Vitamin B12 is essential for 1-C metabolism and cell division. Foods derived from animals are the main sources of vitamin B12. Strict vegetarians have limited sources of vitamin B12 in their diet and therefore likely to have vitamin B12 deficiency( Reference Antony 1 – Reference Jathar, Inamdar-Deshmukh and Rege 3 ). Reduced consumption of cobalamin from food or impaired intestinal absorption leads to severe deficiency when tissue stores of the vitamin are depleted. The clinical consequence of vitamin B12 deficiency includes megaloblastic anaemia and progressive neurologic disease of the central and peripheral nervous system( Reference Obeid, Giesel and Schorr 4 ) and hyperhomocysteinaemia, a risk factor for CVD( Reference Refsum, Smith and Ueland 5 ). Early diagnosis of vitamin B12 deficiency is useful to prevent irreversible neurological damage by cobalamin supplementation( Reference Herrmann, Obeid and Schorr 6 – Reference Weir and Scott 8 ). It has been observed that asymptomatic Indian lactovegetarians, who make up for more than half of the Indian population, had distinctly lower vitamin B12 concentrations than non-vegetarians( Reference Yajnik, Deshpande and Lubree 9 ) and was confirmed by studies from different geographic regions of India. However, total plasma vitamin B12 concentration may not reliably reflect vitamin B12 status. To obtain more specificity and sensitivity in diagnosing vitamin B12 deficiency, the concept of measuring holotranscobalamin (holo-TC) II, a transport protein, has aroused great interest. holo-TC is a biologically active vitamin B12 fraction that promotes the aspecific uptake of vitamin B12 by cells( Reference Nexo and Hoffman-Lücke 10 ).
Vitamin B12 deficiency has been divided into four stages( Reference Herbert 11 ). In stages I and II, indicated by a low plasma level of holo-TC, the plasma and cell stores become depleted. Stage III is characterised by increased plasma levels of total homocysteine (tHcy) and methylmalonic acid (MMA) in addition to lowered holo-TC. In stage IV, clinical signs become recognisable such as macroovalocytosis, elevated mean corpuscular volume (MCV) or lower Hb levels. Stage III of vitamin B12 deficiency has been found in over 60 % of vegetarians( Reference Herrmann and Giesel 12 ). Thus, it is important to monitor vitamin B12 status in this dietary group. Measurement of plasma vitamin B12, holo-TC, tHcy and MMA has been suggested for optimal monitoring of vitamin B12 status in vegetarians( Reference Lindenbaum, Savage and Stabler 13 ). In this study, we investigated the diagnostic cut-off values of plasma vitamin B12, holo-TC and functional marker, tHcy, of vitamin B12 metabolism in Indian vegetarians.
Methods
The study was conducted at Deenanath Mangeshkar Hospital and Research Centre. Young, healthy, postgraduates and staff members of the hospital and their relatives were invited to participate in the study. Self-explained information regarding the participants was captured by an interview, which included age, vitamin supplementation, food habits and routine lifestyle. Study subjects were enrolled after the purpose and requirements of the study were explained. They were clinically examined for gross clinical signs of protein-energy under-nutrition and vitamin deficiencies (vitamin A, B-complex, C and D). Non-vegetarians and pregnant women were not included. Subjects with diabetes, cancer and those taking drugs known to influence vitamin B12 absorption were also excluded. A total of 119 eligible participants (forty-six male and seventy-three female) were enrolled for the study. The study protocol was approved by the Hospital Ethical Committee and all the participants gave written informed consent (2015_APR/SN/169).
Experimental procedure
A volume of 10 ml of fasting blood sample was collected in EDTA vacutainers. Haematological parameters were measured on a five-part differential cell counter (Sysmex) and the remaining blood was centrifuged at 1500 g for 20 min. Separated plasma was stored for biochemical investigations at −20°C. Plasma holo-TC was measured using microparticle enzyme immunoassay. Microparticle enzyme intrinsic factor assay was used for the quantitative determination of plasma vitamin B12. Plasma folate and tHcy were measured by the fluorescence polarisation immunoassay technique. All four biomarkers were analysed on an AxSYM immunoassay analyzer (Abbott Laboratories)( Reference Naik, Bhide and Babhulkar 17 ). Plasma creatinine was measured using the alkaline picrate method with a Daytona analyser (Randox)( 14 ).
Dietary intake data
A 24-h dietary recall questionnaire was administered to capture detailed information about all food, beverages and dietary supplements consumed in the past 24 h from midnight to midnight the previous day by an experienced nutritionist. Energy, protein, fat and vitamin B12 intake was calculated using Dietsoft( Reference Kanade, Rao and Kelkar 15 , Reference Gopalan, Rama Sastri and Balsubramanian 16 ).
Definitions
Folate and vitamin B12 deficiency was defined as concentrations <2 ng/ml and <148 pmol/l, respectively; hyperhomocysteinaemia as plasma tHcy concentrations <15 µmol/l( Reference Naik, Bhide and Babhulkar 17 ); low holo-TC concentration as <35 pmol/l; anaemia as Hb concentration <120 g/l in females and <130 g/l in males; and macrocytosis as MCV>100 fl( Reference Refsum, Yajnik and Gadkari 18 ).
Statistical analysis
The data are presented as medians and 25th and 75th percentiles. Between-group comparisons were calculated using Mann–Whitney U test and associations were tested using Pearson’s correlation coefficient.
Sample size justification
Prevalence of vitamin B12 deficiency in vegetarian Indians is found to be 70 %( Reference Yajnik, Deshpande and Lubree 9 ). Sample size was calculated using Buderer’s method considering prevalence rate( Reference Buderer 19 ). We selected sensitivity and specificity to be 80 and 90 %, respectively. We chose clinically acceptable width of 95 % CI for sensitivity and specificity to be no more than 10 %, significance (a=0·05). The sample size calculated was 88 for the expected sensitivity of 80 % and 116 for the expected sensitivity of 90 %. Taking maximum of both, we arrived at the estimate of 116.
We enrolled 119 subjects. Receiver operating characteristic (ROC) curves and AUC (with 95 % CI) were used to measure the diagnostic accuracy of (cut-offs) vitamin B12, holo-TC and tHcy. ROC decision plots depicting sensitivity and specificity for 100 and 150 pmol/l concentration of vitamin B12 using holo-TC (<35 pmol/l) and tHcy (>15 µmol/l) variables were determined to identify the cut-off values.
Results
The participants had no clinical symptoms of vitamin B12 deficiency (neurological symptoms such as paresthesia, weakness, gait abnormality, tingling of hands and feet or anaemia symptoms such as skin pallor, fatigue, shortness of breath and so on) and were not taking vitamin B12 supplementation or any drugs known to influence vitamin B12 absorption.
All subjects had normal plasma folate and creatinine concentrations (5·9, 6·6 ng/ml and 1·2, 0·95 mg/dl in males and females, respectively). Median plasma vitamin B12, holo-TC and tHcy concentrations were 146·5 and 164 pmol/l, 22·3 and 26·5 pmol/l, and 22·6 and 14·2 µmol/l in males and females, respectively (Table 1). In all, 39 and 30 % had very low concentration (<100 pmol/l), 11 and 20 % had low concentration (100–148 pmol/l), and 50 and 52 % had normal plasma vitamin B12 concentration (>148 pmol/l) in males and females, respectively. holo-TC was lower in three-fourth of the participants (22 and 24 pmol/l). A significant sex difference was found in plasma tHcy concentrations (22·6 µmol/l in males and 14·2 µmol/l in females, P=0·0001), with no sex difference in holo-TC and vitamin B12 concentrations (Table 1). Group-wise distribution of vitamin B12 (<100, 100–150 pmol/l) was strongly related to plasma holo-TC and inversely to tHcy concentrations in both males and females (Table 2, P<0·0001). At normal level of vitamin B12 (>148 pmol/l), the median concentrations of plasma holo-TC concentrations were 27·7 and 29·8 pmol/l in vegetarian males and females, which are lower than those found in non-vegetarians (Enexo). Between plasma vitamin B12 concentrations of 113 and 122 pmol/l, nineteen participants had normal holo-TC (34–52 pmol/l) with higher tHcy (34 μmol/l) concentrations, which is unexplainable with existing cut-offs (Table 3). ROC analysis demonstrated similar AUC at the vitamin B12 concentration of 100 and 150 pmol/l when holo-TC (0·784 and 0·777, respectively) and homocysteine (Hcy) (0·928 and 0·924, respectively) were used as variables. Cut-off value of 100 pmol/l resulted in the highest sensitivity (77·78 %) with acceptable specificity (71·05 %) with a predictive value of 19·6 pmol/l for holo-TC and a sensitivity of 82·72 % and specificity of 89·47 % with a predictive value of 21·2 µmol/l for tHcy (Fig. 1 and 2).
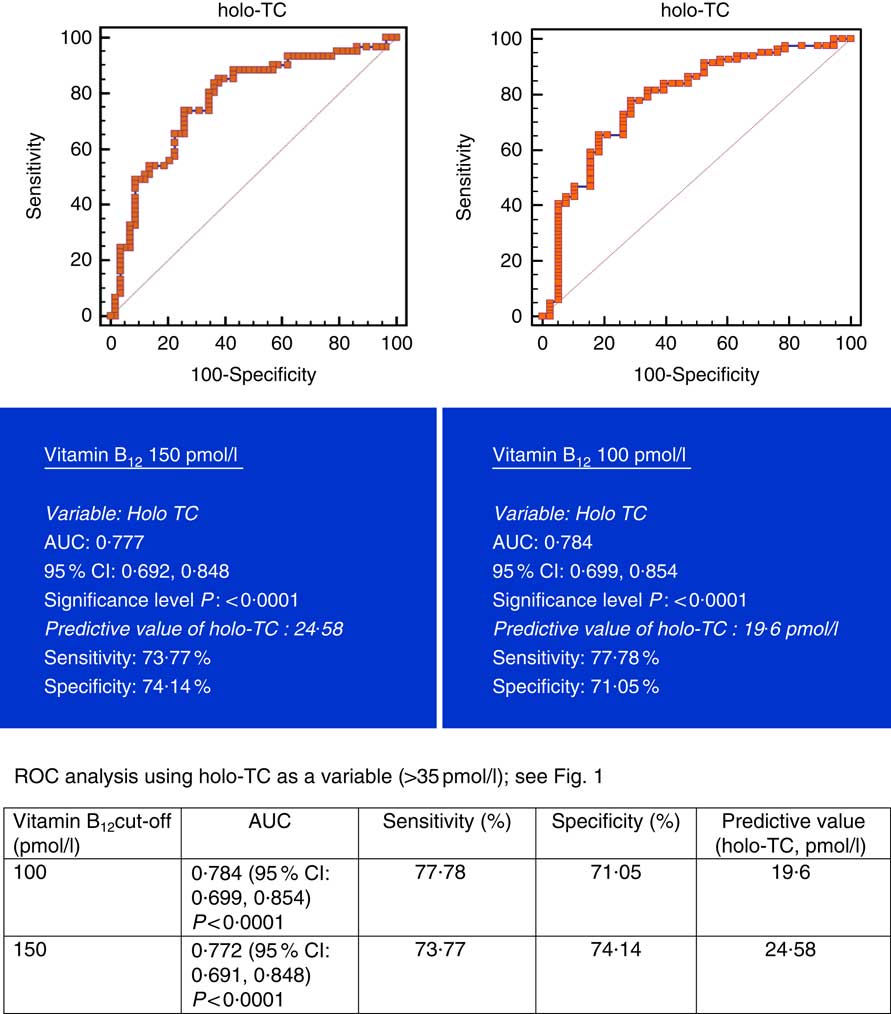
Fig. 1 Plasma vitamin B12 at concentrations of 150 and 100 pmol/l were used for analysis. Metabolic deficiency was defined as plasma holotranscobalamin (holo-TC)<35 pmol/l in 119 vegetarian Indians. Predictive values of plasma holo-TC were 24·58 and 19·6 µmol/l, respectively, with similar sensitivity and specificity at both 150 and 100 pmol/l of vitamin B12. ROC, receiver operating characteristic.
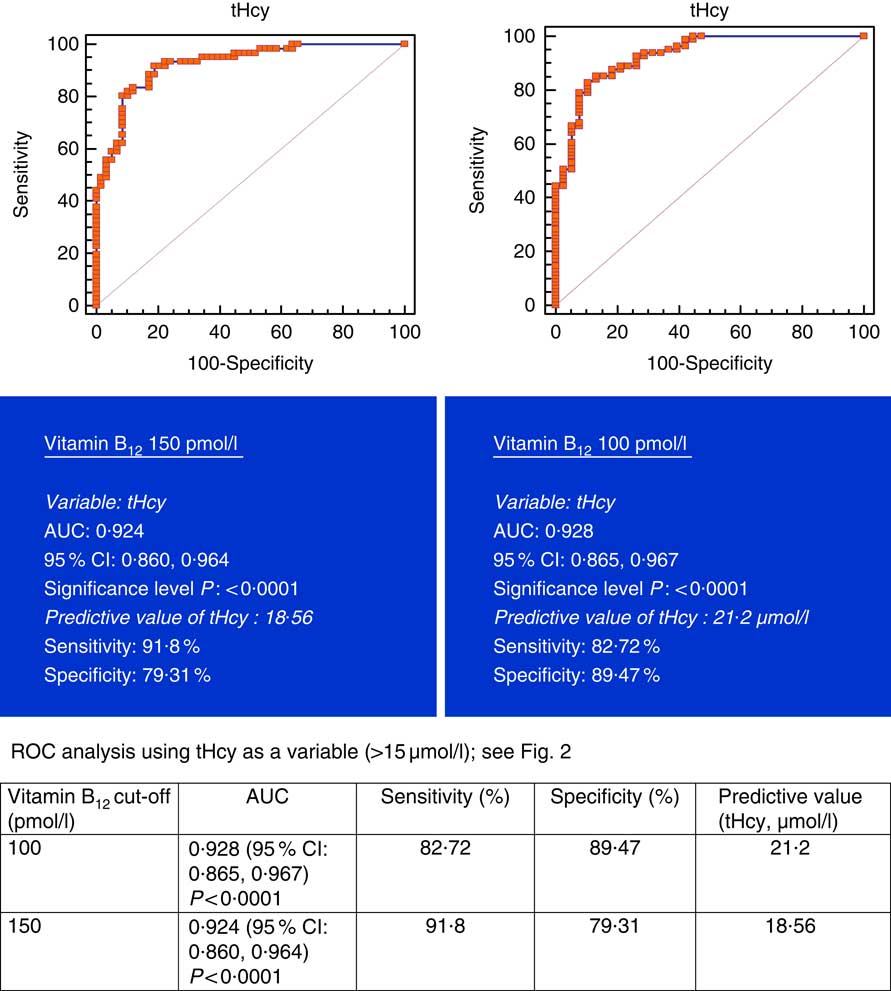
Fig. 2 Plasma vitamin B12 at 150 and 100 pmol/l were used as variables. Metabolic deficiency was defined as total homocysteine (tHcy)>15 µmol/l in 119 vegetarian Indians. Predictive values of plasma tHcy were 18·56 and 21·2 µmol/l, respectively, with better specificity (89·47 %) at 100 pmol/l of vitamin B12. ROC, receiver operating characteristic.
Table 1 Baseline characteristics and biochemistry of the participants (male and female) (Medians and 25th, 75th percentiles)
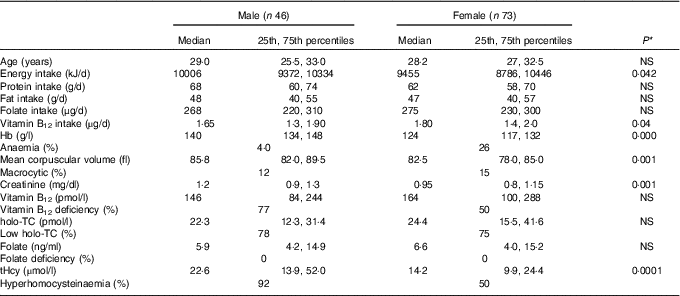
holo-TC, holotranscobalamin; tHcy, total homocysteine.
* Difference between male and female.
Table 2 Sex difference in total homocysteine (tHcy) at different vitamin B12 concentrations (Medians and 25th–75th percentiles)
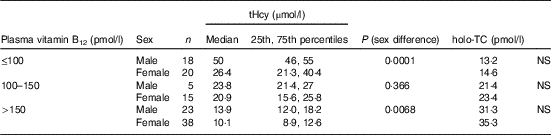
holo-TC, holotranscobalamin.
Table 3 Plasma vitamin B12 concentrations and corresponding concentrations of plasma holotranscobalamin (holo-TC) and total homocysteine (tHcy)

holo-TC, holotranscobalamin; tHcy, total homocysteine.
Discussion
Our data support the concept that the measurement of plasma holo-TC and tHcy along with vitamin B12 provides a better index of cobalamin status than the measurement of vitamin B12 alone( Reference Lysne, Strand and Svingen 20 ). Plasma holo-TC and tHcy are both sensitive markers for cobalamin status( Reference Solomon 21 ). The lowest median levels of holo-TC were observed in the low vitamin B12 concentration group, followed by the 100–150 pmol/l group and then by the normal group (>148 pmol/l): 13·2, 21·4 and 31·3 pmol/l, respectively (P=0·0001). At similar plasma cobalamin concentrations, men had different metabolic effects compared with women. The use of holo-TC and tHcy results enables the differentiation between storage, depletion and functional vitamin B12 deficiency.
Fedosov( Reference Fedosov 22 ) reported that a combination of vitamin B12, holo-TC, MMA and Hcy (cB12) biomarkers is a reliable diagnostic tool. Fedosov et al.( Reference Fedosov, Brito and Miller 23 ) derived equations that combined two, three or four biomarkers into one diagnostic indicator and provided a guidance for treatment. They suggested that adults having plasma vitamin B12 levels between 116 and 119 pmol/l, holo-TC between 8·4 and 20 pmol/l, and Hcy between 19·2 and 51 μmol/l be grouped as low vitamin B12 status and adults with B12 between 119 and 186 pmol/l, holo-TC between 20 and 37 pmol/l, and Hcy between 13·6 and 19·2 μmol/l as transitional vitamin B12 deficiency. A Brito et al.( Reference Brito, Verdugo and Hertrampf 24 ) stated that cB12 could only detect improved neurophysiological function in asymptomatic Chilean elderly with poor vitamin B12 status and also suggested to identify functional indicators of sub-clinical vitamin B12 deficiency.
This is the first study to investigate the diagnostic value of circulating plasma vitamin B12, holo-TC and tHcy (a functional marker) concentrations associated with 1-C metabolism in Indian vegetarians. We found that at similar circulating concentrations of plasma vitamin B12 and holo-TC, there is a sex difference in plasma tHcy concentrations, with men having higher tHcy concentrations than women (P<0·0001). The marked sex difference( Reference Naik, Bhide and Babhulkar 17 ) in plasma tHcy concentrations is confirmed in this study. The difference suggests a higher threshold for supplementation of vitamin B12 to improve reproductive and cardiovascular outcomes( Reference Naik, Joglekar and Bhat 25 ). The sex difference is not significant in participants whose plasma vitamin B12 concentrations are between 105 and 148 pmol/l. The strong inverse relation between plasma vitamin B12 and tHcy and direct relation with holo-TC( Reference Naik, Bhide and Babhulkar 17 ) concentrations are confirmed in this study. Although age and sex reference intervals of tHcy have been established( Reference Naik, Joglekar and Bhat 25 , Reference Hannibal and Blom 26 ), they are usually ignored in reporting tHcy levels, because of dual biochemical origin (B12 and folate). Elevated Hcy in plasma has been very often used as a biomarker, but its relationship to the molecular mechanisms of disease has not been established( Reference Hannibal, Lysne and Monsen 27 ).
In this study, we aimed to meet two criteria: (a) vitamin B12 absorption capacity in Indian vegetarians and (b) optimum plasma vitamin B12 concentration required for methylation of Hcy. At normal plasma vitamin B12 levels (>150 pmol/l), median holo-TC concentrations were 31·3 and 35·3 pmol/l in men and women, respectively, thereby attaining normal tHcy concentrations (13·9 and 10·17 µmol/l, respectively).
A cohort of 100 known patients with CVD and sixty-three normal healthy subjects (median age 44 years) were examined for their vitamin B12 status in a case–control study in Pune. Median plasma vitamin B12, holo-TC and tHcy concentrations were 160 pmol/l, 24 pmol/l and 19·7 µmol/l, respectively, in normal subjects. They stated that hyperhomocysteinaemia and elevated MMA were due to vegetarianism( Reference Refsum, Yajnik and Gadkari 18 ). Similarly, another report from Pune, wherein subjects with low plasma vitamin B12 levels were studied, showed very low plasma holo-TC concentrations (7·7 (SD 4·2) and 9·8 (SD 8·7) pmol/l in men and women, respectively), with tHcy concentrations of 29·2 (SD 19·2) and 15·3 (SD 8·3) µmol/l( Reference Bhat, Thuse and Lubree 28 ). Low vitamin B12 concentration (median 110 pmol/l) and hyperhomocysteinaemia (>15 µmol/l) have been reported to be common in Indian men, particularly in vegetarians and urban middle-class residents. Most of the participants from these studies were vegetarians. In the study by Naik et al.( Reference Naik, Bhide and Babhulkar 17 ), young Indian vegetarian subjects with low plasma vitamin B12 status (<148 pmol/l) were found to have holo-TC and tHcy concentrations of 14·4 pmol/l and 31·9 μmol/l, respectively, and the subjects with normal status (>200 pmol/l) were found to have holo-TC and tHcy concentrations of 27·7 pmol/l and 11·9 μmol/l, respectively. Most of the Indian studies did not use plasma holo-TC measurements. A Dutch study reported 49 (8–388) pmol/l of plasma holo-TC concentrations in healthy subjects with corresponding vitamin B12 concentrations of 217 (119–1210) pmol/l( Reference Bor, Lydeking-Olsen and Moller 29 ). A study from USA reported plasma holo-TC concentration of 85 (SD 48) pmol/l for 495 (SD 119) pmol/l of vitamin B12 concentrations in healthy volunteers( Reference Wokes, Badenoch and Sinclair 30 ). In both these studies the participants were non-vegetarians with high plasma holo-TC concentrations and the referred cut-offs may not be appropriate for vegetarians.
In healthy individuals, all four biomarkers (plasma vitamin B12, holo-TC, tHcy and MMA) had a strong relation to vitamin B12 intake, with steady-state concentrations at a daily intake of 4–7 µg vitamin B12 Reference Bor, von Castel-Roberts and Kauwell (31) . These studies suggest that all four markers may be useful for monitoring a population’s vitamin B12 status over time.
Carmel( Reference Carmel 32 ) categorised available biomarkers as those that directly measured plasma vitamin B12 and those that measured metabolites that accumulated with inadequate amounts of vitamin B12. Plasma holo-TC and vitamin B12 measured circulating vitamin B12 concentrations. These two therefore reflected the broad vitamin B12 status from high risk of severe deficiency to adequacy. Miller et al.( Reference Miller, Garrod and Rockwood 33 ) have stated that holo-TC and total vitamin B12 have equal diagnostic accuracy in screening for metabolic vitamin B12 deficiency. Measurement of both holo-TC and total vitamin B12 provided better screen for vitamin B12 deficiency than either assay alone. According to Green( Reference Green 34 ), low vitamin B12 status was indicated by values lower than the reference range (for vitamin B12<148 pmol/l; for holo-TC <35 pmol/l), whereas for indirect measures of metabolites (MMA or tHcy) low vitamin B12 status measures were indicated by a level above the upper limit of the reference range (for MMA >260 nmol/l; for tHcy >12 µmol/l). However, Valente et al.( Reference Valente, Scott and Cunningham 35 ) suggested a diagnostic strategy using holo-TC as the front-line test. The cut-offs for deficiency were defined as 20 pmol/l for holo-TC and 123 pmol/l for serum vitamin B12 after studying employees and medical students of a local hospital at Dundee, UK.
Conclusions
holo-TC levels may prove most useful if the aim is to monitor a population with a borderline sub-optimal vitamin B12 supply. In contrast, total vitamin B12 may be superior if the goal is to monitor a possible surplus load of vitamin. We advocate a diagnostic cut-off level of plasma vitamin B12 (105 pmol/l), holo-TC (22·6 pmol/l) and Hcy (17·6 µmol/l for females and 27·0 µmol/l for males) in vegetarian Indian population. In addition to this, we recommend that vegetarians do take a supplement of vitamin B12 to ensure adequate supply of the micronutrient. This would be of particular importance for females in reproductive age, to prevent the risks associated with maternal–fetal vitamin B12 deficiency.
The limitation of the study is that plasma MMA has not been measured. However, if none of the participants is folate deficient, the measurement of Hcy shall indicate vitamin B12 status.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Dr Dhanajay Kelkar, Medical Director, extended all the help. Our thanks are due to Swati, Swapnali and Sandeep and Abbott Laboratories for free kits.
S. N. designed the study. V. B. and N. M. prepared the manuscript draft. S. N. prepared the final draft.
The authors declare that there are no conflicts of interest.









Target article
Identification of vitamin B12 deficiency in vegetarian Indians
Related commentaries (1)
Invited commentary in response to: ‘Identification of vitamin B12 deficiency in vegetarian Indians’