High fat intake(Reference Ziegler, Hoover and Pike1–Reference Howe, Hirohata and Hislop3), and possibly high intake of specific fatty acids(Reference Freedman, Potischman and Kipnis4, Reference Bingham, Luben, Welch, Wareham, Khaw and Day5), has been shown to be an important modulator of breast cancer risk in animal studies(Reference Carroll and Khor6–Reference Fay, Freedman, Clifford and Midthune8). These hypotheses were supported by case–control studies conducted in the 1970s and 1980s(Reference Howe, Hirohata and Hislop3). However, cohort studies thus far have been rather inconsistent, with the majority showing no association for total fat intake, reviewed in Hunter et al. (Reference Hunter, Spiegelman and Adami9) and Smith-Warner et al. (Reference Smith-Warner, Spiegelman and Adami10), and some showing direct associations for saturated and n-6 fatty acids(Reference Wirfalt, Mattisson, Gullberg, Johansson, Olsson and Berglund11, Reference Boyd, Stone, Vogt, Connelly, Martin and Minkin12). Recent results of the Women's Health Initiative dietary modification trial suggest a weak, if any, inverse association between a low-fat diet and risk of breast cancer(Reference Prentice, Caan and Chlebowski13).
The aim of the present study was to identify a food pattern which explains variation in fatty acid intake (SFA, MUFA, n-3 PUFA, n-6 PUFA), and to relate this pattern to breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort(Reference Boeing, Wahrendorf and Becker14).
Methods
The study population for the present study stems from the female population of the EPIC-Potsdam cohort(Reference Boeing, Korfmann and Bergmann15), a German population sample of 27 548 participants (10 904 males and 16 644 females) mostly aged 35–65 years, contributing to the large multi-centre EPIC cohort study(Reference Riboli, Hunt and Slimani16). The baseline examination of the study participants took place between August 1994 and September 1998.
We included 15 861 women free of any cancer at baseline recruitment and excluded women with missing information on diet and lifestyle factors, who reported an extreme energy intake (ratio between energy intake and estimated energy expenditure above ninety-ninth or below first percentile) or who had missing follow-up times, leaving 15 351 women for the statistical analysis.
Habitual dietary behaviour of the past 12 months before baseline was assessed with a validated 148-item semi-quantitative FFQ(Reference Kroke, Klipstein-Grobusch and Voss17). Food items were collapsed into thirty-nine food groups based on origin, culinary usage or nutrient profiles, and absolute intakes in gram per day were computed.
Cases of incident invasive breast cancer were identified via a combination of follow-up methods including active follow-up through study participants and their next-of-kin, health insurance, cancer and mortality registries(Reference Bergmann, Bussas and Boeing18). To classify mammary tumours, International Classification of Diseases ICD-10 (code C50) and ICD-O-2 were used. All new diagnoses were physician-verified.
Recently, our group has introduced reduced rank regression into nutritional epidemiology as a new tool for dietary pattern analysis(Reference Hoffmann, Schulze, Schienkiewitz, Nothlings and Boeing19). Reduced rank regression identifies linear functions of predictors (e.g. food group intake) that explain as much variation as possible in a set of so-called response variables, e.g. nutrients(Reference Hoffmann, Schulze, Schienkiewitz, Nothlings and Boeing19) or biomarkers(Reference Hoffmann, Zyriax, Boeing and Windler20), that are presumed to affect disease risk. The principle of the reduced rank regression method has been described in full elsewhere(Reference Hoffmann, Schulze, Schienkiewitz, Nothlings and Boeing19). Briefly, maximum variation in response variables (nutrient densities of fatty acid fractions, i.e. SFA, MUFA, n-3 PUFA, n-6 PUFA, expressed as g/1000 kJ) is explained by linear functions of predictors (thirty-nine food groups). Four factors were generated with the first factor explaining 42·8 % of total response variation (SFA, 26·4 %; MUFA, 58·3 %; n-3 PUFA, 49·1 %; n-6 PUFA, 37·5 %) and the remaining factors explaining 27·8, 7·3 and 2·2 % respectively. Only the first factor showed significant associations in the diet–disease model and was further investigated. To derive a simplified food intake pattern, food groups explaining most of the inter-individual variation in the response score were identified via stepwise linear regression. The score for the simplified food pattern was calculated by summing standardized intakes of the food groups taking into account their positive or inverse association with the original pattern score(Reference Schulze, Hoffmann, Kroke and Boeing21). The simplified pattern explained 41·3 % of variation in response variables.
In Cox's Proportional Hazards models relative risk for the association between tertiles of the simplified pattern score and breast cancer incidence was estimated with age as the primary time variable in the counting process formulation with entry time defined as the subject's age at recruitment and exit time defined as the subject's age at breast cancer diagnosis or censoring date (death, emigration, last complete follow-up). Three sets of models were conducted, one model which was only controlled for age in the strata statement of the PHREG procedure in SAS, one model, additionally controlling for known breast cancer risk factors including BMI, menopausal status, hormone replacement therapy, educational attainment, parity, alcohol consumption, smoking and age at menarche, and one model that additionally adjusted for other dietary factors (total energy intake, dietary fibre, vitamin C, vitamin E and β-carotene) that may confound the diet–disease association.
All statistical analyses were performed with SAS for Windows, version 9.1 (SAS Institute, Cary, NC, USA).
Results
After an average follow-up of 6·0 years, 137 verified incident cases of invasive breast cancer were identified. Baseline characteristics across tertiles of the simplified pattern score are presented in Table 1. Notably, concerning the distribution of fatty acid fractions used as response variables in the applied pattern technique, it became apparent that intakes of all fatty acid fractions (SFA, MUFA, n-3 PUFA and n-6 PUFA) were significantly positively associated with the score, i.e. intakes increased with increasing pattern score. The same direct association was found for total dietary fat. With regard to other dietary characteristics, we observed a decrease in fibre, β-carotene and vitamin C intake, and an increase in alcohol and vitamin E intake. Focusing on foods characterizing the simplified pattern, we found that the consumption of bread and of fruit juices was lower and that the consumption of processed meat, fish, butter and other animal fats, and margarine was higher with higher pattern score.
Table 1 Sample characteristics across tertiles of the simplified pattern score, European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study*
(Mean values and standard deviations)

HRT, hormone replacement therapy.
* Sum of standardized intakes of bread, fruit juices, processed meat, fish, butter and other animal fats, margarine.
† Percentage value where indicated.
‡ P value for linear trend (continuous variables); P value of χ2 test for categorical variables.
§ Age at menarche ≤ 11 years.
∥ Postmenopausal women only (n 5784).
Table 2 shows risk estimates for the association between the simplified pattern score and breast cancer. Both the crude (Model 1) and the adjusted (Model 2) model yielded a significant direct association between the food pattern and risk of breast cancer. Women in the third tertile of the pattern score were at a two-fold increased risk to develop breast cancer compared to women in the first tertile (hazard ratio 2·00; 95 % CI 1·30, 3·09). Further adjustment for other dietary factors strengthened the association in that women in the third tertile displayed a 2·3-fold increase in risk, indicating that these dietary factors were not responsible for the observed association. There was no evidence of effect modification by menopausal status, overweight status and use of hormone replacement therapy when we stratified our analysis according to these factors (data not shown).
Table 2 Crude and adjusted hazard ratios (HR) and 95 % CI for the association between the simplified food pattern score* and risk of breast cancer, European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study (n 15 351)
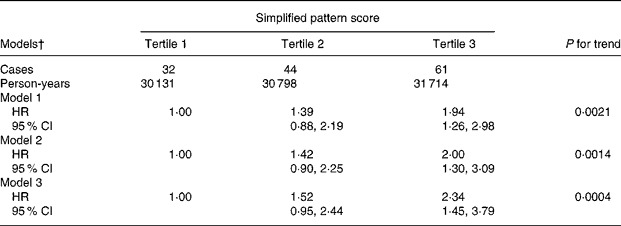
* Sum of standardized intakes of bread, fruit juices, processed meat, fish, butter and other animal fats, margarine.
† Model 1, stratified for age; Model 2, additionally adjusted for BMI, education, alcohol intake, smoking, parity, menopausal status, hormone replacement therapy, age at menarche; Model 3, Model 2 additionally adjusted for other dietary factors (total energy, fibre, β-carotene, vitamin C, vitamin E).
Discussion
The dietary pattern, extracted to explain variation in nutrient densities of fatty acid intake, significantly predicted breast cancer risk in a cohort of >15 000 German middle-aged women. The dietary pattern was positively associated with all fatty acid fractions used to derive the pattern. Thus, the observed association between the food pattern and risk of breast cancer may not be explained by differential effects of specific fatty acid fractions, even though some of them are thought to be protective of breast cancer, but rather by total fat intake related to the pattern. This might have occurred due to correlations between intakes of fatty acids, taking into consideration that with increasing total fat intake, intakes of fatty acids increase correspondingly. Indeed, in the present study, we observed strong positive correlations between total fat and the examined fatty acid fractions. The association of the food pattern with vitamin E can be explained in the same way. Although there is some evidence that vitamin E may protect from cancer, in the present study its intake was highly correlated with overall fat intake and intake of fatty acids.
Given the weak evidence from recent results of the Women's Health Initiative dietary modification in relation to breast cancer risk(Reference Prentice, Caan and Chlebowski13) and the inconclusive results of studies using the single-nutrient approach(Reference Hunter, Spiegelman and Adami9, Reference Smith-Warner, Spiegelman and Adami10), studying dietary patterns in relation to breast cancer risk is of outstanding relevance. The common strategy of considering only individual dietary factors or only a limited number of them may not be sufficient in detecting diet–disease relationships, because it is not capable of modelling and estimating the overall effect of diet on cancer risk. Principal component analysis and the closely related factor analysis were the methods most often used to identify comprehensive food factors (dietary patterns) consisting of highly correlated foods(Reference Schulze and Hu22, Reference Schulze, Hoffmann, Kroke and Boeing23). However, if an underlying hypothesis about the diet–disease pathway exists, the application of these pattern techniques in nutritional epidemiology might be limited, as both methods focus on the variation of food intake only, disregarding the possibly more important variation of nutrients and other food constituents. Since nutrients and other food constituents are considered as the actual biologically active compounds of foods, this disregard might lead to overlooking variation of specific dietary factors which is important for the development or prevention of cancer. Indeed, epidemiologic studies to examine dietary patterns in relation to breast cancer risk have yielded mainly null results(Reference Adebamowo, Hu, Cho, Spiegelman, Holmes and Willett24–Reference Velie, Schairer, Flood, He, Khattree and Schatzkin26). Application of the reduced rank regression method overcomes this flaw, since it combines the two sources of information: a priori knowledge about possible pathways in disease aetiology and the dietary data at hand.
The present study has limitations that are worth mentioning. First, the relatively short follow-up period (6 years) yielded only a limited number of breast cancer cases. Therefore, risk estimates in sub-samples of the study population may be rather unstable and need to be repeated after a longer follow-up. Second, like in many other cohort studies, in the present study dietary intake was measured only once (at baseline recruitment) and not again during the course of follow-up in order to catch dietary changes. For the examination of diet–disease relations this implies the assumption of stable dietary behaviour of the study participants, which may not always be the case.
In conclusion, we found a dietary pattern characterized by a high consumption of processed meat, fish, butter and other animal fats, and margarine, and a low consumption of bread and fruit juices, and associated with dietary fat intake to be a significant risk factor for breast cancer. The present study findings give also new opportunities for a better understanding of the relationships between a high-fat diet and breast cancer risk from a population perspective. The identification of a food pattern generally high in fat being predictive of breast cancer nourishes the current debate about the role of fat intake in breast cancer development.
Acknowledgements
H. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. S. and H. B. were responsible for the study concept and design, and drafting of the manuscript. M. S., C. W. and U. N. acquired the data. Analysis and interpretation of data were performed by M. S., K. H. and M. B. S. Statistical analysis was performed by M. S. and K. H. All the authors were involved in the critical revision of the manuscript for important intellectual content. There were no conflicts of interest.




