in human subjects compared with pigs. This inter-species difference might result from physiological and/or methodological considerations. The digestive and/or absorptive capacity of the gastrointestinal tract may be greater in pigs than in human subjects, and this may be particularly important for more poorly digested proteins such as rapeseed. Forsum et al. (Reference Forsum, Goranzon and Rundgren18) reported a higher (6 %) true faecal digestibility of vegetable proteins in pigs than in human subjects but similar digestibility of a combination of vegetable and animal proteins between the species.
The degree of correlation between pig and human AA and N data was high for both apparent and true digestibility values. Correlations for N data did not reach the level of statistical significance (P = 0·05), with P = 0·09 and P = 0·11 for apparent and true N digestibility, respectively. However, correlations for the AA data were statistically significant. The present dataset was augmented by the inclusion of pig and human ileal digestibility data previously reported by Rowan et al. (Reference Rowan, Moughan and Wilson19) and based on a vegetable–animal protein-based diet. In the augmented dataset, the correlation between pig and human true ileal digestibility coefficients was high (r 0·94) and close to significance (P = 0·06) for N from meals C, HC and R and the vegetable–animal-protein-based diet, and was statistically significant (r 0·83; P < 0·001) for AA from meals C and HC and the vegetable–animal protein-based diet. The linear regression equations derived to allow predictions of human true ileal digestibility values from determined pig true ileal digestibility values were y = 1·47x − 0·47 and y = 1·05x − 0·06 for N (R 2 0·88) and for AA (R 2 0·68), respectively. Caution needs to be exercised in extrapolating from the predictive equation for true ileal digestibility of N beyond the range of data collected. The range of N digestibility assessed here is relatively narrow.
In conclusion, the present results are in general agreement with an earlier observation(Reference Rowan, Moughan and Wilson19) that there is close agreement between pigs and human subjects for the true ileal digestibility of dietary N. The growing pig has been widely promoted as a useful model for human nutrition studies due to a physiologically and anatomically similar digestive tract(Reference Pond and Houpt12–Reference Moughan, Birtles and Cranwell15). In addition, the pig unlike the rat has a meal-eating habit(Reference Miller and Ullrey13) and eats most foods consumed by humans. Another advantage with the pig is the possibility for the collection of large samples of representative digesta(Reference Moughan and Rowan14). For all these reasons, the growing pig has been suggested as being a better model than the growing rat for predicting protein digestibility for the adult human(Reference Darragh and Hodgkinson4, Reference Moughan17, Reference Rowan, Moughan and Wilson19). This is supported by our recent data showing a similar true ileal digestibility for rapeseed and milk proteins in the growing rat (0·955 (sd 0·011) and 0·956 (sd 0·008), respectively(Reference Boutry, Chevalier and Fouillet48)), unlike what was observed here in both the adult human and the growing pig. Overall, the present findings support the use of the growing pig as an animal model for routine determination of true ileal N digestibility in human adults, in particular for ranking protein sources.
Acknowledgements
The study was carried out with the financial support of the Riddet Institute (Palmerston North, New Zealand) and of UMR 914 Nutrition Physiology and Ingestive Behaviour (AgroParisTech/INRA Paris, France). We gratefully acknowledge the Gastroenterology Unit, especially Robert Benamouzig and Gheorge Airinei. We are indebted to Jacques Fauquant (UMR STLO, INRA Rennes, France) for the 15N-labelling of the milk and the casein purification, and Klaus J. Petzke and Shane M. Rutherfurd for their technical assistance.
A. D. designed the study, collected and analysed the data and wrote the manuscript. C. B. assisted in designing the study, collected the data and participated in the writing of the manuscript. D. T. assisted in designing the study. P. J. M. assisted in designing and participated in the writing of the manuscript.
None of the authors had any conflict of interest.








