CVD is the leading cause of mortality both among the general population and among patients with chronic kidney disease (CKD). The rate of mortality from CVD among patients on haemodialysis (HD) is 20-fold higher than that among the general population(Reference Cheung, Sarnak and Yan1), and impaired renal function per se is a very strong cardiovascular prognostic factor(Reference Sarnak, Levey and Schoolwerth2, Reference Go, Chertow and Fan3). Therefore, much attention has been focused on managing risk factors to attempt to reduce the associated CVD risks, especially among patients with CKD. Elevated plasma total homocysteine (Hcy) has been proposed as a risk factor for cardiovascular morbidity and mortality in patients either with normal renal function or with CKD(Reference Eikelboom, Lonn and Genest4, Reference Bayes, Pastor and Bonal5). Among patients with CKD, plasma Hcy levels tend to increase with decreasing glomerular filtration rate(Reference Wollesen, Brattstrom and Refsum6); thus, hyperhomocysteinaemia may potentially further increase the risk of CVD in this special population. In the Cardiovascular Risk Extended Evaluation in Dialysis trial, investigators followed-up on 175 patients with kidney failure for 29 months and found that an increase in Hcy of 10 μmol/l was associated with a 20 % increased risk for CVD events(Reference Mallamaci, Zoccali and Tripepi7). In another study, Hcy was found to be a strong independent predictor of mortality among patients on HD with a 3 % increase in mortality for each 1 μmol/l increase in plasma Hcy concentration(Reference Buccianti, Baragetti and Bamonti8).
Paradoxically, it has not been confirmed that therapy to lower the concentration of Hcy decreases the risk for CVD events among the general population(Reference Lonn, Yusuf and Arnold9, Reference Bazzano, Reynolds and Holder10) or among patients with CKD(Reference Suliman, Lindholm and Barany11). Several small randomised, controlled trials (RCT) of Hcy-lowering therapy for patients on dialysis have produced inconsistent results(Reference Zoungas, McGrath and Branley12–Reference Righetti, Serbelloni and Milani14). Recently, a meta-analysis from seven Hcy-lowering therapy trials in patients with CKD showed that folic acid therapy reduced the risk of CVD by 15 % (RR 0·85, 95 % CI 0·76, 0·96, P = 0·009), and a greater beneficial effect was observed among those trials with a decrease in Hcy levels >20 % (RR 0·83, 95 % CI 0·73, 0·95, P = 0·007)(Reference Qin, Huo and Langman15). However, this meta-analysis did not include three subsequently published RCT of Hcy-lowering and CVD, and so this result leads to a controversy. Considering the inconsistent results obtained with Hcy-lowering therapy in patients with CKD, we thought it important to attempt to resolve the question of whether Hcy-lowering therapy could reduce the risk of CVD morbidity and all-cause mortality in CKD patients who either are or are not on dialysis. Therefore, we conducted a meta-analysis of published RCT to pool the results and, thus, improve the statistical power of the analysis.
Methods
Search strategy
RCT were identified in MEDLINE (source PubMed, 1966–2010), EMBASE (1974–2010), www.clinicaltrials.gov, the Cochrane Controlled Clinical Trials Register Database and Nephrology Filters (http://hiru.mcmaster.ca/hiru/HIRU_Hedges_Nephrology_Filters.aspx). We used the following terms to search all trial registers and databases: ‘homocysteine OR HCY’ AND ‘chronic kidney disease OR End Stage Renal Disease (ESRD)’ AND ‘cardiovascular events’ AND ‘Folic acid’, presence of a randomised control group, duration of intervention ≥ 12 months, availability of primary CVD or secondary CVD outcome data for morbidity, stroke and all-cause mortality among patients with CKD, including those on dialysis and those not on dialysis. We obtained 134 papers. In the second step, we then kept all of the clinical trials and deleted review papers and those involving animal experiments; thus, 124 studies were excluded. The detailed steps are shown in Fig. 1. The bibliographies of all retrieved articles were also checked manually. Finally, ten studies met the inclusion criteria for our meta-analysis. All included trials were published in English-language medical journals.

Fig. 1 Flow diagram of selection process for inclusion of studies in the meta-analysis. RCT, randomised, controlled clinical trials.
Eligibility criteria
Studies were eligible for inclusion if the study was: a randomised, controlled trial; duration of intervention was more than 12 months; availability of primary CVD or second CVD outcomes, stroke and all-cause death among patients with CKD including those on dialysis and those not on dialysis; the intervention with folic acid and/or in combination with vitamins available for change of Hcy concentrations during therapy.
Data collection
Data were collected by all authors and extracted independently by two authors (H. M. Jin and Y. Pan) for participants' characteristics (study design, participant's age, sex, duration of follow-up, co-interventions (folic acid and/or vitamin (Vit) B6 and VitB12), baseline concentrations of Hcy and net change in Hcy from baseline). Any disagreement in data extraction was resolved by discussion between them and in consultation with the other authors (Table 1).
Table 1 Baseline characteristics of participants in randomised, controlled trials
(Mean values and standard deviations)

NR, not reported.
Summary measures and synthesis of results
The primary outcome was relative risk (RR) for cardiovascular events, CHD, stroke and all-cause mortality from the pooled trials. We also used subgroup analysis to assess the confounding factors to affect the outcomes such as whether on dialysis or not on dialysis, prescription of VitB6 or VitB12 and the rate of Hcy-lowering. To assess the potential for publication bias, we constructed funnel plots for each outcome in which the log RR was plotted against their standard errors. We also conducted a sensitivity analysis in which each study was extracted in turn to evaluate the influence of the study on the estimate.
Statistical analyses
All different units of Hcy were converted to μmol/l, using the conversion factor 1 mg/l = 7·397 μmol/l(Reference Bazzano, Reynolds and Holder10). RR were used as a measure of the association between the treatment group v. the control group and risk of CVD, CHD, stroke and all-cause mortality. RR for each trial were based on the number of events in each group. RR and 95 % CI for each side effect were calculated. We also carried out subgroup analyses based on whether the patients were on dialysis or not on dialysis, prescription of VitB6 or VitB12, and the extent of decrease in Hcy levels to further analyse the potential confounding factors in exploration of the different CVD outcomes. STATA version 10.0 (STATA Corporation) was used to pool the data and calculate RR. Statistical significance was set at P < 0·05 for all analyses. All analyses were conducted in parallel by two investigators (Y. Pan and H. M. Jin).
Results
Trial flow and study characteristics
The decision process that was used to differentiate among studies considered for inclusion is shown in Fig. 1. Overall, ten eligible studies with a total of 4836 participants were included in the review (2511 participants in the treatment group and 2325 participants in the control group)(Reference Zoungas, McGrath and Branley12–Reference Righetti, Serbelloni and Milani14, Reference Righetti, Ferrario and Milani16–Reference Heinz, Kropf and Domröse22). Of these, five of the selected trials focused on patients on dialysis, three focused on patients not on dialysis, and the remaining trial included patients among whom 60 % were on HD, 25 % were on peritoneal dialysis and 15 % were not on dialysis(Reference Zoungas, McGrath and Branley12). Effectively, nine of these trials provided data on the number of target events (including CVD, CHD, stroke and all-cause mortality) in each group, and one trial reported myocardial infarction as the cardiovascular event(Reference Jamison, Hartigan and Kaufman20). The net changes in blood Hcy are shown in Table 2. All trials showed a reduction in Hcy levels, in the range − 2·4 to − 26·0 μmol/l ( − 8·9 % to − 53·8 %). However, the characteristics of baseline Hcy existed in a hung range from 14·7 (se 4·9) to 50·3 (se 6·0) among included studies. However, there was no significant association between the baseline of Hcy concentrations and the extent of Hcy-lowering.
Table 2 Reported changes in homocysteine (Hcy) levels
(Mean values and standard deviations; mean values and 95 % confidence intervals)

Vit, vitamin.
Relative risk for CHD, CVD, stroke and all-cause mortality
The results from random-effect models pooling the RR for CHD, CVD, stroke and all-cause mortality are shown in Figs. 2–5. As noted, Jamison et al. (Reference Jamison, Hartigan and Kaufman20) reported myocardial infarction as the cardiovascular event. The RR estimated were not statistically significantly different for any outcomes. The total number of CHD events was ninety-five among 981 in the treatment group and ninety among 820 in the control group, respectively (RR 1·00, 95 % CI, 0·75, 1·31, P = 0·97; Fig. 2); the total number of cardiovascular events was 501 among 2511 in the treatment group and 522 among 2325 in the control group (RR 0·94, 95 % CI 0·84, 1·05, P = 0·30; Fig. 3); the total number of strokes was eighty-five among 2013 in the treatment group and ninety-three among 1844 in the control group (RR 0·83, 95 % CI 0·57, 1·19, P = 0·31; Fig. 3); and the total all-cause mortality was 810 among 2320 in the treatment group and 757 among 2156 in the control group (RR 1·00, 95 % CI 0·92, 1·09, P = 0·98; Fig. 4).

Fig. 2 Relative risk (RR) for CHD in pooled trials. The RR estimated were not significantly different for CHD (RR 1·00, 95 % CI 0·75, 1·31, P = 0·974).

Fig. 3 Relative risk (RR) for CVD in pooled trials. The RR estimated were not significantly different for CVD (RR 0·94, 95 % CI 0·84, 1·05, P = 0·30).
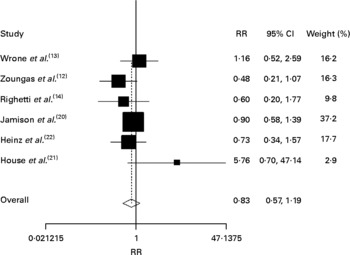
Fig. 4 Relative risk (RR) for stroke in pooled trials. The RR estimated were not significantly different for stroke (RR 0·83, 95 % CI 0·57, 1·19, P = 0·31).

Fig. 5 Relative risk (RR) for all-cause mortality in pooled trials. The RR estimated were not significantly different for all-cause mortality (RR 1·00, 95 % CI 0·92, 1·09, P = 0·98).
Subgroup analysis of relative risk for CVD
Whether on dialysis or not on dialysis, prescriptions of VitB6 or VitB12, or the degree of Hcy-lowering were potential confounders related to both the outcome and the primary variable of interest. As Fig. 6 shows, there were six trials focused on patients on dialysis and four trials focused on pre-dialysis. The estimated RR indicated that there was no statistical association between Hcy-lowering and incidence of CVD events in both pre-dialysis and dialysis conditions (RR 0·91, 95 % CI 0·74, 1·12, P = 0·39; RR 0·94, 95 % CI 0·74, 1·20, P = 0·62, respectively). Furthermore, there was also no significant relevance between incidence of CVD events and whether the degree of Hcy decreased by < 20 % or ≥ 20 % (RR 1·20, 95 % CI 0·78, 1·86, P = 0·41; RR 0·87, 95 % CI 0·76, 1·01, P = 0·06, respectively), although there was a declining tendency of CVD incidence in Hcy-lower ≥ 20 % subgroup (P = 0·06). Similarly, there was no significant difference in CVD incidence whether folic acid was alone or was in combination with VitB6 and VitB12 (RR 0·88, 95 % CI 0·75, 1·03, P = 0·12; RR 0·98, 95 % CI 0·76, 1·26, P = 0·88, respectively).

Fig. 6 Relative risk (RR) for CVD associated with dialysis condition, homocysteine (Hcy)-lowering degree, and in combination with vitamin (Vit) B6/12.
Sensitivity analyses and publication bias
In sensitivity analyses, exclusion of any one study from the analysis did not alter the overall findings of the non-significant association between Hcy-lowering therapy and the risk of cardiovascular outcome. Within the sensitivity analysis, we considered variance in results as a possible source of small heterogeneity. Publication bias was assessed by visually examining a funnel plot of precision against hazard ratio (not shown), with asymmetry being formally assessed with the Egger test; and no significant bias was found (bias = − 0·07, P = 0·94).
Discussion
In this meta-analysis, ten RCT were combined to assess the effects of folic acid-based therapy with or without other vitamins on the risk for CVD events and all-cause mortality among patients with CKD who were either on or not on dialysis. The pooled results confirmed no significant benefit of Hcy-lowering therapy for the risk of CVD, stroke and all-cause mortality among this population of patients with CKD.
Numerous conflicts and inconsistencies have obscured the interpretation of the literature on this topic. For example, the plausibility of a link between the levels of Hcy and the risk of CVD was supported by a number of studies. One report demonstrated that endothelial cells can develop abnormal vasoreactivity and may adopt a prothrombotic phenotype and secrete leucocyte chemotactic factors, adhesion molecules and inflammatory cytokines when exposed to elevated Hcy(Reference Widlansky, Gokce and Keaney23). Endothelial dysfunction is a key process in atherosclerosis, which independently predicts CVD events(Reference Schachinger, Britten and Zeiher24); and it becomes progressively more common as renal function declines(Reference Tatematsu, Wakino and Kanda25). However, studies of supplementation with either 5 mg/d folic acid or 5 mg/d folic acid combined with 4 g/d betaine for 12 weeks, followed by either 1 or 5 mg/d folic acid for 40 weeks showed no improvement of endothelial function in patients on peritoneal dialysis or HD(Reference van Guldener, Janssen and Lambert26, Reference van Guldener, Janssen and Lambert27).
Another problem that complicates the interpretation of the literature on Hcy-lowering therapy is the lack of data supporting either an optimal dose of folic acid, an optimal target level of Hcy, or the required duration of intervention in order to see an effect. Arnadottir et al. (Reference Arnadottir, Gudnason and Hultberg28) observed that an average dose of 2·14 mg/d folic acid was as effective as either 5 or 10 mg/d over a 6-week period among HD patients for lowering Hcy; so 2 mg of folic acid has been recommended as the optimum dose for CKD patients. However, in clinical trials, attempts to normalise Hcy with either 1 or 5 mg/d folic acid were unsuccessful over a 40-week period among HD patients(Reference van Guldener, Janssen and Lambert27). In fact, reduction to a normal Hcy level occurs in only about 20 % of treated patients. Also, to the best of our knowledge, there are no studies that have determined a threshold value for Hcy level for effective prevention of cardiovascular events in the general population or in patients with CKD. Observational data in a meta-analysis show that a 25 % reduction in Hcy was associated with a 32 % lower risk of CVD among women and a 15 % lower risk among men(Reference Boushey, Beresford and Omenn29). However, the reported decreases in Hcy levels vary substantially; and the ten papers in our meta-analysis reported a wide range of Hcy-lowering from 8·9 % to 53·8 %, which is consistent with the observed range of decrease in patients without CKD. In this meta-analysis, we could not observe the benefits when Hcy levels decreased by 20 %, which was in contrast to a previous meta-analysis(Reference Qin, Huo and Langman15). It is unclear whether there are benefits if the reduction of Hcy levels is greater. In fact, the degree of Hcy-lowering in each patient in response to folic acid therapy is different. It has been proposed that the mutant TT genotype at nucleotide 677 of the gene regulating methylenetetrahydrofolate reductase activity is associated with increased Hcy levels and increased mortality in general and CKD populations. In the methylenetetrahydrofolate reductase TT group, the greatest reductions of Hcy concentration after folate therapy were observed(Reference Tremblay, Bonnardeaux and Geadah30). However, it could not be observed in other studies(Reference Billion, Tribout and Cadet31, Reference Födinger, Mannhalter and Wölfl32), since Hcy concentration decreased in all genotypes.
Another confounding factor that could affect the outcomes of Hcy-lowering therapy among patients on dialysis might be the dialysis procedure and membrane pore size. However, dialysis kinetics does not affect the levels of Hcy reduction(Reference House, Wells and Donnelly33, Reference Biasioli, Schiavon and Petrosino34). Furthermore, studies comparing non-albumin-leaking with albumin-leaking high-flux membranes also failed to find a significant difference in plasma Hcy levels before dialysis despite greater clearance per dialysis session(Reference Galli, Benedetti and Buoncristiani35, Reference Mudge, Rogers and Hollett36). Finally, a recent study found no difference in Hcy levels between patients on HD compared to those on peritoneal dialysis(Reference Helal, Smaoui and Hamida37).
A third unresolved issue is whether elevated plasma Hcy is paralleled by increased Hcy concentration in the coronary arteries of CKD patients, and few studies have addressed this question. In patients with coronary artery disease (CAD)(Reference Mehrabi, Huber and Serbecic38, Reference Jadad, Moore and Carroll39), it was observed that although the CAD group presented with high plasma Hcy levels (27·7 (se 12·8) μmol/l), the media and intimal layers containing the endothelium exhibited the lowest enrichment of Hcy (media: 20·8 (se 4·4) %; intima: 6·1 (se 2·3) %). In contrast, the control group (from heart donors) revealed an extensive Hcy enrichment that co-localised with vascular smooth cells by immunohistochemical staining. Similar investigations in patients with CKD have not been performed.
Now that there are no benefits for the prevention of CVD events and mortality in individuals during treatment with folic acid and/or in combination with vitamins, another concern has also been raised about the safety of folic acid, particularly in relation to CVD and mortality risk. Small trials were designed to deal with this negative effect. In a double-blind, placebo-controlled trial with a total of 6837 patients with IHD, after a median 39 months of follow-up, it had been observed that treatment with folic acid plus VitB12 was associated with increased all-cause mortality, with a hazard ratio of 1·18 (95 % CI 1·04, 1·33, P = 0·01)(Reference Ebbing, Bønaa and Nygård40). In the House et al. (Reference House, Eliasziw and Cattran21) study, a multicentre, randomised, double-blind, placebo-controlled trial, it was also found that high doses of B vitamins resulted in a greater decrease in glomerular filtration rate and an increase in vascular events as myocardial infarction, stroke, revascularisation (peripheral, cardiac angioplasty or cardiac bypass). Considering this potential side effect, more RCT are needed to confirm the results.
There are several limitations in this meta-analysis to improve the statistical power to assess the effect of Hcy-lowering therapy on CVD events and mortality in patients with CKD. One is that there are still relatively few trials. Only ten RCT compared folic acid-based active therapy with or without other vitamins with placebo, and were included in the meta-analysis. Second, there was considerable variation among patients (CKD without dialysis and on dialysis) and the degree of reduction in plasma Hcy concentration. Third, the study by Nanayakkara et al. (Reference Nanayakkara, van Guldener and ter Wee18) was included in the meta-analysis although it was not mainly designed to examine the clinical end points.
Despite these limitations, the present meta-analysis supports the conclusion that Hcy-lowering therapy was not associated with a significant decrease in the risk for CVD events, stroke and all-cause mortality among patients with CKD. Although these results cannot rule out the possibility that normalisation of Hcy in CKD would benefit patients with CKD or under dialysis, it seems to be very difficult to normalise the Hcy concentration in this special population. In conclusion, more large-scale clinical trials evaluating the effect of Hcy-lowering therapy on cardiovascular risk are expected to clarify whether Hcy-lowering therapy has the potential to clearly decrease cardiovascular risk among this special population.
Acknowledgements
The authors are solely responsible for the design and conduct of the present study; as also all study analyses, and drafting and editing of the paper. Y. P., X. J. Z., L. L. G., L. L. C., J. L. S., X. L. L. and H. M. J. participated in the design of the study, collected the data and contributed to the analysis. H. M. J. designed the study, was responsible for the data analysis and interpretation, and wrote the manuscript. All authors have approved the final version of the manuscript and agreed to the submission. None of the authors had any conflicts of interest. This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.










