Obesity is a pro-inflammatory state characterised by an increased production of different cytokines by the adipose tissue( Reference Greenberg and Obin 1 , Reference Trayhurn and Wood 2 ). It has been reported that obesity-associated inflammation and oxidative stress are the key and early contributing factors for the occurrence of many chronic diseases, including type 2 diabetes, hypertension and CVD( Reference Greenberg and Obin 1 , Reference Mokdad, Ford and Bowman 3 , Reference Esposito and Giugliano 4 ). Therefore, the suppression of low-grade chronic pro-inflammation and oxidative stress is an effective strategy for reducing the risk of these diseases.
Histidine is an important amino acid for humans. It has been reported that chronic kidney disease patients have a low plasma concentration of histidine, which is associated with protein-energy wasting, inflammation and oxidative stress( Reference Watanabe, Suliman and Qureshi 5 ). In a previous study, we had found that serum histidine concentrations in obese women were lower than those in non-obese women and had negative relationships with inflammation and oxidative stress( Reference Niu, Feng and Hou 6 ). The recovery of serum histidine concentrations to normal levels through histidine supplementation could significantly attenuate inflammation and oxidative stress in obese women( Reference Feng, Niu and Sun 7 ). In addition, plasma histidine concentrations have been found to be lower in diabetic BALB/cA mice than in non-diabetic control mice, and histidine supplementation has been found to markedly reduce the concentrations of IL-6, TNF-α and C-reactive protein (CRP)( Reference Lee, Hsu and Lin 8 ). Histidine has been reported to inhibit the TNF-α-induced IL-8 expression at the transcriptional level in intestinal epithelial cells( Reference Son, Satsu and Shimizu 9 ). However, the potential in vivo mechanisms involved in the anti-inflammatory and anti-oxidative stress functions of histidine in the obese state are still unclear.
The transcription factor NF-κB is a central regulator of various cellular genes involved in immune and inflammatory responses( Reference Tak and Firestein 10 , Reference Li and Verma 11 ). Under basal conditions, NF-κB is an inactive cytoplasmic heterotrimer consisting of p50, p65 and inhibitor of κBα (IκBα) subunits. In response to stimulation by factors such as lipopolysaccharides and TNF-α, IκBα undergoes phosphorylation and an ubiquitination-dependent degradation by a proteasome complex, which leads to the p65 subunit being transferred into the nucleus and stimulating the transcription of its target genes, such as IL-6, TNF-α and CRP ( Reference Tak and Firestein 10 ). The reported studies have revealed that the NF-κB signalling pathway could be a therapeutic target in chronic inflammation, evidenced by the results that the inhibition of this pathway could attenuate inflammatory responses( Reference Yamamoto and Gaynor 12 , Reference D'Acquisto, May and Ghosh 13 ).
Adiponectin, one of the most abundant gene transcripts of the adipose tissue, is an important link among obesity, inflammation and chronic diseases( Reference Finelli and Tarantino 14 , Reference Kwon and Pessin 15 ). It has been reported that an increase in adiponectin concentrations could attenuate inflammation in obese individuals( Reference Ouchi and Walsh 16 ). In a previous study, we had found that histidine supplementation could increase the serum concentrations of adiponectin in obese women( Reference Feng, Niu and Sun 7 ). Therefore, we sought to study whether histidine could attenuate inflammation by increasing the serum concentrations of adiponectin. The expression of adiponectin has been found to be significantly enhanced by PPARγ at the transcriptional level( Reference Maeda, Takahashi and Funahashi 17 ). PPARγ is a ligand-activated transcription factor that is most widely expressed in the adipose tissue. Importantly, PPARγ is a central regulator of adipocyte differentiation and controls many adipocyte genes by binding to specific PPAR response elements in the promoters of these genes( Reference Monsalve, Pyarasani and Delgado-Lopez 18 ).
Therefore, in the present study, we focused on the NF-κB- and PPARγ-involved pathways to investigate the mechanisms underlying the effects of histidine on inflammation and oxidative stress in a high-fat diet (HFD)-induced female obese rat model.
Experimental methods
Ethics statement
All protocols used in the present study were approved by the Medical Ethics Committee of Harbin Medical University (Harbin, China) and were implemented in accordance with the National Institutes of Health regulations for the care and use of animals in research.
Animals and obese model
A total of fifty adult female Sprague–Dawley rats with body weight ranging from 160 to 200 g were purchased from Shanghai SLACK Laboratory Animal. The rats were allowed to acclimatise for 1 week before conducting the experiment. Among these rats, eight were randomly selected and fed the AIN-93M diet for 12 weeks until the end of the experiment( Reference Reeves 19 ). The remaining forty-two rats were fed a HFD for 8 weeks to establish an obese model (OM group). The HFD was based on the AIN-93M diet with lard and maize starch contents being adjusted to 180 and 286·5 g/kg, respectively. To ensure that both diets had the same amount of amino acids, especially histidine, the average food intake per d of rats fed the AIN-93M diet was restricted to the same amount of food intake of the OM group, and the eight rats were designated as the diet-restricted group for obese model (OM DR group). The rats were housed individually in a temperature-controlled room under a 12 h light–12 h dark cycle and given free access to water.
Histidine supplementation
A total of ten HFD-fed rats with body weight less than the mean body weight plus 1-fold of the standard deviation of the OM DR group were considered to be obesity resistant and excluded from the study. The remaining obese rats (n 32) were randomly assigned to four groups: (1) OM group (no additional histidine included); (2) low-histidine dose group (histidine 0·375 g/kg·body weight); (3) high-histidine dose (H-His) group (histidine 1·875 g/kg·body weight); (4) diet-restricted group for high histidine dose (HH DR) group (no additional histidine included and with food intake the same as that of the H-His group). Histidine (purity ≥ 95 %) was purchased from Yuan Cheng Gong Chuang. The four groups were fed the same HFD mentioned above until the end of the experiment. Suspensions containing different doses of histidine in an aqueous solution of carboxymethyl cellulose were orally administered to rats at a dose of 1 ml/100 g·body weight. These doses were chose on the basis of the protocols described in previous reports( Reference Yan, Wu and Yin 20 , Reference Liu, Liu and Yin 21 ). Treatment was carried out for four consecutive weeks (Fig. 1). Food intake was recorded daily and body weight was measured weekly.
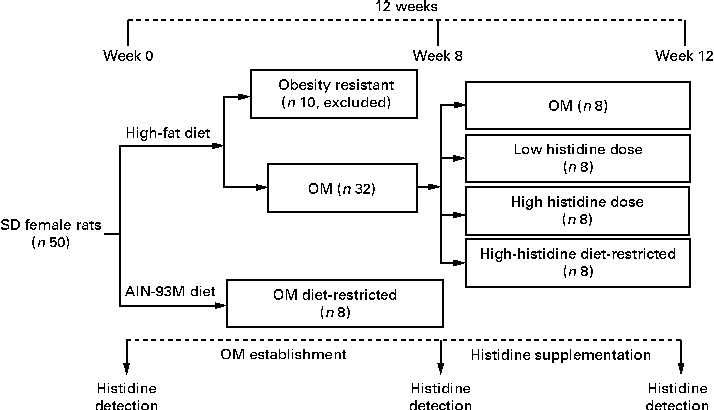
Fig. 1 Flowchart depicting obese model (OM) establishment and histidine supplementation protocol. SD, Sprague–Dawley.
Sample collection
Tail blood samples were collected from each rat at the beginning and at the end of week 8. At week 12, the rats were killed after an overnight fast and pentobarbital anaesthesia. Blood samples were obtained from the abdominal aorta and centrifuged (3000 rpm for 15 min). Livers, kidneys and white adipose tissues (parametrium, perirenal fat and omental fat pads) were removed, weighed, quick-frozen in liquid N2 and stored at − 80°C for further analysis.
Serum analysis
The serum concentrations of histidine were measured at weeks 0, 8 and 12 according to the method reported previously( Reference Niu, Feng and Hou 6 ). The serum concentrations of TNF-α, IL-6, CRP and adiponectin were determined by ELISA using commercial kits (TNF-α and IL-6, R&D Systems Europe; CRP, Biocheck, Inc.; and adiponectin, AdipoGen) according to the manufacturers' protocols. The serum concentrations of superoxide dismutase (SOD) and malondialdehyde were measured with enzymatic methods using commercial kits (Jiancheng Technology).
RNA isolation and real-time PCR
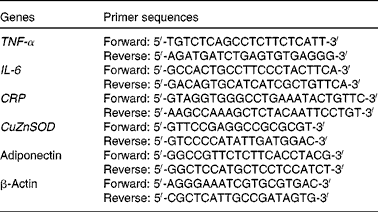
The mRNA levels of TNF-α, IL-6, CRP, adiponectin and CuZnSOD in white adipose tissue were determined by real-time PCR. Total mRNA was extracted from the white adipose tissue using the TRIzol reagent (Invitrogen). Real-time PCR was carried out using the SYBR Green PCR Master Mix and the 7500 FAST Real-time PCR System (Applied Biosystems). Relative quantification of mRNA expression was performed using the ΔΔC t (2− ΔΔC t ) method( Reference Li, Li and Ning 22 ). Primer sequences used in the PCR are listed in Table 1. All reactions were carried out at least in triplicate.
Table 1 Primer sequences for TNF-α, IL-6, C-reactive protein (CRP), copper-zinc superoxide dismutase (CuZnSOD), adiponectin and β-actin used in the PCR
Western blot analysis
Nuclear protein extracts and total protein extracts of the white adipose tissue were prepared using protein extraction kits (Biotype Institute of Biotechnology) according to the manufacturers' protocol. Western blot analysis was carried out as described previously to measure the concentrations of proteins involved in the NF-κB and PPARγ pathways( Reference Dong, Li and Ning 23 ). Antibodies used for protein detection were bought from the following sources: NF-κB p65, phospho-IκBα and IκBα from Cell Signaling Technology; PPARγ and Histone H3 from Abcam; β-actin from Santa Cruz Biotechnology, Inc.; secondary antibody from Promega Corporation. Data are presented as the relative intensity of the protein bands. Experiments were replicated at least three times, and representative blots are shown in the figures.
Statistical analysis
Data are presented as means with their standard errors. Data were analysed using one-way ANOVA followed by post hoc test, with P< 0·05 considered to be significant. All P values were two-sided. Statistical analysis was carried out using the SPSS software (version 16; Beijing Stats Data Mining).
Results
Histidine reduces high-fat diet-induced body weight increase and food intake
An obese rat model was successfully established after 8 weeks of HFD feeding, evidenced by the obvious increase in body weight in the OM group when compared with that in the OM DR group (P< 0·001; Table 2). After histidine supplementation for another 4 weeks, HFD-induced body weight increase was markedly reduced by high-dose histidine supplementation compared with that in OM group (P= 0·042; Table 2). The food intake of the H-His group was significantly lower than that of the OM group from week 10 (P= 0·026) after histidine supplementation (Fig. 2). However, no significant differences in body weight and food intake were observed between the H-His and HH DR groups.
Table 2 Serum histidine concentration and body weight before and after histidine supplementation in rats (Mean values with their standard errors)
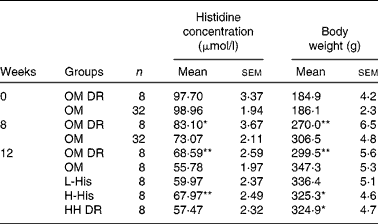
OM, obese model group; OM DR, diet-restricted group for obese model; L-His, low-histidine dose group; H-His, high-histidine dose group; HH DR, diet-restricted group for high histidine dose.
Mean values were significantly different from those of the OM group: * P< 0·05; ** P< 0·01 (ANOVA).

Fig. 2 Comparison of weekly food intake of each group after histidine supplementation. OM (![]() ), obese model group; OM DR (
), obese model group; OM DR (![]() ), diet-restricted group for obese model; L-His (
), diet-restricted group for obese model; L-His (![]() ), low-histidine dose group; H-His (
), low-histidine dose group; H-His (![]() ), high-histidine dose group; HH DR (
), high-histidine dose group; HH DR (![]() ), diet-restricted group for high histidine dose. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the OM group (P< 0·05; ANOVA).
), diet-restricted group for high histidine dose. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the OM group (P< 0·05; ANOVA).
Histidine supplementation reverses high-fat diet-lowered serum histidine concentrations in obese rats
There was no difference in serum histidine concentrations between the OM and OM DR groups at the start of the experiment (Table 2). After HFD feeding for 8 weeks, serum histidine concentrations were decreased by 12 % in the OM group, compared with those in the OM DR group (P= 0·036; Table 2). Compared with those in HH DR group, the HFD-lowered serum concentrations of histidine were significantly reversed by histidine supplementation in the H-His group (P= 0·032; Table 2).
Histidine alleviates high-fat diet-induced adverse changes in serum inflammatory and oxidative biomarkers
As shown in Fig. 3, compared with those in the OM DR group, the serum concentrations of inflammatory factors, including TNF-α, IL-6 and CRP, were significantly elevated by HFD feeding in the OM group (P= 0·007, P= 0·004 and P= 0·005, respectively). The serum concentrations of adiponectin were reduced by HFD feeding (P= 0·002). After histidine supplementation, inflammation was not significantly attenuated in the low-histidine dose group (P>0·05). The HFD-induced adverse changes in inflammatory factors mentioned above were significantly alleviated in the H-His group than in the HH DR group (P =0·020, Fig. 3(A); P= 0·035, Fig. 3(B); P= 0·031, Fig. 3(C); and P= 0·028, Fig. 3(D), respectively). The results also revealed that histidine supplementation significantly improved HFD-lowered SOD concentrations (P= 0·017) and HFD-increased malondialdehyde concentrations (P= 0·039) in the serum when compared with those in the HH DR group (Fig. 3(E) and (F)).

Fig. 3 Effects of histidine supplementation on serum inflammatory and oxidative biomarkers. The serum concentrations of (A) TNF-α, (B) IL-6, (C) C-reactive protein (CRP) and (D) adiponectin were determined using commercial ELISA according to the manufacturers' protocols and those of (E) superoxide dismutase (SOD) and (F) malondialdehyde (MDA) were measured with enzymatic methods using commercial kits. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA). OM, obese model group; OM DR, diet-restricted group for obese model; L-His, low-histidine dose group; H-His, high-histidine dose group; HH DR, diet-restricted group for high histidine dose.
Histidine ameliorates the mRNA expressions of inflammatory and oxidative biomarkers in the adipose tissue
The mRNA expressions of TNF-α, IL-6 and CRP in the adipose tissue were markedly increased by HFD feeding (P< 0·001). Histidine supplementation significantly alleviated HFD-increased TNF-α, IL-6 and CRP expressions in the adipose tissue in the H-His group than in the HH DR group (P= 0·011, Fig. 4(A); P= 0·043, Fig. 4(B); and P= 0·037, Fig. 4(C), respectively). In addition, HFD-lowered adiponectin and CuZnSOD expressions in the adipose tissue were also reversed by high-dose histidine supplementation at the mRNA level compared with those in the HH DR group (P= 0·019, Fig. 4(D), and P= 0·013, Fig. 4(E)).

Fig. 4 Effects of histidine supplementation on the mRNA expressions of inflammatory and oxidative biomarkers in the adipose tissue. Adipose tissue mRNA was extracted and the mRNA expressions of (A) TNF-α, (B) IL-6, (C) C-reactive protein (CRP), (D) adiponectin and (E) copper-zinc superoxide dismutase (CuZnSOD) were measured by quantitative real-time PCR. Expression was normalised to that of β-actin. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA). OM, obese model group; OM DR, diet-restricted group for obese model group; L-His, low-histidine dose group; H-His, high-histidine dose group; HH DR, diet-restricted group for high histidine dose.
NF-κB pathway is involved in the anti-inflammatory role of histidine
The HFD stimulated strong transference of p65 into the nucleus in the adipose tissue compared with that in the OM DR group (P< 0·001). High-dose histidine supplementation significantly reduced the nuclear p65 content, accompanied by a decreased ratio of p65 protein content in the nucleus to that in the cytoplasm in the adipose tissue (P= 0·032; Fig. 5(A) and (B)). Histidine supplementation also blocked the phosphorylation of IκBα in the adipose tissue (P= 0·024; Fig. 5(C) and (D)). These results indicate that the histidine-inhibited NF-κB pathway contributes to its anti-inflammatory role in the adipose tissue.

Fig. 5 Inhibitory effects of histidine supplementation on the NF-κB-involved pathway in the adipose tissue. Nuclear protein extracts and total cell protein of the white adipose tissue were prepared and measured by Western blot analysis. (A) Blot depicting the reduction of p65 transference into the nucleus by histidine. (B) Quantification of the protein expression of p65 in the nucleus and cytoplasm shown as the relative intensity of the protein bands. (C) Blot showing the inhibition of the phosphorylation (p) of the inhibitor of κBα (IκBα) by histidine. (D) Quantification of the protein expression of p-IκBα shown as the relative intensity of the protein bands. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA). OM, obese model group; OM DR, diet-restricted group for obese model; L-His, low-histidine dose group; H-His, high-histidine dose group; HH DR, diet-restricted group for high histidine dose.
Histidine induces PPARγ expression in the adipose tissue
After 8 weeks of HFD feeding, the protein expression of PPARγ in the adipose tissue of the OM group was decreased compared with that in the adipose tissue of the OM DR group (P< 0·001). High-dose histidine supplementation significantly improved the HFD-down-regulated expression of PPARγ at the protein level (P= 0·021), suggesting that the PPARγ pathway is probably involved in the up-regulation effects of histidine on adiponectin expression (Fig. 6(A) and (B)).

Fig. 6 Effects of histidine supplementation on the protein expression of PPARγ in the adipose tissue. Nuclear protein extracts of the white adipose tissue were prepared and measured by Western blot analysis. (A) Blot depicting the induction of PPARγ protein expression in adipose tissue by histidine. (B) Relative densities normalised to Histone H3 expression. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA). OM, obese model group; OM DR, diet-restricted group for obese model; L-His, low-histidine dose group; H-His, high-histidine dose group; HH DR, diet-restricted group for high histidine dose.
Discussion
The present study demonstrates for the first time that histidine supplementation ameliorates HFD-induced inflammation in the adipose tissue of obese rats. The results of the present study revealed that histidine exerted its beneficial effects on obesity-associated inflammation by inactivating the NF-κB signalling pathway. The results also revealed that histidine reversed HFD-lowered adiponectin expression via a PPARγ-involved pathway, which might contribute to its role in anti-inflammation.
Histidine is generally considered to be a dietary essential amino acid for human infants and an important amino acid for adults. It has been reported that chronic kidney disease patients and obese and type 2 diabetic youth have low plasma concentrations of histidine( Reference Watanabe, Suliman and Qureshi 5 , Reference Mihalik, Michaliszyn and de las Heras 24 ). In our previous study, we had reported serum histidine concentrations were lower in obese women( Reference Niu, Feng and Hou 6 ). A similar result was obtained in the present study, i.e. serum concentrations of histidine in the HFD-induced obese rats were lower than those in the normal diet-fed non-obese rats, suggesting that the serum amino acid metabolism is correlated with the occurrence of obesity. In addition, we also observed that food intake and weight gain were lower in the H-His group than in the OM group, which could probably be explained by the suppression of appetite by histidine through its conversion into neuronal histamine in the hypothalamus( Reference Kasaoka, Tsuboyama-Kasaoka and Kawahara 25 , Reference Yoshimatsu, Chiba and Tajima 26 ). To eliminate the food intake-induced disturbance, we established a HH DR group for further excluding the effects of the reduced body weight on the alleviation of inflammation and, thereby, focused on the inhibitory effects of histidine on inflammation and oxidative stress.
TNF-α, IL-6 and CRP are pro-inflammatory cytokines, and the elevation of their concentrations has been confirmed in obese subjects( Reference Greenberg and Obin 1 , Reference Trayhurn and Wood 2 ). Previous studies have suggested that TNF-α and IL-6 are involved in obesity-related insulin resistance and atherosclerosis( Reference Gustafson 27 , Reference Kern, Ranganathan and Li 28 ). CRP is the most extensively studied marker of systemic inflammation in humans, which is also related to the insulin resistance syndrome and endothelial dysfunction( Reference Yudkin, Stehouwer and Emeis 29 ). In the present study, HFD induced an increase in the serum concentrations of TNF-α, IL-6 and CRP in the OM group but not in the OM DR group. Moreover, we found that high-dose histidine supplementation could decrease the serum concentrations of TNF-α, IL-6 and CRP in the H-His group but not in HH DR group, in spite of the same amount of food being consumed by both groups. These results were consistent with the results of our previous study in which histidine supplementation was found to significantly lower the serum concentrations of the inflammatory biomarkers TNF-α and IL-6 in obese women( Reference Feng, Niu and Sun 7 ). Correspondingly, a previous study has reported that plasma histidine concentration is significantly lower in chronic kidney disease patients with a history of CVD and inflammation, which is associated with inflammation, oxidative stress and mortality( Reference Watanabe, Suliman and Qureshi 5 ). The results of an animal study revealed that diabetic BALB/cA mice had lower plasma histidine concentrations than non-diabetic control mice and histidine supplementation could reduce the concentrations of IL-6, TNF-α and CRP in diabetic animal models( Reference Lee, Hsu and Lin 8 ). Besides, an in vitro study has also reported that histidine has a potential to attenuate intestinal inflammation by inhibiting the H2O2 and TNF-α-induced IL-8 secretion at the transcriptional level in intestinal epithelial cells( Reference Son, Satsu and Shimizu 9 ).
Adipose tissue is a crucial endocrine organ that secretes a wide variety of cytokines and has a central role in obesity-associated complications such as dyslipidaemia, insulin resistance and type 2 diabetes. Several studies have revealed that attenuation of inflammation could reduce the occurrence of inflammation-related diseases( Reference Kang, Goto and Han 30 , Reference Chuang and McIntosh 31 ). It has been reported that pre-intake of histidine and carnosine decreases the hepatic concentrations of IL-6, IL-10, and TNF-α during acetaminophen-induced liver injury in BALB/cA mice( Reference Yan, Wu and Yin 20 ). Our previous study has also demonstrated that histidine could inhibit the expressions of IL-6 and TNF-α in human preadipocytes( Reference Feng, Niu and Sun 7 ). However, little is known about the anti-inflammatory effects of histidine in the adipose tissue. Therefore, we quantified the mRNA expressions of inflammatory biomarkers in the adipose tissue using real-time PCR. The results revealed that the mRNA expressions of TNF-α, IL-6, and CRP in the adipose tissue were down-regulated by high-dose histidine supplementation, which indicates that histidine could alleviate HFD-induced inflammation in the adipose tissue. A number of recent reports have demonstrated the key role of the NF-κB signalling pathway in the development of inflammation-associated metabolic disorders in the liver and adipose tissue( Reference Baker, Hayden and Ghosh 32 , Reference Arkan, Hevener and Greten 33 ). The transcription factor NF-κB is a primary regulator of inflammatory responses. It has been reported that histidine could be a novel therapeutic agent for Crohn's disease by inhibiting NF-κB activation and down-regulating inflammatory cytokine production in macrophages( Reference Andou, Hisamatsu and Okamoto 34 ). Besides, in the previous study, we had observed that histidine could inhibit the translocation of NF-κB into the nucleus in human preadipocytes( Reference Feng, Niu and Sun 7 ). So, we quantified the expression of proteins involved in the NF-κB pathway in the adipose tissue by Western blot analysis, and the results revealed that histidine significantly inhibited NF-κB p65 translocation and IκBα degradation, which suggests that NF-κB might be responsible for the anti-inflammatory effects of histidine.
Adiponectin is an anti-inflammatory factor secreted by adipocytes( Reference Ouchi and Walsh 16 ). In contrast to most adipocyte hormones, the concentrations of circulating adiponectin are decreased in obesity and increased in response to weight loss( Reference Yang, Lee and Funahashi 35 ). We had found that histidine supplementation could increase the serum concentrations of adiponectin in obese women( Reference Feng, Niu and Sun 7 ). In the present study, we quantified the concentration of adiponectin in the serum and its mRNA expression in the adipose tissue. The results revealed that the expression of adiponectin was significantly increased by histidine supplementation. It is well accepted that the expression of adiponectin is markedly stimulated by the activation of PPARγ in adipocytes( Reference Maeda, Takahashi and Funahashi 17 ). So, we quantified the protein expression of PPARγ in the adipose tissue, and the results revealed that the expression of PPARγ was up-regulated by histidine supplementation, which might at least partially explain the results obtained for the effects of histidine supplementation on adiponectin. Moreover, PPARγ and NF-κB interact in several ways to oppose their respective activities. PPARγ can form a transcriptionally inhibitory complex with NF-κB in mouse macrophages( Reference Chung, Kang and Kim 36 ). Another study has revealed that the activation of PPARγ suppresses cytokine-induced NF-κB transcriptional activity and target gene expression in skeletal muscle cells( Reference Remels, Langen and Gosker 37 ). NF-κB also inhibits the binding of PPAR to genomic response elements, thereby reducing the transcriptional activity of PPAR and the expression of PPAR-related transcripts( Reference Seymour, Bennink and Watts 38 ).
In addition, histidine has been reported to protect human LDL against oxidation and glycation and to have beneficial effects on liver in rats with acetaminophen-induced liver injury through its actions against oxidative stress( Reference Lee, Hsu and Lin 8 , Reference Yan, Wu and Yin 20 ). Histidine can also restrict the accumulation of free radicals and delay the activation of extracellular signal-regulated kinase and c-jun N-terminal kinase in neuronal cells( Reference Kulebyakin, Karpova and Lakonsteva 39 ). Previous studies have suggested that the anti-oxidative effect of histidine is based on its free radical-scavenging and divalent metal ion-chelating abilities( Reference Babizhayev, Seguin and Gueyne 40 , Reference Lee, Miyawaki and Bobst 41 ). In the present study, we quantified two oxidative biomarkers, SOD and malondialdehyde, and the results revealed that the concentrations of SOD in the serum and the mRNA expression of CuZnSOD in the adipose tissue were increased and the concentrations of malondialdehyde in the serum were decreased by histidine supplementation, indicating that histidine is a potential antioxidant in obese individuals.
All these findings indicate that histidine supplementation could attenuate inflammation and oxidative stress in obese women. The results of the present study also provide a new insight for understanding the relationship between amino acid metabolism disorder and inflammation and/or oxidative stress, which suggests that more attention has to be paid to uncovering amino acid metabolism in obese individuals in a further study. In addition, previous studies have shown that women are more sensitive to dietary histidine and energy intake than men( Reference Kasaoka, Kawahara and Inoue 42 , Reference Okubo and Sasaki 43 ). Therefore, we chose female rats as the experimental animals in the present study. We established a HH DR group for excluding the effects of the reduced body weight on the alleviation of inflammation. However, artificially controlling food intake cannot completely imitate food intake reduction and body weight loss caused by histidine supplementation-induced appetite suppression. The suppression of appetite might lead to some unknown effects, which might be associated with the regulation of inflammation. Therefore, we cannot completely exclude the possibility that some of the findings might be related to the suppression of appetite, and more studies are required to be conducted in the future.
In summary, the present study provides evidence for the first time that by inhibiting NF-κB and activating PPARγ, histidine supplementation protects the adipose tissue from inflammation and oxidative stress induced by HFD feeding, suggesting that histidine is a potential candidate for ameliorating inflammation and oxidative stress in obese individuals.
Acknowledgements
The present study was supported by the National Natural Science Fund of China (81130049 and 81202184), the 12th China Five-Year Scientific and Technical Plan (grant no. 2012BAI02B00), and the Research Fund for Innovation Talents of Science and Technology in Harbin City (2013RFQXJ068).
The authors' contributions are as follows: S. Li, Ying Li and C. S. were responsible for the study concept and design; X. S., R. F. and W. Z. performed the experiment and collected the data; Yanchuan Li and S. Lin carried out the analysis and interpretation of the data; S. Lin and W. Z. performed the statistical analysis; X. S. and R. F. wrote the article; Ying Li and C. S. were responsible for obtaining funds and supervision; S. Li, X. S. and C. S. also contributed to the critical revision of the manuscript for its intellectual content; X. S. and S. Li had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.










